Abstract
Background
With prolonged life expectancy and more frequent use of biological prostheses, an increasingly higher proportion of patients are undergoing aortic valve replacement (AVR) after previous sternotomy. We critically appraised the quantity and quality of evidence to demonstrate the efficacy and safety of the minimally invasive (MIrAVR) versus conventional (CrAVR) approaches for reoperative AVR.
Methods
Electronic searches were performed using six databases from their inception to April 2014. Relevant studies utilizing a MIrAVR were identified. Data were extracted and analyzed according to predefined clinical endpoints.
Results
Four single-arm and seven comparative observational studies including a total of 441 MIrAVR patients were included for quality assessment, data extraction and analysis. In-hospital mortality ranged from 0-9.5%, and was similar between the MIrAVR and CrAVR groups (RR, 0.77; 95% CI, 0.39-1.54; P=0.46). Stroke rates ranged from 2.6-8% and were also similar between the two cohorts. The rates of pacemaker implantation, renal failure and reoperation for bleeding were not significantly different between the two groups. There were no reports of myocardial infarctions in the included studies. No significant difference in hospital stay was observed for the MIrAVR versus CrAVR group.
Conclusions
The current literature suggests that MIrAVR has similar efficacy and mortality outcomes compared to CrAVR without compromise to myocardial protection or hospitalization duration. It appears to be a valid alternative option for patients requiring reoperative AVR.
Keywords: Minimally invasive, aortic valve replacement (AVR), reoperative, systematic review
Introduction
Given extended life expectancies and improved survival rates of modern procedures, there is an increasing number of patients undergoing reoperative cardiac surgery (1). Conventional reoperative aortic valve replacement (CrAVR) is particularly challenging, often due to severe calcified aortic stenosis following previous sternotomy for coronary artery bypass grafting (CABG) (2) or degeneration of aortic bioprostheses (3). These complicated cases are often associated with prolonged cross-clamp and cardiopulmonary bypass durations, increased blood loss and poorer survival rates (4,5).
Minimally invasive rAVR (MIrAVR) is an alternative approach to conventional sternotomy with comparable mortality rates but reduced hospitalization, intensive care stay and improved cosmesis (6). While minimally invasive valvular surgery is becoming increasingly accepted for primary operations, MIrAVR has not been well defined. MIrAVR may be advantageous in avoiding large, open dissections, minimizing trauma and reducing injury to cardiac structures such as previous patent grafts (7,8), while still having the benefits of reduced intensive care and hospital stay. On the other hand, MIrAVR procedures are technically more demanding for the operating surgeon and myocardial protection may be a concern (9), especially in patients with patent coronary artery bypass grafts. A systematic review and meta-analysis was carried out to assess the current evidence on the efficacy and safety of MIrAVR versus CrAVR.
Methods
Literature search strategy
Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from their date of inception to April 2014. To achieve the maximum sensitivity of the search strategy, we combined the terms: “minimally invasive OR ministernotomy OR hemisternotomy OR partial upper sternotomy OR minithoracotomy” AND “aortic valve replacement OR AVR” AND “reoperative OR redo OR resternotomy” as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies, assessed using the inclusion and exclusion criteria. Expert academic cardiothoracic surgeons (M.D.E, T.D.Y) were consulted as to whether they knew of any unpublished data.
Selection criteria
Eligible studies for the present systematic review and meta-analysis were those in which patient cohorts underwent MIrAVR after a previous sternotomy and operation. Studies that did not include mortality or complications as endpoints were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article (K.P, J.J.Z). Discrepancies between the two reviewers were resolved by discussion and consensus (K.P, J.J.Z, N.N). If the study provided medians and interquartile ranges instead of means and standard deviations (SDs), we imputed the means and SDs as described by Hozo et al. (10). As quality scoring is controversial in meta-analyses of observational studies, two reviewers (K.P, J.J.Z) independently appraised each article using the criteria for case series quality assessment recommended by the National Health Service Center for Reviews and Dissemination (11) (University of York, Heslington, United Kingdom). The final results were reviewed by senior investigators (M.D.E, T.D.Y).
Statistical analysis
The relative risk (RR) was used as a summary statistic. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that the treatment effect in each study was the same, whereas in the random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. The I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. In the present meta-analysis, the results using the random-effects model were reported to take into account the possible clinical diversity and methodological variation between studies. Specific analyses considering confounding factors were not possible because raw data were not available. Data are presented as means ± SD. Weighted means (WM) were calculated by determining the total number of events divided by total sample size. Pearson’s statistic was used to calculate correlation for meta-regression meta-analysis. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.2.1 (Cochrane Collaboration, Software Update, Oxford, United Kingdom) and the metafor package for R version 3.01.
Results
Literature search
A total of 685 references were identified through the six electronic database searches (Figure 1). After exclusion of duplicate or irrelevant references, 566 potentially relevant articles were retrieved. After detailed evaluation of these articles, 27 studies remained for assessment. After applying the selection criteria, 11 articles (7,8,12-20) were selected for qualitative analysis. Of these, 7 observational studies (7,8,14,15,17,19,20) were included for quantitative analysis. The study characteristics are summarized in Table 1. Of the 11 included articles, 441 patients underwent rAVR via a MIrAVR, and 1,145 patients via the conventional sternotomy approach. Baseline patient characteristics and myocardial protection strategies are summarized in Tables 2 and 3, respectively.
Figure 1.
PRISMA search strategy for the present systematic review and meta-analysis.
Table 1. Summary characteristics of included studies in the present systematic review and meta-analysis.
| First author | Year | Treatment center | Study period | Study design | n (MIrAVR) | n (CrAVR) | Minimally invasive reoperative approach | Mean follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| Byrne | 1999 | Boston | 1996-1998 | OS, R | 20 | 19 | Partial upper ministernotomy | – |
| Grossi | 2000 | New York | 1996-2000 | OS, R | 42 | 0 | Right minithoracotomy | – |
| Svensson | 2001 | Burlington | – | OS, R | 18 | 0 | J ministernotomy | – |
| Mihaljevic | 2004 | Boston | 1996-2003 | OS, R | 63 | 134 | Upper ministernotomy | 39M (IR 1-84) |
| Sharony | 2006 | New York | 1995-2002 | OS, P | 61 | 160 | Minithoracotomy | – |
| Bakir | 2007 | Aalst | 1999-2005 | OS, R | 19 | 0 | J ministernotomy | 23.6±19.7 |
| Totaro | 2009 | Milan | 1997-2007 | OS, R | 77 | 695 | Upper ministernotomy (T and L) | – |
| Gaeta | 2010 | Messina | 1997-2007 | OS, R | 16 | 0 | J or reversed-T ministernotomy | 58M (IR 11-124) |
| Pineda | 2013 | Florida | 2005-2011 | OS, R | 36 | 41 | Right minithoracotomy | 24 |
| Mikus | 2013 | Cotignola | 2007-2012 | OS, R | 38 | 42 | Upper J ministernotomy | – |
| Kaneko | 2014 | Boston | 1997-2011 | OS, R | 51 | 54 | Upper ministernotomy | 36M (IR 14-64) |
MIrAVR, minimally invasive reoperative aortic valve replacement; CrAVR, conventional reoperative aortic valve replacement; n, number of patients; OS, observational study; R, retrospective; P, prospective; M, median; IR, interquartile range; NR, not reported; Dashed lines indicate value was not reported.
Table 2. Baseline characteristics of patients undergoing reoperative aortic valve replacement via a minimally invasive approach.
| First author | n | Mean age (years) | Male | LVEF | NYHA class III-IV | AS | AR | DM | PVD | Strokes | CVA | Renal failure | COPD | Previous CABG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Byrne | 20 | 69M (IR 39-93) | 80 | 54M (IR 20-78) | 50 | – | – | – | – | – | – | – | – | 55 |
| Grossi | 42 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Svensson | 18 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Mihaljevic | 63 | 75M (IR 31-93) | 75 | –* | 37 | – | 63 | – | – | – | – | – | – | 63 |
| Sharony | 61 | 63.4±1.3 | 64 | – | – | – | – | 14.3 | – | – | – | – | 13 | – |
| Bakir | 19 | 73.6±11.4 | – | 58.1±15 | 47.3 | – | – | 15.8 | – | – | – | 15.8 | 15.8 | 63.2 |
| Totaro | 77 | 62±13 | 61 | 53±12 | – | – | – | – | – | – | – | – | – | – |
| Gaeta | 16 | – | – | – | – | – | – | – | – | 6.3 | – | – | – | 100 |
| Pineda | 36 | 75.3±9 | 92 | 55M (IR 41-60) | – | – | – | 64 | 17 | – | 14 | – | – | 86 |
| Mikus | 38 | 76±11 | 55.3 | 55M | – | 73.6 | – | 21.1 | 31.6 | – | 2.6 | – | 21.1 | 46 |
| Kaneko | 51 | 83.3±2.7 | 70.6 | 55M (IR 50-60) | 62.7 | 78.4 | 5.9 | 31.4 | – | 9.8 | 9.8 | 2 | – | 92.2 |
| Minimum | – | 62 | 55.3 | 53 | 37 | 73.6 | 5.9 | 14.3 | 17 | 6.3 | 2.6 | 2 | 13 | 46 |
| Maximum | – | 83.3 | 92 | 55 | 62.7 | 78.4 | 63 | 64 | 31.6 | 9.8 | 14 | 15.8 | 21.1 | 100 |
| Weighted Mean | – | 71.2 | 69.2 | 49.9 | 48.7 | NA | NA | 30.0 | NA | NA | 8.8 | NA | NA | 71.7 |
Data presented are expressed in % unless otherwise stated; n, number of patients; PVD, peripheral vascular disease; AS, aortic stenosis; AR, aortic regurgitation; M, median; IR, interquartile range; DM, diabetes mellitus; *, 19 out of 63 patients had LVEF ≤40%; Dashed lines indicate value was not reported; NA, not appropriate due to low reporting of outcome; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass graft.
Table 3. Myocardial protection strategies for included studies.
| Study | Cardioplegia strategy | CPB temperature (°C) | Patent CABG grafts occluded |
|---|---|---|---|
| Byrne | Retrograde, after initial antegrade dose | 20-25 | No |
| Grossi | Retrograde | NR | NR |
| Svensson | Antegrade + retrograde | 22 | No |
| Mihaljevic | Antegrade or retrograde | 20-22 | No |
| Sharony | Antegrade + retrograde | 25-28 | NR |
| Bakir | Antegrade + retrograde | 26.2±4 | NR |
| Totaro | Antegrade | NR | No |
| Gaeta | Antegrade +/or retrograde | 24-33 | Yes |
| Pineda | Retrograde, after initial antegrade dose | 28 | No |
| Mikus | Antegrade | Normothermia | No |
| Kaneko | Antegrade + retrograde | 28-32 (without patent LITA) 20-30 (with patent LITA) |
No |
CPB, cardiopulmonary bypass; NR, not reported; +, indicates combined strategy; LITA, left internal thoracic artery; CABG, coronary artery bypass graft.
Quality appraisal
All included studies except one (15) were retrospective, observational studies, seven of which had comparative control groups. There were four studies which included greater than 50 patients undergoing rAVR by a MIrAVR (7,14,15,17), while seven studies assessed fewer than 50 patients (8,12,13,16,18-20). A partial upper sternotomy or ministernotomy approach for resternotomy was used in eight studies (7,8,13,14,16-18,20) (n=302, while a minithoracotomy approach was employed by three studies (12,15,19) (n=139). Only five studies reported mean or median follow-up, which were all greater than or equal to 24 months (7,14,16,18,19).
The cardioplegia strategy was reported in all included studies, with three studies using retrograde approach (7,11,18), two studies using antegrade approach (16,19), five studies using the combined approach (6,12,14,15,17), and one study using either approach (13). Temperatures used during cardiopulmonary bypass (CPB) were reported in all included studies except for two (12,17). CPB duration was reported in all but three studies (12,13,15), while cross-clamp duration was not reported in three studies (13,15,17). In-hospital mortality was reported in all included studies. Stroke outcomes were reported in seven studies (7,13,14,16,18-20), reoperations for bleeding in six studies (7,14,16,18-20) and myocardial infarctions in four studies (13,14,18,20). The percentage of patients with previous coronary bypass grafting (CABG) operations was reported in seven studies (7,8,14,16,18-20).
Assessment of mortality and morbidity
In-hospital mortality outcomes are outlined in Table 4, with a WM of 4.1% (range: 0-9.5%) for the 11 included studies. Seven comparative observational studies investigating rAVR via MIrAVR versus CrAVR were available for meta-analysis. The risk of in-hospital mortality was not significantly different between MIrAVR and CrAVR groups (3.8% vs. 5.4%; RR, 0.77; 95% CI, 0.39-1.54; P=0.46; I2=17%; Figure 2). There was also a significant negative correlation between midpoint of study period and in-hospital mortality (r2=0.6884; P=0.021; Figure 3).
Table 4. Assessment of morbidity and mortality in reoperative aortic valve replacement via a minimally invasive approach.
| First author | n | In-hospital mortality | Strokes | MI | Renal failure | Prolonged ventilation | Reop for bleeding | Blood transfusion | RBC units transferred | Pace-maker | Endocarditis | Wound infection | Sepsis | Hospital stay (days) | ICU stay (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Byrne | 20 | 0 | – | – | 0 | – | – | – | 3.1±3 | 10 | 0 | 0 | – | 6.9±2.9 | – |
| Grossi | 42 | 9.5 | – | – | – | – | – | – | – | – | – | – | – | 8M* | 1.1 |
| Svensson | 18 | 0 | 5.6 | 0 | – | – | – | – | – | – | – | – | – | – | – |
| Mihaljevic | 63 | 5 | 6 | 0 | – | – | 0 | – | – | – | – | 2 | – | 7M (IR 3-44) | – |
| Sharony | 61 | 6.6 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Bakir | 19 | 5.3 | 5.3* | – | 5.2 | – | 21 | – | 2.4±3.7 | – | – | 5.3 | – | 12.9±5.7 | 2.9±2.6 |
| Totaro | 77 | 3.8 | – | – | – | 9 | – | – | – | – | – | – | – | 10±7 | 2.1±3.7 |
| Gaeta | 16 | 0 | 6.3^ | 0 | 0 | 6.3 | 0 | 0 | 0.9±1.2 | 0 | 0 | 0 | 0 | 7.5±2.6 | 1.6±1.1 |
| Pineda | 36 | 0 | 8 | – | 3 | 11 | 0 | 72 | 1M (IR 0-2) | – | – | 0 | – | 7M (IR 5-10) | 2M (IR 1.71-4.04) |
| Mikus | 38 | 2.6 | 2.6 | 0 | 5.3 | – | 7.9 | – | 2M | 10.5 | 2.6 | – | 0 | – | 2M |
| Kaneko | 51 | 3.9 | 5.9 | – | 0 | – | 7.8 | 62.7 | 3M (IR 2-4) | – | 0 | – | 2 | 9M (IR 7-15) | 3M (IR 1.38-5.38) |
| Minimum | – | 0 | 2.6 | 0 | 0 | 6.3 | 0 | 0 | 0.9 | 0 | 0 | 0 | 0 | 6.9 | 1.1 |
| Maximum | – | 9.5 | 8 | 0 | 5.3 | 11 | 21 | 72 | 3.1 | 10.5 | 2.6 | 5.3 | 2 | 12.9 | 3 |
| Weighted mean | – | 4.1 | 5.7 | 0 | 2.3 | 9.2 | 4.9 | 56.2 | 2.2 | 8.1 | 0.8 | 1.5 | 1.0 | 8.5 | 2.1 |
Data presented are expressed in % unless otherwise stated; n, number of patients; *, due to CVA; ^, due to TIA; M, median; RBC, red blood cells; MI, myocardial infarction; ICU, intensive care unit; dashed lines indicate value was not reported; IR, interquartile range.
Figure 2.

Forest plot of the relative risk of in-hospital mortality after minimally invasive reoperative aortic valve replacement (MIrAVR) versus conventional reoperative aortic valve replacement (CrAVR).
Figure 3.

Bubble chart showing correlation between the midpoint of the study period and in-hospital mortality rate. Studies with n ≤20 and with mixed concomitant valve and arch surgery were excluded. The solid line indicates the correlation trend line, while dotted lines indicate the 95% confidence interval.
The rates of stroke ranged from 2.6-8%, with a WM of 5.7%. Meta-analysis also showed similar rates of stroke between MIrAVR and CrAVR cohorts (5.9% vs. 3.2%; RR, 1.88; 95% CI, 0.75-4.68; P=0.18; I2=0%; Figure 4). No myocardial infarctions were reported by the included studies. The WM incidence of renal failure was 2.3% (range: 0-5.3%), and this was not significantly different between MIrAVR versus CrAVR (1.3% vs. 5.7%; RR, 0.33, 95% CI, 0.10-1.04; P=0.06; I2=0%). Reoperation for bleeding ranged from 0-21%, but was not significantly different between MIrAVR and CrAVR cohorts (3.0% vs. 4.4%; RR, 0.72; 95% CI, 0.25-2.06; P=0.55; I2=21%). Blood transfusion requirements were reported in three studies, with a WM of 56.2% (range: 0-72%). Pacemaker implantation requirements ranged from 0-10.5%, while wound infection occurred in a WM of 1.5% of cases (range: 0-5.3%). Hospital stay duration was reported by eight studies, and ranged from 6.9-12.9 days (WM: 8.5 days). Intensive care unit (ICU) stay duration was reported by seven studies, and ranged from 1.1-3 days (WM: 2.1 days). No difference in ICU stay was observed between MIrAVR versus CrAVR cohorts. Hospital stay was also similar for the MIrAVR versus CrAVR group (WMD, −0.62 days; 95% CI, −2.20-0.96; P=0.44; I2=91%; Figure 5).
Figure 4.

Forest plot of the relative risk of perioperative stroke after minimally invasive reoperative aortic valve replacement (MIrAVR) versus conventional reoperative aortic valve replacement (CrAVR).
Figure 5.

Forest plot for the mean difference in length of hospital stay after minimally invasive reoperative aortic valve replacement (MIrAVR) versus conventional reoperative aortic valve replacement (CrAVR).
Assessment of operative outcomes
Cross-clamp duration ranged from 51-93 minutes, with a WM of 78.4 minutes. CPB duration ranged from 67-156 minutes, with a WM of 133.6 minutes. No significant difference between MIrAVR and CrAVR was observed for cross-clamp duration (P=0.67; Figure 6) or CPB duration (P=0.40; Figure 7). The rate of conversion was low, with a WM of 0.9%, ranging from 0-2.8%. The proportion of patients using aortic, femoral arterial, femoral venous and right atrial cannulation is reported in Table 5.
Figure 6.

Forest plot for the mean difference in cross-clamp duration with minimally invasive reoperative aortic valve replacement (MIrAVR) versus conventional reoperative aortic valve replacement (CrAVR).
Figure 7.
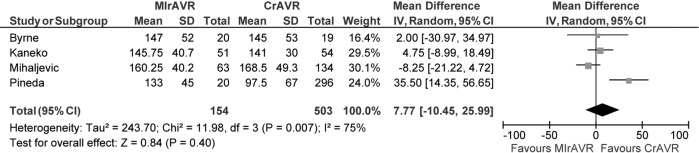
Forest plot for the mean difference in cardiopulmonary bypass duration with minimally invasive reoperative aortic valve replacement (MIrAVR) versus conventional reoperative aortic valve replacement (CrAVR).
Table 5. Operative outcomes of patients undergoing reoperative aortic valve replacement via a minimally invasive approach.
| First author | n | Cross-clamp (min) | CPB (min) | Rate of conversion | Aortic cannulation | Femoral artery cannulation | Femoral vein cannulation | Direct right atrium cannulation | Other |
|---|---|---|---|---|---|---|---|---|---|
| Byrne | 20 | 93±48 | 147±52 | – | 10 | 40 | 40 | 10 | 50 |
| Grossi | 42 | 86±36 | – | 0 | – | – | – | – | – |
| Svensson | 18 | – | – | – | – | – | – | – | – |
| Mihaljevic | 63 | 82M (IR 38-229) | 141M (IR 59-300) | – | – | – | – | 100 | – |
| Sharony | 61 | – | – | – | – | – | – | – | – |
| Bakir | 19 | 87.4±32.7 | 133.1±54.4 | 0 | 53 | 47 | 94.7 | 5.3 | – |
| Totaro | 77 | – | 156±52 | 1.2 | 88 | 12 | – | – | – |
| Gaeta | 16 | 72±20 | 119.7±38.1 | – | 75 | 25 | – | – | – |
| Pineda | 36 | 90M (IR 76-99) | 134M (IR 119-146) | 2.8 | – | – | – | – | – |
| Mikus | 38 | 51M | 67M | 0 | 100 | – | – | 100 | – |
| Kaneko | 51 | 73M (IR 62-92) | 139M (IR 125-180) | – | 11.8 | 21.6 | 94.1 | 5.9 | 66.6 |
| Minimum | – | 51 | 67 | 0 | 10 | 12 | 40 | 5.9 | – |
| Maximum | – | 93 | 156 | 2.8 | 100 | 47 | 94.7 | 100 | – |
| Weighted mean | – | 78.4 | 133.6 | 0.9 | 61.5 | 22.5 | 82.1 | 61.6 | – |
n, number of patients; CPB, cardiopulmonary bypass duration; M, median; IR, interquartile range; dashed lines indicate value was not reported.
Discussion
Reoperative aortic valve surgery represents a surgical challenge associated with increased mortality rates and complications (21). This is particularly pertinent for patients with previous sternotomy for CABG operations, where injury to patent grafts represents a serious concern (8). In this setting, the MIrAVR may avoid hazardous tissue dissections and reduce surgical trauma but is more technically demanding and potentially associated with sub-optimal myocardial protection strategies (7,9). The safety and efficacy of MIrAVR were investigated in the present meta-analysis and systematic review.
While comparable mortality rates and complications have been reported for MIrAVR and CrAVR, the outcomes for rAVR are not well established. From the results of the present meta-analysis, in-hospital mortality was not found to be significantly different between MIrAVR and CrAVR groups. Mortality rates ranged from 0-9.5% for the MIrAVR. The in-hospital mortality rates also negatively correlated with the midpoint of the study period, suggesting improvement in survival outcomes over time (Figure 3), a trend that may continue in the future. This may be partially explained by the learning curve associated with minimally invasive techniques, with lower mortality rates reported in recent years (0-3.9%). The incidences of stroke were also comparable between the MIrAVR and CrAVR cohorts (5.9% vs. 3.2%; P=0.18 and were similar to values reported by previous meta-analyses on primary AVR cases. While it is expected that the reduced invasiveness of MIrAVR would reduce reoperations required for bleeding and transfusions, there were no significant differences found between the cohorts, a result possibly attributable to the low statistical power and small sample size of the included studies. No significant reduction in hospital stay was noted in the MIrAVR group. Overall, MIrAVR appears to have comparable complication rates and length of stay compared with CrAVR, lending support to its role as a safe alternative to median sternotomy for reoperative AVR.
The procedural duration of AVR is of great clinical interest, as prolonged cross-clamp and CPB durations have been shown to be associated with inflammation and poorer surgical outcomes (22,23). In the present study, the cross-clamp and CPB durations were similar between minimally invasive and conventional sternotomy cohorts for rAVR. Considering limitations in both surgical vision and maneuverability in a limited working space, this outcome is unexpected. However, by minimizing the surgical isolation of the heart often completed on CPB during CrAVR, MIrAVR may reduce the overall CPB time. In addition, disparities in operational duration may also be mitigated with the evolution of technical skill and experience in minimally invasive surgery, traversing the initial learning curve phase. The introduction of sutureless AVR technologies will further obviate and alleviate the technical challenges involved in traditional AVR (24,25). Given that annular sutures do not need to be securely tied down to hold the valve in place, the sutureless approach facilitates smaller incisions and shorter cross-clamp, CPB and procedural durations, ideal for reoperations (26). However, it remains to be seen if these newer sutureless valve technologies are suitable for reoperative aortic valve operations.
The major concern in minimally invasive reoperations is the optimal myocardial protection strategy (8,9,20). Typically, the standard approach involves isolation and occlusion of patent CABG grafts, antegrade or retrograde cardioplegia infusion, and moderate or mild hypothermia. These conditions are easily met during conventional reoperative aortic valve surgery. However, during minimally invasive reoperative surgery, given the reduced surgical field of ministernotomy and minithoracotomy incisions, it is difficult to isolate and control internal thoracic artery (ITA) grafts during clamping (9). In these cases, an alternative approach is to leave patent grafts unoccluded, resulting in constant perfusion of the myocardium with oxygenated blood. Cardioplegia is well delivered and deeper levels of systemic hypothermia are used to compensate for the sub-optimal conductance of myocardial protection. As a result, perfect arrest is not always achieved, and with the heart still fibrillating, the risk of postoperative myocardial infarction becomes a serious concern. Notably, the present study showed the incidence of myocardial infarction to be nil in all studies. As such, the current evidence seems to suggest that, in carefully selected patients, such ‘no-touch’ (9) hypothermic cardioplegia provides acceptable myocardial protection. Furthermore, proponents of MIrAVR have also suggested that by avoiding dissection and occlusion of grafts, there is reduced risk of ITA injury and embolism due to manipulation of atherosclerotic vein grafts (8,13,19,27).
Limitations
The current meta-analysis is limited by small, retrospective studies with inadequate statistical power, which may have underestimated complication rates. Resource-related outcomes such as economic costs, pain scores and quality of life outcomes were not reported by the included studies. The heterogeneity of cross-clamp and CPB outcomes, as well as hospitalization time, may be accounted for by considering the inherent variation in the patient populations, which comprise patients with a wide variety of previous cardiac operations. This ranged from prior CABG and patent grafts to prior sternotomy for AVR or mitral valve surgery with concurrent surgical ablation, with the latter known to have different postoperative outcomes (28,29).
Given the technical challenges involved in minimally invasive reoperative surgery, it is likely that the current evidence is based on outcomes from highly experienced expert surgeons at high-volume academic centers. As such, the current results may only be representative of carefully selected patient and surgeon populations, and may not be reproducible for surgeons with lesser experience. Variation in procedural outcome may also be due to inherent differences between the ministernotomy and minithoracotomy approaches employed. Furthermore, long-term outcomes were not available, making it difficult to comprehensively evaluate the comparative risks and benefits of MIrAVR and CrAVR. Given the promising data to date, future registry or prospectively randomized trials should be carried out to more definitively assess the MIrAVR.
Conclusions
Minimally invasive approaches to rAVR represent a potential alternative to median sternotomy, with similar mortality and morbidity outcomes, and adequate myocardial protection. MIrAVR appears to have acceptable outcomes in carefully selected patients, and these are likely to further improve with the learning curve of the procedure and emergence of sutureless valve technology. However, there remains a lack of robust clinical evidence and adequately powered, randomized studies are warranted to comprehensively evaluate the efficacy and safety of MIrAVR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [DOI] [PubMed] [Google Scholar]
- 2.Hirose H, Gill IS, Lytle BW. Redo-aortic valve replacement after previous bilateral internal thoracic artery bypass grafting. Ann Thorac Surg 2004;78:782-5. [DOI] [PubMed] [Google Scholar]
- 3.Vogt PR, Brunner-LaRocca H, Sidler P, et al. Reoperative surgery for degenerated aortic bioprostheses: predictors for emergency surgery and reoperative mortality. Eur J Cardiothorac Surg 2000;17:134-9. [DOI] [PubMed] [Google Scholar]
- 4.Maganti M, Rao V, Armstrong S, et al. Redo valvular surgery in elderly patients. Ann Thorac Surg 2009;87:521-5. [DOI] [PubMed] [Google Scholar]
- 5.Langanay T, Verhoye JP, Ocampo G, et al. Current hospital mortality of aortic valve replacement in octogenarians. J Heart Valve Dis 2006;15:630-7; discussion 637. [PubMed] [Google Scholar]
- 6.Phan K, Xie A, Di Eusanio M, et al. A Meta-Analysis of Minimally Invasive Versus Conventional Sternotomy for Aortic Valve Replacement. Ann Thorac Surg 2014;98:1499-511. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko T, Loberman D, Gosev I, et al. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2014;147:155-62. [DOI] [PubMed] [Google Scholar]
- 8.Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko T, Nauta F, Borstlap W, et al. The “no-dissection” technique is safe for reoperative aortic valve replacement with a patent left internal thoracic artery graft. J Thorac Cardiovasc Surg 2012;144:1036-40. [DOI] [PubMed] [Google Scholar]
- 10.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien WT, Norman I. The effectiveness and active ingredients of mutual support groups for family caregivers of people with psychotic disorders: a literature review. Int J Nurs Stud 2009;46:1604-23. [DOI] [PubMed] [Google Scholar]
- 12.Grossi EA, LaPietra A, Bizekis C, et al. Minimal access reoperative mitral and aortic valve surgery. Curr Cardiol Rep 2000;2:572-4. [DOI] [PubMed] [Google Scholar]
- 13.Svensson LG, Nadolny EM, Kimmel WA. Minimal access aortic surgery including re-operations. Eur J Cardiothorac Surg 2001;19:30-3. [DOI] [PubMed] [Google Scholar]
- 14.Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive reoperative isolated valve surgery: early and mid-term results. J Card Surg 2006;21:240-4. [DOI] [PubMed] [Google Scholar]
- 16.Bakir I, Casselman FP, De Geest R, et al. Should minimally invasive aortic valve replacement be restricted to primary interventions? Thorac Cardiovasc Surg 2007;55:304-9. [DOI] [PubMed] [Google Scholar]
- 17.Totaro P, Carlini S, Pozzi M, et al. Minimally invasive approach for complex cardiac surgery procedures. Ann Thorac Surg 2009;88:462-6; discussion 467. [DOI] [PubMed] [Google Scholar]
- 18.Gaeta R, Lentini S, Raffa G, et al. Aortic valve replacement by ministernotomy in redo patients with previous left internal mammary artery patent grafts. Ann Thorac Cardiovasc Surg 2010;16:181-6. [PubMed] [Google Scholar]
- 19.Pineda AM, Santana O, Reyna J, et al. Outcomes of reoperative aortic valve replacement via right mini-thoracotomy versus median sternotomy. J Heart Valve Dis 2013;22:50-5. [PubMed] [Google Scholar]
- 20.Mikus E, Calvi S, Tripodi A, et al. Upper ‘J’ ministernotomy versus full sternotomy: an easier approach for aortic valve reoperation. J Heart Valve Dis 2013;22:295-300. [PubMed] [Google Scholar]
- 21.Potter DD, Sundt TM, 3rd, Zehr KJ, et al. Operative risk of reoperative aortic valve replacement. J Thorac Cardiovasc Surg 2005;129:94-103. [DOI] [PubMed] [Google Scholar]
- 22.Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23:223-31. [DOI] [PubMed] [Google Scholar]
- 23.Rossaint J, Berger C, Van Aken H, et al. Cardiopulmonary bypass during cardiac surgery modulates systemic inflammation by affecting different steps of the leukocyte recruitment cascade. PLoS One 2012;7:e45738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic Valve System: multicenter experience. J Thorac Cardiovasc Surg 2010;140:878-84,884.e1. [DOI] [PubMed]
- 25.Santarpino G, Pfeiffer S, Concistrè G, et al. REDO aortic valve replacement: the sutureless approach. J Heart Valve Dis 2013;22:615-20. [PubMed] [Google Scholar]
- 26.Phan K, Tsai YC, Nithya N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dell’Amore A, Del Giglio M, Calvi S, et al. Mini re-sternotomy for aortic valve replacement in patients with patent coronary bypass grafts. Interact Cardiovasc Thorac Surg 2009;9:94-7. [DOI] [PubMed] [Google Scholar]
- 28.Phan K, Xie A, La Meir M, et al. Surgical ablation for treatment of atrial fibrillation in cardiac surgery: a cumulative meta-analysis of randomised controlled trials. Heart 2014;100:722-30. [DOI] [PubMed] [Google Scholar]
- 29.Phan K, Xie A, Tian DH, et al. Systematic review and meta-analysis of surgical ablation for atrial fibrillation during mitral valve surgery. Ann Cardiothorac Surg 2014;3:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]



