Abstract
Background
In 2009, a large meningitis A epidemic affected a broad region of northern Nigeria and southern Niger, resulting in more than 75 000 cases and 4000 deaths. In collaboration with state and federal agencies, Médecins Sans Frontières (MSF) intervened with a large-scale vaccination campaign using polysaccharide vaccine. Here the authors analyze the impact (cases averted) of the vaccination response as a function of the timing and coverage achieved.
Methods
Phenomenological epidemic models were fitted to replicate meningitis surveillance data from the Nigerian Ministry of Health/WHO surveillance system and from reinforced surveillance conducted by MSF in both vaccinated and unvaccinated areas using a dynamic, state–space framework to account for under-reporting of cases.
Results
The overall impact of the vaccination campaigns (reduction in meningitis cases) in Katsina State, northern Nigeria, ranged from 4% to 12%. At the local level, vaccination reduced cases by as much as 50% when campaigns were conducted early in the epidemic.
Conclusions
Reactive vaccination with polysaccharide vaccine during meningitis outbreaks can significantly reduce the case burden when conducted early and comprehensively. Introduction of the conjugate MenAfriVac vaccine has reduced rates of disease caused by serogroup A Neisseria meningitidis in the region. Despite this, reactive campaigns with polysaccharide vaccine remain a necessary and important tool for meningitis outbreak response.
Keywords: Meningitis surveillance, Neisseria meningitidis serogroup A, Nigeria, Polysaccharide vaccine, Vaccination outbreak response
Introduction
The epidemiology of meningitis in the African meningitis belt is complex, with seasonal increases in invasive disease and periodic, though unpredictable, large-scale outbreaks.1–3 Many bacteria contribute to seasonal cases of cerebrospinal meningitis (Neisseria spp, Haemophilus influenzae, Streptococcus pneumoniae), but large-scale epidemics are predominantly caused by Neisseria meningitidis serogroup A.1,2,4,5
Meningitis vaccination campaigns with a polysaccharide vaccine that provided short-term immunity1,6 have traditionally been reactive, beginning only after incidence reaches an outbreak threshold.7,8 Given the limited vaccine supplies and resources for conducting campaigns, this reactive approach also avoids resources being allocated to areas where they would have minimal impact. The threshold level for response has been based on observed outbreaks to maximize the sensitivity and the specificity of response.8 The threshold strategy necessarily allows epidemics to progress to a detectable level by the time a vaccination response is endorsed, resulting in a lag between outbreak identification and vaccination that can limit the impact of campaigns. A new conjugate vaccine against N. meningitidis serogroup A that is hoped to result in long-term immunity has recently been deployed in the meningitis belt.1,6 Full coverage with the conjugate vaccine is likely to take many years to achieve and the possibility of epidemics caused by serogroups for which the conjugate vaccine does not provide protection means that the risk of epidemics, and the need for reactive vaccination strategies, may continue for several years.
There has been little formal evaluation of the impact, in terms of cases averted, of reactive vaccination campaigns for managing meningitis outbreaks (though see references8–11). Model-based evaluation of the impact of vaccination campaigns12 is hampered by uncertainties about the link between endemic carriage and local or widespread outbreaks and a by a lack of detailed data on meningitis carriage.1–3 Mechanistic models of carriage have been developed for European settings3,13 but it is not clear that these can be directly applied in the African context, because of the differing environmental conditions and because European disease outbreaks have been dominated by N. meningitidis serogroup C.
In the study described here we took a comparative approach to the quantitative evaluation of the impact of reactive vaccination during the 2009 meningitis A outbreak in Katsina State, northern Nigeria (Figure 1A). We estimated the meningitis cases averted as a result of vaccination campaigns by fitting two phenomenological epidemic models that represent the potential extremes of meningitis epidemiology to meningitis surveillance data from areas with and without reactive campaigns: the first model assumes a time-varying attack rate independent of incidence and the second assumes that the attack rate is an increasing function of the incidence of infection. As the reality of meningitis epidemiology is likely to be intermediate to these two models, the two together reflect bounds on the possible impact of vaccination campaigns. By simulating from these two models, fitted to the 2009 outbreak in northern Nigeria, we compared the potential impact of a simultaneous vaccination strategy to that achieved using the current sequential strategy.
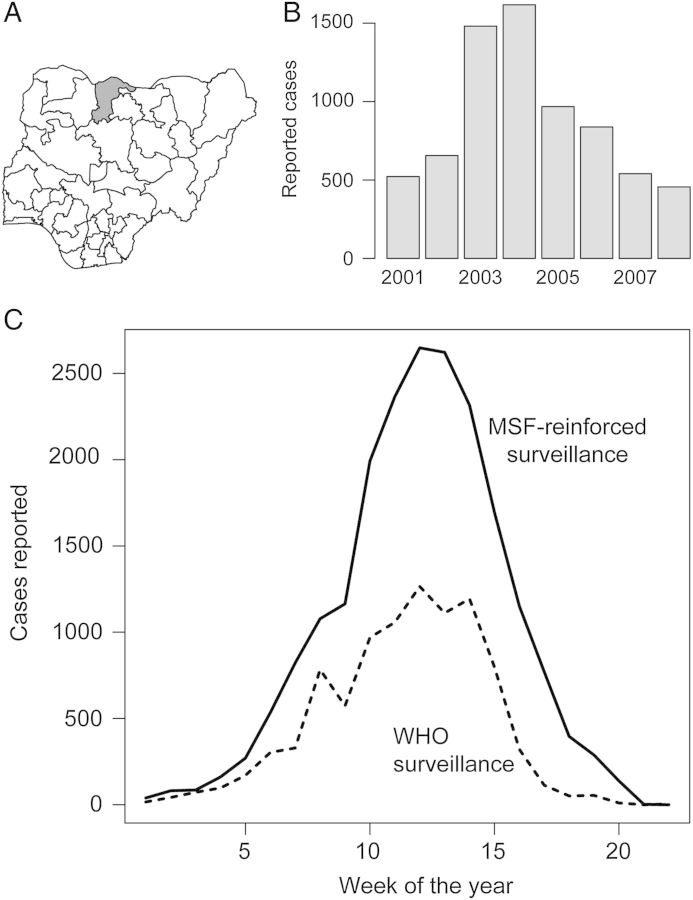
Figure 1.
(A) Katsina State (shaded) in northern Nigeria shares borders with the states of Jigawa, Kano, Kaduna and Zamfara, and with the Republic of Niger to the north. It has a population of 6 million. (B) Annual reported cases of meningococcal meningitis in Katsina, 2001–2008. (C) Weekly cases of meningococcal meningitis reported in Katsina State in 2009 through Médicines Sans Frontières-reinforced and WHO surveillance.
Materials and methods
Study area
In 2009, a large meningitis epidemic affected a broad region of northern Nigeria and southern Niger, resulting in more than 75 000 cases and 4000 deaths.14 Neisseria meningitidis serogroup A was identified as the main causative agent. In collaboration with the Nigerian Federal and State Ministries of Health, the Nigerian National Primary Health Care Development Agency and Nigeria's national program of immunization, Médecins sans Frontières Operational Center Paris (MSF-OCP) intervened in three states in northern Nigeria (Katsina, Jigawa and Bauchi), supporting case management, strengthening surveillance and organizing mass vaccination campaigns.
Following guidance prepared by the International Coordinating Group on Vaccine Provision for Epidemic Meningitis Control (composed of representatives from MSF, the International Federation of the Red Cross and Red Crescent Societies [IFRC], UNICEF and WHO), MSF-OCP conducted vaccination campaigns in Katsina. The campaigns targeted local government areas (LGAs) selected on the basis of the attack rate (cases per 100 000 population), and wards within each LGA selected by evaluating epidemic risk on the basis of number of cases reported, population size and density. The first vaccination campaign started on week 10 of 2009 and the last started on week 19 in three LGAs. Campaigns used a mix of trivalent (A+C+W135) and bivalent (A+C) polysaccharide vaccine. Campaigns targeted all individuals aged between 2 and 30 years. A total of 1 302 951 individuals were vaccinated in 134 (of 361) wards in 18 (of 34) LGAs.
Weekly cerebrospinal meningitis cases and death reports for the 34 LGAs of Katsina State were obtained from the WHO state office. These data were collected through the usual Nigerian state Ministry of Health (MoH) surveillance system, following the WHO Integrated Disease Surveillance and Response (IDSR) technical guidelines. In brief, during the meningitis epidemic, health facilities were required to record information for each suspected meningitis case in specific IDSR line-lists. Each week, the data were transmitted to the Disease Surveillance and Notification Officer (DSNO) at the LGA level, where the data were aggregated before being shared with the WHO state office. As part of its support to the epidemic response, MSF reinforced this surveillance system to ensure timely (weekly) monitoring of the epidemic. MSF data collection started in week 6, with support in six health facilities. Thereafter, MSF increased their support to cover 29 LGAs on week 11 (91 heath facilities), and then the entire state by week 14 (up to 127 health facilities). As health facilities were enrolled, MSF retrospectively reviewed health facility registers or IDSR reporting forms (line-lists or weekly reporting forms) according to the registration procedure in place in the health facility. Following enrollment, prospective data collection was supported through review of the WHO-recommended case definition, completion of line-lists, weekly reporting, and regular visitation by MSF case management teams. Both WHO/MoH and the MSF-reinforced surveillance relied on the same case definition and source information; however, MSF teams collected the data directly from the health facilities whereas WHO/MoH relied on transmission and compilation of these lists through a multilevel reporting system, with the possibility of loss in transmission.
The case definition of a suspected meningitis case was: any person with sudden onset of fever (temperature of >38.5°C rectal or 38.0°C axillary) and one of the following signs: neck stiffness, altered consciousness or other meningeal signs. The WHO data included weekly cases per LGA up to week 37 of 2009, though no cases were reported after week 22, and recorded a total of 9331 cases (Figure 1C). Data obtained from MSF-OCP were the cases per ward from week 50 of 2008 to week 22 of 2009, a total of 20 617 cases. Throughout, we distinguish these two data sources as WHO surveillance and MSF-reinforced surveillance, respectively.
Yearly cases of cerebrospinal meningitis for 2001–2006 and weekly cases of cerebrospinal meningitis for 2007 and 2008 were obtained from the State Ministry of Health (Figure 1B). LGA and ward population sizes were obtained from Katsina State authorities and are a linear extrapolation of the general population census conducted in 2006 assuming a 3% growth rate. The administrative vaccine coverage achieved during the 2009 vaccination campaigns per ward were obtained from MSF-OCP. For LGAs where only a fraction of wards were vaccinated, the LGA-level vaccine coverage was calculated as follows: vaccination coverage (%) in the vaccinated wards multiplied by the percentage of the LGA population residing in the wards in which campaigns were conducted.
Description of models
Pinner et al.9 proposed estimating the number of meningitis cases averted as a result of vaccination campaigns by estimating a weekly attack rate (AR) in the absence of vaccination from regions where campaigns were not conducted and applying that attack rate to those regions where campaigns were conducted. The AR is assumed to vary with time, but is independent of incidence; hence the model can be written as:
where It is the number of cases in location i and week t, St is the number of susceptible individuals, and Vt is the proportion of the population vaccinated in location i and week t. We assumed that vaccine efficacy is 0.85;9 we assessed the sensitivity of our results to this assumption by re-analyzing the data assuming vaccine efficacy ranging from 0.5 to 1.0 (see Supplementary Data). The form of this model assumes that the vaccination protects only those individuals that were vaccinated and has no effect on carriage or transmission. Given that in Katsina State there had been no major outbreaks of meningitis A nor any significant vaccination campaigns in several years, we assume that the entire population was susceptible at the start of the epidemic, which is consistent with the assumptions of Pinner et al.9
The Pinner model ignores the role of transmission in the progression of a meningitis outbreak, and is equivalent to a model of temporally varying environmental exposure. A recent longitudinal study in Burkina Faso showed seasonal increase in N. meningitidis carriage in the dry season, coincident with increased incidence of disease, which is at least consistent with increased transmission.15 Recognizing that N. menigitidis is directly transmissible, we construct an alternative model to evaluate the number of cases averted as a result of vaccination campaigns based on a model of directly transmitted infection. We can caricature the dynamics of meningitis as an S–E–I–R–S type pathogen (susceptible–exposed [carrier, non-infectious]–infectious–recovered–susceptible). In the context of a single outbreak we can assume that transition from temporary immunity to susceptibility is trivial. Further, in the absence of data on carriage, we collapse the carrier (E) and infectious (I) states into a single category and treat the outbreak as a standard susceptible–infectious–recovered (SIR)-type outbreak. This simplification of meningitis epidemiology as an SIR-type outbreak assumes that all cases during an epidemic are the result of transmission on the time-scale of the epidemic; i.e. no cases are attributable to asymptomatic carriers progressing to severe disease. Therefore we can write the number of new infections in location i and week t+1 as:
where βt is a time varying transmission rate, and I, S, and V are as above, and N is the LGA population size. As above, we assume that vaccine efficacy is 0.85 (see Supplementary Data for sensitivity to this assumption). As with the Pinner model, the transmission term is estimated using both the unvaccinated and vaccinated LGA populations. Here, because infection is directly transmitted, the probability of new infection is 0 when there are no cases in the LGA. We allow the introduction of infection to LGAs through migration from neighboring LGAs where the number of migrants, m, is an exponentially decaying function of distance between LGAs and the parameter γ controls the rate of decay with distance.
As above, all individuals are considered susceptible at the start of the epidemic. The time from infection to disease onset (infectious generation period) is set at 1 week, which was coincident with the time scale of the reported data. There is considerable uncertainty about the infectious generation period for meningitis, with published reports ranging from a few days16 to several months.3 Greenwood et al.17 reported that >50% of secondary cases occurred within 7 days of the index case in households, which provides further justification for this time scale.
In practice, the epidemiology of a meningitis outbreak is likely to lie somewhere between the Pinner model and the SIR-type model. The Pinner model ignores the role of transmission and therefore should result in a relatively low estimate of campaign impact, while the SIR-type model emphasizes transmission and therefore should result in a relatively high estimate of campaign impact. We present the results of these two analyses as measure of the potential bounds on the cases averted as a result of vaccination campaigns.
Model fitting
We fitted the parameters of the Pinner and SIR-type model using a state–space modeling framework.18,19 State–space models provide a probabilistic framework to predict the unobserved elements of a dynamic process (i.e. meningitis incidence), given observed elements of the dynamic process such as reported meningitis cases.18–22 In addition to predicting the unobserved elements of the system, state–space models facilitate the efficient calculation of the likelihood function for the observed elements of the system, thus allowing for statistical inference of the parameters of the dynamic process. State–space models are characterized by two inter-related sets of equations with unknown parameters: a ‘process model’ that represents the evolution of a dynamic process through time (i.e. the true disease incidence through time as a function of infection risk and immunization coverage) and an ‘observation model’ that represents the observation of that process (i.e. the cases reported through the two surveillance systems). Therefore the Pinner and SIR-type model constitute alternate process models of the epidemic dynamics through time. For the observation model, we made two assumptions: that the number of cases reported under the WHO surveillance system each week is a binomial sample from the true number of cases, with observation probability piWHO, and the number of cases reported in the MSF-reinforced data each week is a binomial sample from the true number of cases, with observation probability pR. The superscript i indicates that we estimated a separate observation probability for the WHO surveillance system for each LGA. We assumed that the MSF-reinforced data, because of the direct data collection on site, had the same observation probability in all areas. The observation probability for both data sources was considered as constant throughout the epidemic and estimated from the surveillance data by fitting the state–space model.
We fitted the full state–space model using a Bayesian Markov chain Monte Carlo framework.19,20 Markov chains of the states, Iit, the weekly attack rates, ARt, and the observation rates, pWHO and pR were generated using the Metropolis–Hastings algorithm with candidate values proposed using a random walk. We used non-informative uniform priors for all parameters except the migration parameter, γ, which was modeled as gamma distributed with shape and scale parameter equal to three. Markov chains were run for 100 000 iterations and the posterior was sampled following 50 000 iterations to allow for convergence.
Evaluation of campaign impact
For both the Pinner and the SIR-type model, we can evaluate the expected epidemic by simulating realizations from models 1 and 2, respectively, under the assumption that Vit is 0 for all locations and all weeks. Because of the variability in the epidemic outcomes without vaccination, the simulation was iterated 10 000 times each with parameters drawn independently from their respective posterior distributions. The mean of the predicted cases for each LGA and week provided an estimate of the number of cases in the absence of vaccination.
In practice, LGA populations were vaccinated after the incidence had crossed a threshold level, leading to campaigns that were sequential in time. An alternative would have been to vaccinate all LGA populations simultaneously; such a strategy would have resulted in more prophylactic vaccination in areas that were not yet experiencing outbreaks, but could have been criticized for not focusing effort on areas in crisis. To evaluate the potential impact of simultaneous vaccine strategies we simulated from the fitted models. To simplify the comparison to the fitted models we considered only vaccination in those LGAs where vaccination was carried out in 2009; thus, if the campaign had been a true mass campaign in all LGAs the predicted number of cases would have been lower. We simulated mass campaigns occurring on weeks 10–15.
Results
The outbreak of meningococcal meningitis began in the north of Katsina State and spread through the entire state (and neighboring states of Jigawa, Bauchi and Kano) by week 12 of 2009 (Figure 1). The outbreak peaked in week 14 of the year and no cases were reported after week 22.
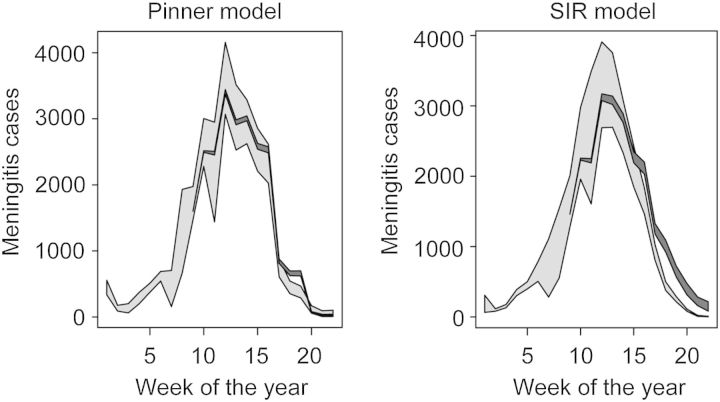
The Pinner model estimated that the true number of cases, correcting for under-reporting, was larger (26 531cases) than did the SIR-type model (21 742 cases), but that the percentage of cases averted as a result of the vaccination campaigns was smaller (4.1% vs 11.7% cases averted; Table 1, Figure 2). The greater predicted impact of vaccination campaigns in the SIR-type model is expected, as the structure of this model presumes that vaccination limits onward transmission as well as protecting the vaccine recipient from disease. For both models, the estimated LGA-level reporting rate for the WHO surveillance data was highly variable; point estimates ranged from 0.06 to 0.97 under the SIR model and 0.04 to 0.86 under the Pinner model. Estimates were not significantly affected by the assumption of 0.85 vaccine efficacy (see Supplementary Data).
Table 1.
Mean and quantiles (2.5th and 97.5th) of the posterior distribution of values estimated for the Pinner model9 and the SIR-type model for the 2009 meningitis outbreak in Katsina State, Nigeria
| Pinner model9 | SIR-type model | |
|---|---|---|
| Epidemic size | 26 531 (26 041, 27 095) | 21 742 (21 456, 22 079) |
| Reporting rate from MSF-reinforced surveillance | 0.778 (0.76, 0.79) | 0.936 (0.92, 0.95) |
| Mean WHO reporting rate | 0.430 (0.42, 0.44) | 0.538 (0.52, 0.55) |
| Standard deviation for WHO reporting rate | 0.215 (0.20, 0.23) | 0.253 (0.24, 0.26) |
| % cases averted | 4.1 (1.9, 6.2) | 11.7 (8.6, 14.9) |
MSF: Médicines Sans Frontières; SIR: susceptible–infectious–recovered.
Figure 2.
Model projections of meningococcal meningitis cases in Katsina State, Nigeria in 2009, corrected for estimated under-reporting, for the Pinner model9 and the SIR-type model. Light shading: 95% prediction intervals for the true cases. Dark shading: 95% prediction intervals for the epidemic in the absence of vaccination. Pinner model: see text; SIR: susceptible–infectious–recovered.
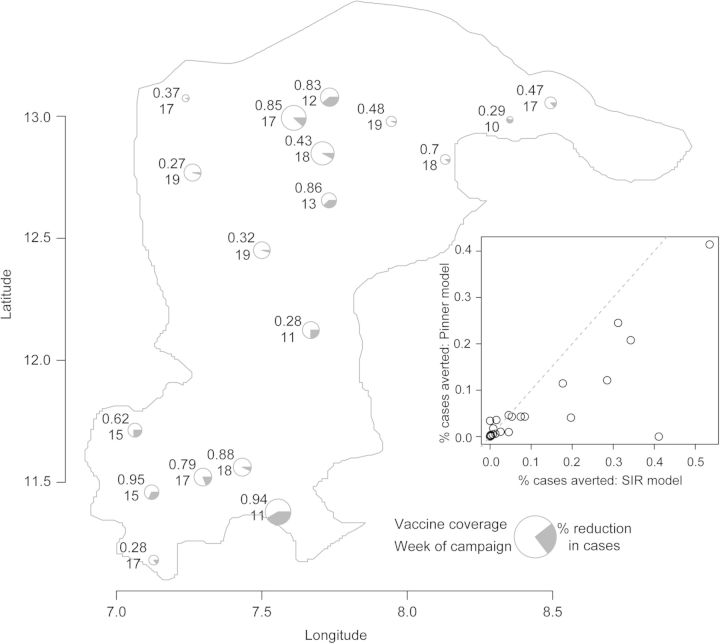
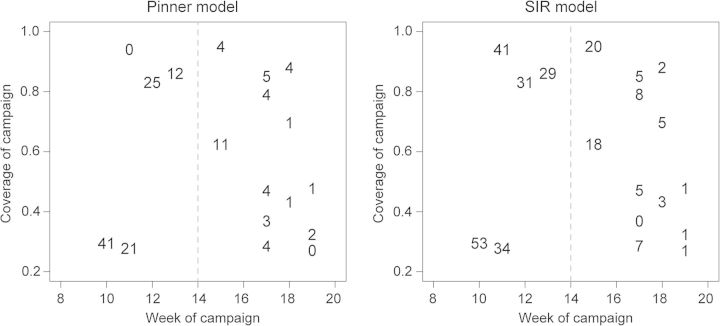
At the scale of individual LGAs, the predicted impact of the campaigns was highly variable (Figure 3). The SIR-type model predicted greater impact of the vaccine campaigns in all LGAs, though the magnitude of the predicted impact from both models was strongly correlated (Figure 3 inset). In general, the estimated impact of vaccination was positively correlated with the value of vaccine efficacy that we assumed (Supplementary Data); under the Pinner model estimated impact ranged from 0.018 to 0.065 and under the SIR model estimated impact ranged from 0.076 to 0.150 for vaccine efficacy of 0.5–1.0. The predicted impact of the vaccination campaigns under both models was greatest for campaigns that started early (Figure 4). In particular, campaigns that started before the epidemic peak (week 14) were predicted to have a higher percentage of cases averted. For both models there was no significant positive correlation between vaccine coverage and predicted impact. Note that our measure of vaccine coverage reflects the total population of the LGA that was vaccinated as not all wards in each LGA were vaccinated. Thus, LGAs with low coverage may reflect campaigns that targeted only a few wards. An alternative analysis using the coverage achieved in the targeted wards similarly finds no significant correlation between predicted impact and vaccination coverage (not shown). For those campaigns conducted after the epidemic peak (after week 14), there was a significant positive correlation (p<0.05) between vaccine coverage and campaign impact.
Figure 3.
Estimated impact of 2009 vaccine campaigns in Katsina State, Nigeria, under the SIR-type model in each local government area (LGA). Pie graphs are located at a central point for each LGA. The size of each circle is proportional to the estimated number of cases. The numbers to the left of each pie graph give the proportion of the target population vaccinated in the campaign (top) and the week of the year (2009) when the campaign began (bottom). Grey wedges represent the estimated impact of the campaign (proportional reduction of cases). Inset shows the correlation between the predicted impact under the Pinner9 and SIR-type models; dashed line represents a 1-to-1 relationship. SIR: susceptible–infectious–recovered.
Figure 4.
Impact of the 2009 meningitis vaccination campaigns (% cases averted) in Katsina State, Nigeria, as a function of the start week of the campaign (x-axis) and the vaccine coverage achieved (y-axis) for the Pinner model9 and the SIR-type model. SIR: susceptible–infectious–recovered.
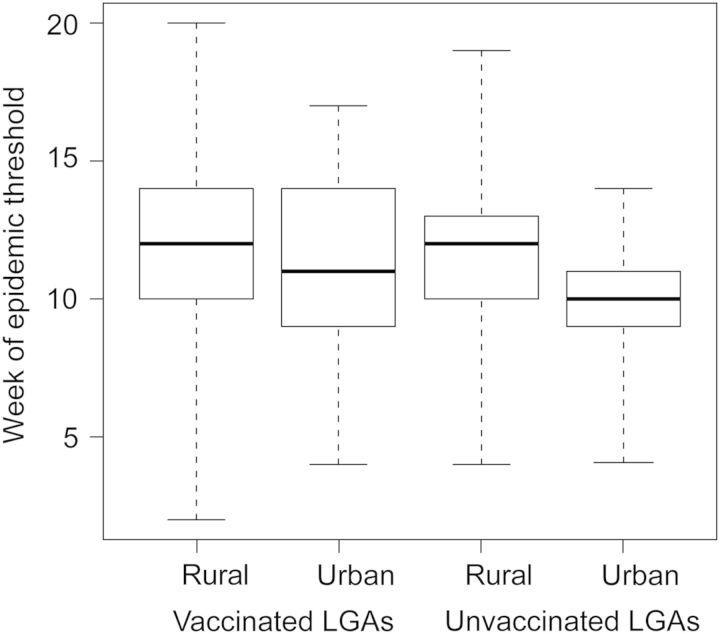
Urban wards tended to cross the epidemic threshold faster than did rural wards (ANOVA; p<0.05; Figure 5). Within each LGA the vaccination response was prioritized to the urban wards and those areas that were most heavily affected. The faster epidemics (mean=1 week earlier) in urban wards and prioritized vaccination response at the ward level was not reflected in the LGA-level epidemic models and may lead to some under-estimation of campaign impact.
Figure 5.
Distribution of the timing of the meningitis epidemic threshold (10 cases per 100 000) in Katsina State, Nigeria, in 2009 at ward level. Box plots are grouped into urban and rural wards in local government areas (LGAs) that received polysaccharide vaccination (left) in 2009 and those that did not (right). Boxes indicate the median, 25th and 75th quantiles of the distribution of weeks; dashed lines extend to the earliest and latest week in each category.
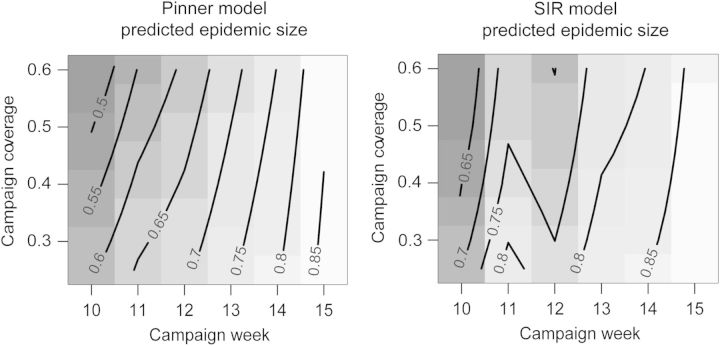
Under the predictions of both models, carrying out vaccination in all remaining LGAs in week 15 with coverage as low as 25% would have resulted in as many cases as in the staggered vaccination strategy that was used (Figure 6). Increasing vaccine coverage or earlier campaigns are predicted to have resulted in further cases averted, though earlier campaigns are predicted to be more effective than campaigns with higher coverage. Note that though the Pinner model projects a greater reduction in terms of overall cases, the projected size of the 2009 outbreak was larger under this model as well.
Figure 6.
The expected size of the meningitis outbreak (as a proportion of that observed in 2009), predicted by the Pinner model9 and the SIR-type model if all local government areas (LGAs) had been vaccinated simultaneously rather than in sequence. The x-axis gives the week of the simultaneous campaign and the y-axis gives the vaccination coverage achieved in each LGA (assuming all LGAs receive the same coverage). Grey shading and contour lines: size of the total outbreak as a proportion of the observed outbreak in 2009 in Katsina State, Nigeria. SIR: susceptible–infectious–recovered.
Discussion
Before the rollout of the new conjugate vaccine, reactive vaccination with polysaccharide vaccine has been the standard response to meningitis A outbreaks in sub-Saharan Africa. Polysaccharide vaccines remain the only tool available to control epidemics attributable to other serogroups that are circulating in the region.5 Further, experience with measles vaccine suggests that improved vaccination can result in increasingly episodic outbreaks.19 Therefore, while the immune landscape of N. meningitidis is changing in response to the introduction of a new vaccine, reactive campaigns are likely to remain a relevant public health tool for the near term. While timely response is always the goal, reactive campaigns are constrained by delays in reporting, vaccine availability and logistics; in Niger and Nigeria >60% of doses used in 2009 were received at or after the peak of incidence.14 The relative impact of these campaigns is often difficult to assess, because the impact of vaccination on meningitis transmissibility and epidemiology is disputed, and because comparative surveillance from nearby areas without campaigns is rarely available. We addressed these two limitations by taking a comparative approach to assessing the impact of vaccination campaigns, using two models that reflect extremes of the potential epidemiology and incorporating observations from areas with and without vaccination campaigns.
While this comparative, empirical approach allows a basis for the estimation of vaccination campaign impact, it remains a fairly naïve characterization of meningitis epidemiology. Though debate continues about the relative contribution of transmission, environmental and strain dynamic effects on the epidemiology of epidemic meningitis,2,3,5,13,23 the application of more mechanistic models in the African context is severely limited by a lack of data on the role of meningococcal carriage. However, this situation is changing through the efforts of the African Meningitis Carriage Consortium. To develop predictive models of meningitis outbreaks or consider a prospective evaluation of alternative response strategies would require the development of models that explicitly address these competing mechanisms and incorporate data on bacterial carriage.
While the goal should always be to attain the highest coverage possible, our comparative analysis of campaign impact across LGAs suggests that speed of response should be emphasized. In particular, under both models the number of potential cases averted dropped significantly for campaigns that began after week 14. The availability of good surveillance data is a significant limitation to the speed of vaccination response. While the WHO surveillance system tracked the progression of the epidemic well in all LGAs, our analysis suggests that the epidemic was significantly under-reported and highly variable at the LGA level. The reinforcement of the surveillance system by MSF took time–it was week 14 before all health facilities had MSF support for surveillance; however, the reinforced surveillance revealed a higher incidence of cases than was reported through the existing system.This implies that, had these cases been reported immediately, the response threshold and the resulting campaigns would have occurred earlier, resulting in improved outcomes. In many settings good data on population size are poorly available, so that vaccination response decisions based on incidence rates are limited by uncertainties in both the numerator and the denominator.
In practice, some vaccination campaigns occurred later because the outbreaks occurred later in those LGAs. The 2009 MSF-OCP campaign in Nigeria vaccinated >3 million individuals and delivered >28 000 courses of treatment at a cost of €5.4 million. The magnitude of these campaigns imposes significant logistical constraints for the size of a simultaneous response. Further, limited supply of polysaccharide vaccine necessitates real-time decisions about allocation, to ensure the availability of vaccine throughout the dry season. However, a strategy of responding only after an LGA has crossed an epidemic threshold results in consistent lags in vaccination response. A vaccination response over a larger area, even if it achieves lower overall coverage, may be a viable strategy if it tends to reduce the lag between the onset of an epidemic and the campaign in areas where outbreaks have not yet been detected. Simulations from the fitted models suggest that the reduction in case burden achieved in 2009 might also have been achieved with a lower coverage vaccination strategy in all LGAs, if it were conducted by week 15. Thus, as an outbreak spreads, the cost of moving vaccination teams to new locations before high coverage is achieved (e.g. at 75% rather than 90% coverage) might be offset by the gains achieved by moving them more rapidly to areas where outbreaks are just beginning. Clearly this recommendation does not account for any additional costs of such a coordinated campaign, or any political resistance to limiting vaccination response in already affected areas and directing resources to areas not yet experiencing large epidemics. However, it does suggest that this trade-off between campaign timing and campaign coverage is worth considering when planning the scope of a vaccination response. Further, the observation that outbreaks progressed faster in urban wards confirms the benefit of prioritized vaccination response at the finest scale possible.
The recent introduction of a new conjugate vaccine in the meningitis belt has the potential to significantly change both meningitis epidemiology and outbreak response policy. The conjugate vaccine is not currently approved for use in outbreak response outside of those areas where it has been already introduced; the characteristics that make it attractive as a prophylactic vaccine also recommend its utility for outbreak response. The new vaccine is predicted to limit transmission of meningitis,24–27 which would suggest an impact of the conjugate vaccine in the outbreak setting more like that predicted by the SIR model. Further, the predicted long-lasting immunity would provide protective benefits beyond the reduction of a current outbreak.24–27 The potential for longer lasting immunity also makes strategies such as the vaccination of neighboring, but not yet affected, areas more cost effective because of the additional, long-term benefit of protection from future outbreaks.
As with many vaccine-preventable diseases (e.g. measles, polio) meningitis outbreaks remain a significant public health threat even as vaccination coverage improves. Even if the conjugate meningococcal vaccine is incorporated into routine vaccination schedules, meningitis outbreaks may continue to be a threat. Further, N. meningitidis serogroups not covered by the conjugate vaccine (W135, X, C, B) continue to circulate in the meningitis belt and may increase in prevalence with reduced competition from serogroup A. The results presented here suggest that lags in outbreak detection attributable to imperfect reporting remain a significant limitation to timely and effective campaigns. To that end, it is useful to consider the limitations of previous vaccination campaigns and evaluate strategies to improve timely reporting of cases and rapid implementation of outbreak response in the future.
Supplementary data
Acknowledgments
Authors' contributions: MF developed modeling analyses. FF, FN, AL, RFG organized and contributed to field collection of data. MF, FF, FN, AL, CM and RFG contributed to the writing of the text. All authors read and approved the final version of the paper. MF is the guarantor of this paper.
Acknowledgements: We acknowledge the contributions of many local and state officials in Katsina who facilitated the collection of surveillance data. In particular the support of Dr Halliru Idriss (State Ministry of Health), Dr Bwaka Mpia Ado (WHO regional office) and Dr Chirstopher Mambula (Medical Coordinator for MSF in Abuja) was instrumental in facilitating data collection and in the evaluation of the analyses.
Funding: Funding for this project was provided by the Médicines Sans Frontières Operational Center, Paris. MF is supported by the Vaccine Modeling Initiative through the Bill and Melinda Gates Foundation and the Research and Policy for Infectious Disease Dynamics (RAPIDD) Program of the Science and Technology Directorate, US Department of Homeland Security and the Fogarty International Center, National Institutes of Health (NIH) and Ecology and Evolution of Infectious Diseases Initiative (EEID) award [1 R01 GM105247-01] from NIH, National Science Foundation (NSF) and the UK Biotechnology and Biological Sciences Research Council (BBSRC).
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.LaForce FM, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine. 2009;27(Suppl. 2):B13–9. doi: 10.1016/j.vaccine.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 2.Mueller JE, Gessner BD. A hypothetical explanatory model for meningococcal meningitis in the African meningitis belt. Int J Infect Dis. 2010;14:e553–9. doi: 10.1016/j.ijid.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162:89–100. doi: 10.1093/aje/kwi160. [DOI] [PubMed] [Google Scholar]
- 4.Broutin H, Philippon S, de Magny GC, et al. Comparative study of meningitis dynamics across nine African countries: a global perspective. Int J Health Geogr. 2007;6:6–29. doi: 10.1186/1476-072X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl.2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 6.Kieny MP, LaForce EM. The promise of conjugate vaccines for Africa. Vaccine. 2007;25(Suppl. 1):A108–10. doi: 10.1016/j.vaccine.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Geneva: WHO; 2001. WHO strategy for prevention and control of meningococcal disease in Africa. [Google Scholar]
- 8.Leake JAD, Kone ML, Yada AA, et al. Early detection and response to meningococcal disease epidemics in sub-Saharan Africa: appraisal of the WHO strategy. Bull WHO. 2002;80:342–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Pinner RW, Onyango F, Perkins BA, et al. Epidemic meningococcal disease in Nairobi, Kenya, 1989. J Infect Dis. 1992;166:359–64. doi: 10.1093/infdis/166.2.359. [DOI] [PubMed] [Google Scholar]
- 10.Lewis R, Nathan N, Diarra L, et al. Timely detection of meningococcal meningitis epidemics in Africa. Lancet. 2001;358:287–93. doi: 10.1016/S0140-6736(01)05484-8. [DOI] [PubMed] [Google Scholar]
- 11.Woods CW, Armstrong G, Sackey SO, et al. Emergency vaccination against epidemic meningitis in Ghana: implications for the control of meningococcal disease in West Africa. Lancet. 2000;355:30–33. doi: 10.1016/S0140-6736(99)03366-8. [DOI] [PubMed] [Google Scholar]
- 12.Grais RF, Conlan AJK, Ferrari MJ, et al. Time is of the essence: exploring a measles outbreak response vaccination in Niamey, Niger. J RSoc Interface. 2008;5:67–74. doi: 10.1098/rsif.2007.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotter C. Epidemiology of meningococcal disease and the impact of vaccination in Europe. 2006. Wurzburg, Germany: 58th Annual Conference, German Society for Hygiene and Microbiology (DGHM)
- 14.WHO. Meningitis in Chad, Niger and Nigeria: 2009 epidemic season. Wkly Epidemiol Rec. 2010;85:57–68. [PubMed] [Google Scholar]
- 15.Kristiansen PA, Diomande F, Wei SC, et al. Baseline meningococcal carriage in Burkina Faso before the Introduction of a meningococcal serogroup A conjugate vaccine. Clin Vaccine Immunol. 2011;18:435–43. doi: 10.1128/CVI.00479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27(Suppl.2):B71–7. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwood BM, Hassanking M, Whittle HC. Prevention of secondary cases of meningococcal disease in household contacts by vaccination. Br Med J. 1978;1:1317–9. doi: 10.1136/bmj.1.6123.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breto C, He DH, Ionides EL, King AA. Time series analysis via mechanistic models. Ann Appl Stat. 2009;3:319–48. [Google Scholar]
- 19.Ferrari MJ, Grais RF, Bharti N, et al. The dynamics of measles in sub-Saharan Africa. Nature. 2008;451:679–84. doi: 10.1038/nature06509. [DOI] [PubMed] [Google Scholar]
- 20.Clark J, Bjornstad ON. Population inference from messy data: errors, missing values and hidden states. Ecology. 2004;85:3140–50. [Google Scholar]
- 21.Ionides EL, Breto C, King AA. Inference for nonlinear dynamical systems. Proc Natl Acad Sci U S A. 2006;103:18438–43. doi: 10.1073/pnas.0603181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jandarov R, Haran M, Ferrari M. A compartmental model for meningitis: separating transmission from climate effects on disease incidence. J Agr Biol Envir Stat. 2012;17:395–416. [Google Scholar]
- 23.Trotter CL, Maiden MCJ. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8:851–61. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiden MCJ, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359:1829–30. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 25.Moore MR, Hyde TB, Hennessy TW, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004;190:2031–8. doi: 10.1086/425422. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Br Med J. 2003;326:365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestrheim DF, Hoiby EA, Aaberge IS, Caugant DA. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol. 2010;17:325–34. doi: 10.1128/CVI.00435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.