Abstract
Background:
Solid organ transplant recipients have elevated risks of virus-related cancers, in part because of long-term immunosuppression. Merkel cell carcinoma (MCC) is an aggressive skin cancer recently found to have a viral origin, but little is known regarding the occurrence of MCC after transplant.
Methods:
We linked the US Scientific Registry of Transplant Recipients with data from 15 population-based cancer registries to ascertain MCC occurrence among 189498 solid organ transplant recipients from 1987 to 2009. Risks for MCC following transplantation were compared with the general population using standardized incidence ratios, and Poisson regression was used to compare incidence rates according to key patient and transplant characteristics. All statistical tests were two-sided.
Results:
After solid organ transplantation, overall risk of MCC was increased 23.8-fold (95% confidence interval = 19.6 to 28.7, n = 110). Adjusted risks were highest among older recipients, increased with time since transplantation, and varied by organ type (all P ≤ .007). Azathioprine, cyclosporine, and mTOR inhibitors given for maintenance immunosuppression increased risk, and non-Hispanic white recipients on cyclosporine and azathioprine experienced increasing MCC risk with lower latitude of residence (ie, higher ultraviolet radiation exposure, P = .012).
Conclusions:
MCC risk is sharply elevated after solid organ transplant, likely resulting from long-term immunosuppression. Immunosuppressive medications may act synergistically with ultraviolet radiation to increase risk.
Merkel cell carcinoma (MCC) is an uncommon skin cancer of neuroendocrine differentiation. MCC behaves aggressively, and five-year relative survival is only 62% (1). Like other skin cancers, MCC largely affects light-skinned populations (2,3), especially those highly exposed to ultraviolet radiation (UVR) (4). Recently, a previously unknown virus, Merkel cell polyomavirus (MCV), was detected in most but not all MCC tumors tested (5). This discovery has revived interest in MCC epidemiology, especially regarding the role of impaired immunity in promoting viral carcinogenesis. However, details regarding the relevant type of immunosuppression are poorly understood.
Immunosuppression is suspected as important to MCC causation, as risk is increased among persons with human immunodeficiency virus (HIV) (6,7), chronic lymphocytic leukemia, (3,8) and other hematologic malignancies (8). MCC risk is also elevated following solid organ transplantation (9–12), after which patients must be pharmacologically immunosuppressed to prevent graft rejection. Also, some immunosuppressant medications used in transplantation may have direct skin carcinogenic effects, including interacting with UVR to enhance DNA damage (13–18). These direct effects may relate to the very high risks of squamous cell skin cancers in transplant recipients (19). Prior studies of transplant-related MCC have included fewer than 50 case patients and have not provided information on how risk differs by age, timing of transplant, or specific immunosuppressive medications (9–12).
In the present study, we evaluated the occurrence of MCC among solid organ transplant recipients in the Transplant Cancer Match (TCM) Study, a large, population-based cohort of US transplant recipients for which cancer ascertainment was conducted uniformly via linkage with cancer registries. We quantified MCC risk overall and according to recipient demographic characteristics, transplanted organ, UVR exposure based on place of residence, length of time since transplant, and type of immunosuppressive drugs received.
Methods
Transplant Cancer Match Study
The TCM Study (http://transplantmatch.cancer.gov) is described in detail elsewhere (20). Briefly, computer-based linkages were performed between the Scientific Registry of Transplant Recipients (SRTR) and 15 US central cancer registries. The SRTR includes structured data regarding all US solid organ transplants since 1987, including recipient demographic characteristics, characteristics of the transplanted organs, and immunosuppressive medications prescribed at time of transplant. Transplants performed on the same person at different times are considered separately.
Serial record linkages were completed between the SRTR and 15 cancer registries, altogether covering 46% of US transplants: California (years of coverage: 1988–2008), Colorado (1988–2009), Connecticut (1973–2009), Florida (1981–2009), Georgia (1995–2008), Hawaii (1973–2007), Illinois (1986–2007), Iowa (1973–2009), Michigan (1985–2009), New Jersey (1979–2006), New York (1976–2007), North Carolina (1990–2007), Seattle (1974–2008), Texas (1995–2006), and Utah (1973–2008). Linkages were performed using a computer algorithm (incorporating name, sex, date of birth, and social security number), followed by manual review and confirmation of potential matches. Analyses were restricted to transplant recipients residing in geographic areas covered by cancer registries during the specified time periods. The TCM Study was approved by human subjects research review committees at the National Cancer Institute (NCI) and, as required, the participating cancer registries.
For the present study, we considered a cohort of 208096 solid organ transplants performed from 1987 to 2009, from which we successively excluded five transplants with a pretransplant history of MCC and two groups of transplants among which no MCC cases were observed: 18379 in persons age 0 to 19 years at transplant, and 214 in persons with known HIV infection. The final cohort thus included n = 189498 transplants.
MCC Outcome and Follow-up
MCC cases in transplant recipients were identified through linked cancer registry data, using the International Classification of Diseases (ICD) for Oncology, 3rd edition histology code 8247 (introduced in 1986). Patient follow-up began at the latest of transplantation or start of cancer registry coverage and ended at the earliest of MCC diagnosis, death, failure of a transplanted organ, subsequent transplant, loss to follow-up, or end of cancer registry coverage.
Variable Definitions
We obtained cancer-related information from cancer registries regarding cancer diagnoses, including date and age at diagnosis and tumor characteristics. We obtained transplant-related information from the SRTR, including organ(s), date, age at transplant, and baseline immunosuppressive medications. We grouped medications that are often prescribed together as follows: the “cyclosporine/azathioprine” category included recipients prescribed both cyclosporine and azathioprine but not tacrolimus or mycophenolate mofetil (MMF); the “tacrolimus/MMF” category included recipients prescribed both tacrolimus and MMF but not cyclosporine or azathioprine; and remaining recipients were categorized as “other.”
Neither the SRTR nor cancer registries collect information regarding patient history of UVR exposure. Thus, we derived proxy, ecologic measures of UVR exposure based on their residence at time of entry onto the transplant waitlist or transplant, when available. We assigned latitude to each transplant using a public database providing latitudes for US zip codes (21). Separately, we linked transplants by county of residence to AVGLO, a measure of potential UVR exposure previously shown to be associated with melanoma risk (22,23). AVGLO is based on the predicted 30-year average daily global solar radiation, defined as the total direct and diffuse solar radiation received on a horizontal surface measured in watt-hours per square kilometer (Wh/km2). Latitude and AVGLO measures were categorized into quintiles by dividing the range of each measure into equal segments (see footnote to Table 2 for details, including imputation for n = 2282 transplants for whom county information was not available).
Table 2.
Risk of Merkel cell carcinoma according to demographic characteristics, transplanted organ, and time since transplant
| Demographic and clinical characteristics | MCC patients | Incidence rate (per 100 000 person-years) | Incidence rate ratio (IRR) (95% CI) | P |
|---|---|---|---|---|
| Sex | .009 | |||
| Female | 30 | 8.9 | Ref. | |
| Male | 80 | 15.3 | 1.7 (1.1 to 2.6) | |
| Age at transplant, y | P trend < .001 | |||
| 20–34 | 7 | 4.5 | Ref. | |
| 35–49 | 23 | 7.2 | 1.6 (0.7 to 3.7) | |
| 50–64 | 64 | 19.7 | 4.3 (2.0 to 9.5) | |
| 65+ | 16 | 25.7 | 5.7 (2.3 to 13.8) | |
| Race/ethnicity | <.001 | |||
| White | 100 | 17.6 | Ref. | |
| Nonwhite | 10 | 3.4 | 0.2 (0.1 to 0.4) | |
| Transplanted organ | .019 | |||
| Kidney only | 70 | 13.8 | Ref. | |
| Liver only | 15 | 8.4 | 0.6 (0.3 to 1.1) | |
| Heart only | 20 | 20.5 | 1.5 (0.9 to 2.4) | |
| All other | 5 | 6.7 | 0.5 (0.2 to 1.2) | |
| Time since transplant | P trend < .001 | |||
| <1 year | 6 | 3.4 | Ref. | |
| 1 to <3 y | 23 | 9.5 | 2.8 (1.1 to 6.9) | |
| 3 to <5 y | 17 | 10.0 | 2.9 (1.2 to 7.5) | |
| 5 to <10 y | 44 | 21.0 | 6.2 (2.6 to 14.5) | |
| ≥10 y | 20 | 33.1 | 9.8 (3.9 to 24.3) | |
| Attained calendar year | P trend = .096 | |||
| 1987–1998 | 13 | 6.5 | Ref. | |
| 1999–2002 | 32 | 14.5 | 2.2 (1.2 to 4.2) | |
| 2003–2005 | 38 | 17.6 | 2.7 (1.4 to 5.0) | |
| 2006–2009 | 27 | 12.0 | 1.8 (0.9 to 3.6) | |
| Residential latitude quintile* | P trend = .478 | |||
| 1 (lowest UVR) | 12 | 16.8 | Ref. | |
| 2 | 43 | 11.1 | 0.7 (0.3 to 1.2) | |
| 3 | 29 | 13.0 | 0.8 (0.4 to 1.5) | |
| 4 | 20 | 14.7 | 0.9 (0.4 to 1.8) | |
| 5 (highest UVR) | 2 | 38.9 | 2.3 (0.5 to 10.4) | |
| Residential AVGLO quintile* | P trend = .308 | |||
| 1 (lowest UVR) | 3 | 13.2 | Ref. | |
| 2 | 36 | 11.6 | 0.9 (0.3, 2.9) | |
| 3 | 17 | 11.1 | 0.8 (0.2 to 2.9) | |
| 4 | 41 | 13.9 | 1.1 (0.3 to 3.4) | |
| 5 (highest UVR) | 8 | 16.9 | 1.3 (0.3 to 4.8) |
* For latitude (in degrees), quintiles corresponded to ≥42.8 (lowest UV exposure), 36.6–42.7, 30.4–36.5, 24.2–30.3, and <24.2 (highest UV exposure). For AVGLO (predicted 30-year average daily global solar radiation, defined as the total amount of direct and diffuse solar radiation received on a horizontal surface measured in watt-hours per square kilometer), quintiles corresponded to <3552 (lowest UV exposure), 3552–4091, 4092–4631, 4632–5171, and ≥5172 (highest UV exposure). AVGLO was imputed for transplant recipients for whom county was not available, but who lived in states whose range of county-level mean AVGLO values were within a single quintile, (ie, all Connecticut, New Jersey, and New York residents were assigned to quintile 2, and North Carolina residents to quintile 3). For Hawaii residents, AVGLO was not available but the AVGLO measures for Mexico City (at a similar latitude to Hawaii) fell within our highest quintile, so all Hawaii residents were assigned to quintile 5. The analysis cohort was reduced for these two variables because of missing zip code data or AVGLO that could not be imputed (n = 183 703 recipients included for latitude analyses and n = 184 721 included for AVGLO analyses). Poisson regression analysis; All statistical tests were two-sided. CI = confidence interval; UVR = ultraviolet radiation.
Statistical Analysis
We measured MCC risk in transplant recipients relative to the general population using the standardized incidence ratio (SIR) (ie, observed/expected number of case patients). Expected numbers were calculated by applying general population MCC incidence rates, based on MCC cases ascertained in cancer registries, to person-time at risk among transplant recipients, stratified by sex, five-year age group, race/ethnicity, calendar year, and registry. Rates for non-Hispanic whites, non-Hispanic blacks, and Asians/Pacific Islanders were obtained from participating registries. Rates for Hispanics (available 1992 onward) were obtained from the NCI Surveillance, Epidemiology, and End Results program. We derived exact confidence intervals around each SIR.
To compare MCC risk between categories of transplant recipients, we calculated incidence rates (ie, cases/100000 person-years) and corresponding incidence rate ratios (IRRs). We compared incidence across categories defined by age at transplant, transplanted organ, successive time intervals after transplant, and calendar year of follow-up attained by the recipient (ie, attained calendar year). We calculated P values using a likelihood ratio test.
To explore possible interactions of photosensitizing medications and UVR exposure, we analyzed trends in MCC incidence across categories of latitude and AVGLO, stratified by maintenance medication category. These analyses were restricted to non-Hispanic whites, who are most susceptible to UVR damage. Finally, to identify independent predictors of MCC risk, we constructed a multivariable Poisson model of MCC incidence restricted to non-Hispanic whites. Covariables included sex, age at transplant, transplanted organ, time since transplantation, and variables capturing the effects of medications and UVR exposure observed in univariate analyses. We included a reduced, two-level maintenance medication regimen variable comparing cyclosporine/azathioprine recipients (as described above) to all other regimens, and further included latitude quintile (modeled with one degree of freedom) and an interaction term between cyclosporine/azathioprine maintenance regimen and latitude quintile. Accordingly, we present IRRs representing the adjusted trend in MCC incidence across latitude quintile, separately for cyclosporine/azathioprine and other maintenance regimens. All statistical tests were two-sided.
Results
We evaluated risk of MCC in 189498 solid organ transplants with 859789 person-years of follow-up (Table 1). Sixty-two percent of transplant recipients were male, and the median age at transplant was 49 years. Recipients were racially and ethnically diverse, with 37% of transplants occurring in persons who were not non-Hispanic white. Kidney transplants were most common, but 35% of transplants were liver, heart, or lung transplants, and 6% included less common or multiple organs.
Table 1.
Characteristics of 189 498 US solid organ transplants evaluated for risk of Merkel cell carcinoma
| Characteristic | No. of transplants | % of total |
|---|---|---|
| Total | 189498 | 100 |
| Sex | ||
| Male | 116933 | 61.7 |
| Female | 72565 | 38.3 |
| Age at transplant, y | ||
| 20–34 | 31621 | 16.7 |
| 35–49 | 64868 | 34.2 |
| 50–64 | 75431 | 39.8 |
| 65+ | 17578 | 9.3 |
| Race/ethnicity | ||
| Non-Hispanic white | 119419 | 63.0 |
| Non-Hispanic black | 31865 | 16.8 |
| Hispanic | 27674 | 14.6 |
| Asian/Pacific Islander | 10540 | 5.6 |
| Transplanted organ | ||
| Kidney only | 111775 | 59.0 |
| Liver only | 40238 | 21.2 |
| Heart only | 17693 | 9.3 |
| Lung only | 7946 | 4.2 |
| Other or multiple | 11846 | 6.3 |
| Calendar year of transplantation | ||
| 1987–1994 | 34249 | 18.1 |
| 1995–1999 | 47609 | 25.1 |
| 2000–2004 | 59409 | 31.4 |
| 2005–2009 | 48231 | 25.5 |
A total of 110 MCC diagnoses among transplant recipients were identified. The median age at diagnosis of MCC was 62 years (range = 34–80). More than half of all MCC case patients were diagnosed on the head or neck (51%, n = 56), while smaller proportions were diagnosed on the upper extremities (26%, n = 29), the trunk (12%, n = 13), lower extremities (8%, n = 9) or unknown sites (3%, n = 3). Among MCCs with known stage at diagnosis (n = 95), the majority (62%) were diagnosed at localized stage, while 29% had regional and 9% had distant stage; this distribution was similar to the expected distribution (data not shown).
The overall incidence rate of MCC was 12.8 (95% confidence interval [CI] = 10.5 to 15.4) per 100000 person-years. When compared with the general population, this corresponded to a nearly 24-fold elevation in risk (SIR = 23.8, 95% CI = 19.6 to 28.7). As shown in Table 2, incidence rates increased steeply with increasing age at transplant, and 73% of cases were in people older than age 50 years at transplant. MCC incidence was 70% higher in male compared with female transplant recipients (IRR = 1.7, 95% CI = 1.1 to 2.6). Ninety-one percent of MCC cases were in whites, and incidence was five-fold higher in white than in non-white recipients.
MCC incidence rates increased statistically significantly with time since transplant, with highest incidence occurring 10 or more years after transplant (Table 2). MCC rates also increased over the time period studied, with the highest rate observed from 2003 to 2005. For the cohort overall, MCC risk did not vary statistically according to ecologic proxies of UVR exposure, including quintiles of AVGLO and latitude of residence. Incidence also was not different between recipients of kidneys and other organs, although the test for heterogeneity (P = .019) suggested statistically significant variation among organ types, as did subsequent multivariable regression (see below).
We examined MCC rates according to receipt of specific medications (Table 3). About 44% of the transplant population received induction medications at the time of transplantation. We observed a statistically significantly protective association of monoclonal antibody induction with MCC, as fewer than 2% (n = 2) of MCC case patients had received monoclonal antibodies, but a higher proportion (5.4%) without MCC had received them. With respect to individual maintenance immunosuppressive medications, we observed higher incidence among persons receiving cyclosporine, azathioprine, or mTOR inhibitors, and decreased incidence in those receiving tacrolimus or MMF, compared with recipients not receiving these medications. The combination regimen of cyclosporine/azathioprine was associated with highest MCC risk (Table 3).
Table 3.
Risk of Merkel cell carcinoma according to induction and maintenance medications prescribed at time of transplantation*
| Medications | MCC patients | Incidence rate (per 100 000 person-years) | Incidence rate ratio (IRR)(95% CI) | P |
|---|---|---|---|---|
| Induction medications | ||||
| Monoclonal antibodies (T-cell depleting) | .007 | |||
| No | 108 | 13.6 | Ref. | |
| Yes | 2 | 3.1 | 0.2 (0.1 to 0.9) | |
| Polyclonal antibody | .630 | |||
| No | 94 | 13.0 | Ref. | |
| Yes | 16 | 11.5 | 0.9 (0.5 to 1.5) | |
| IL2 receptor antagonist | .727 | |||
| No | 96 | 13.0 | Ref. | |
| Yes | 14 | 11.7 | 0.9 (0.5 to 1.6) | |
| Maintenance medications | ||||
| Cyclosporine | .031 | |||
| No | 39 | 10.0 | Ref. | |
| Yes | 71 | 15.2 | 1.5 (1.0 to 2.3) | |
| Tacrolimus | .002 | |||
| No | 84 | 15.6 | Ref. | |
| Yes | 26 | 8.1 | 0.5 (0.3 to 0.8) | |
| Azathioprine | .059 | |||
| No | 66 | 11.2 | Ref. | |
| Yes | 44 | 16.3 | 1.5 (1.0 to 2.1) | |
| MMF | .036 | |||
| No | 71 | 15.1 | Ref. | |
| Yes | 39 | 10.0 | 0.7 (0.4 to 1.0) | |
| mTOR inhibitors | .069 | |||
| No | 100 | 12.2 | Ref. | |
| Yes | 10 | 23.6 | 1.9 (1.0 to 3.7) | |
| Corticosteroids | .919 | |||
| No | 10 | 12.4 | Ref. | |
| Yes | 100 | 12.8 | 1.0 (0.5 to 2.0) | |
| Maintenance medication regimen | .021 | |||
| Tacrolimus/MMF | 19 | 9.0 | Ref. | |
| Cyclosporine/Azathioprine | 43 | 18.1 | 2.0 (1.2 to 3.5) | |
| Other | 48 | 11.7 | 1.3 (0.8 to 2.2) | |
* Poisson regression analysis. All statistical tests were two-sided. CI = confidence interval; IL2 = interleukin 2; MMF = mycophenolate mofetil; mTOR = mammalian target of rapamycin.
We hypothesized that the effects of UVR would be most apparent in non-Hispanic whites who were given cyclosporine and azathioprine, given the reported photosensitizing and carcinogenic effects of these medications (18,24). Figure 1 shows that latitude and AVGLO were not associated with statistically significant variations in incidence among non-Hispanic white persons receiving tacrolimus/MMF or other regimens. However, for those receiving cyclosporine/azathioprine, MCC incidence increased statistically significantly with decreasing latitude (P = .003) (Figure 1A) and increasing AVGLO (P = .023) (Figure 1B), ie, conditions of higher average UVR exposure.
Figure 1.
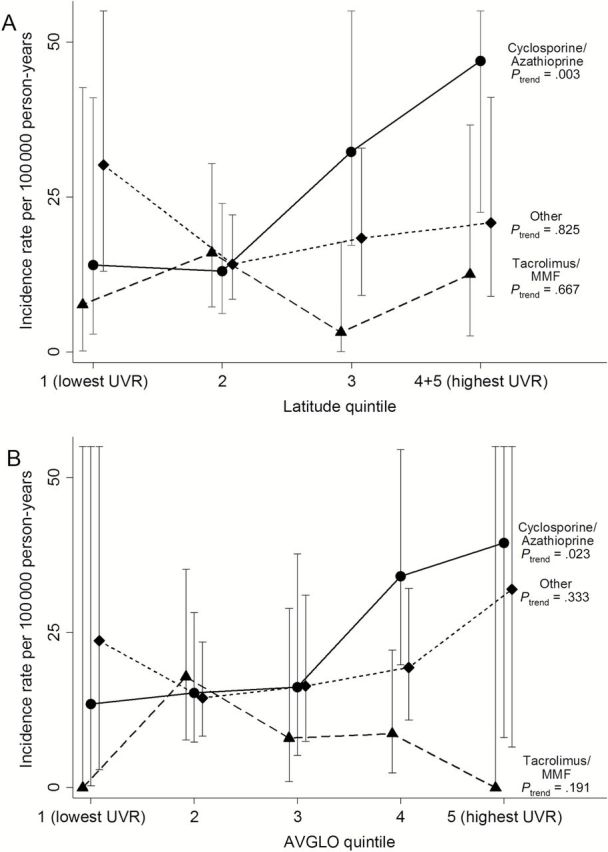
MCC incidence rates among non-Hispanic whites according to maintenance medication type and ultraviolet radiation as measured by quintile of residential A) latitude and B) AVGLO. AVGLO is predicted 30-year average daily total global solar radiation (Wh/km2). Results are presented for transplants associated with maintenance immunosuppression with cyclosporine/azathioprine, tacrolimus/mycophenolate mofetil, and other regimens (see Methods for details). Vertical lines depict 95% confidence intervals of incidence rate estimates (some upper limits are out of range and so are not shown). P trend values are shown, and P interaction values are .016 for residential latitude and .086 for AVGLO. Latitude quintiles 4 and 5 are combined because of small numbers (5139 person-years in quintile 5). All statistical tests were two-sided.
In a multivariable model restricted to non-Hispanic white recipients, we confirmed that most of these characteristics were independent risk factors for MCC (Table 4). Specifically, MCC risk was higher with male sex, older age at transplant (older than age 50 years vs 18–49 years), receipt of kidney transplant, longer time since transplant, receipt of no induction therapy or induction therapy other than monoclonal antibodies, maintenance therapy with mTOR inhibitors, and lower residential latitude (ie, higher UVR) among those receiving cyclosporine and azathioprine (P = .012).
Table 4:
Multivariable analysis of risk factors for MCC (n = 100) among non-Hispanic white transplant recipients
| Characteristic | Incidence rate ratio (IRR) (95% CI) | P |
|---|---|---|
| Sex | .002 | |
| Female | Ref. | |
| Male | 2.1 (1.3 to 3.4) | |
| Age at transplant, y | <.001 | |
| 20–34 | Ref. | |
| 35–49 | 2.0 (0.8 to 5.4) | |
| 50–64 | 5.4 (2.1 to 13.5) | |
| 65+ | 7.4 (2.7 to 20.7) | |
| Organ | .007 | |
| Kidney only | Ref. | |
| Liver only | 0.4 (0.2 to 0.7) | |
| Heart only | 0.6 (0.4 to 1.1) | |
| Other/multiple | 0.5 (0.2 to 1.3) | |
| Time since transplant, y | <.001 | |
| <1 | Ref. | |
| 1 to <3 | 5.4 (1.6 to 18.0) | |
| 3 to <5 | 5.3 (1.5 to 18.3) | |
| 5 to <10 | 12.0 (3.7 to 39.0) | |
| ≥10 | 21.0 (6.0 to 73.4) | |
| Induction therapy with T-cell–depleting monoclonal antibodies | 0.2 (0.1 to 0.9) | .008 |
| Maintenance therapy with mTOR inhibitors | 3.0 (1.5 to 5.9) | .006 |
| Maintenance regimen including cyclosporine and azathioprine, and interaction with latitude Maintenance therapy with cyclosporine/azathioprine* |
1.6 (1.0 to 2.5) | .076 |
| Latitude quintile, among recipients of cyclosporine/azathioprine† |
1.6 (1.1 to 2.2) | .012 |
| Latitude quintile, among recipients of other maintenance medication regimens† |
1.0 (0.7 to 1.3) | .808 |
* Incidence rate ratio corresponds to the effect of cyclosporine/azathioprine in the middle quintile of latitude. Analyses are adjusted for all variables shown in table. CI = confidence interval. Poisson regression analysis. All statistical tests were two-sided.
† Latitude quintile is modeled with 1 degree of freedom such that the highest quintile is the lowest latitude ie, experiences the highest UVR exposure. Incidence rate ratios correspond to the effect of an increase of one quintile of latitude. The P interaction between latitude and medication = 0.033.
Discussion
In this study of transplant-related MCC, we found that solid organ transplant recipients had steeply elevated risk, with incidence almost twenty-four times higher than in the general population. MCC incidence rose with advancing time since transplant, suggesting an etiologic role of long-term, chronic immunosuppression, as opposed to the more acute, intense immunosuppression induced at the time of transplantation. Given the nature of transplant-related immunosuppression, and the increased risk of MCC seen in HIV-infected people (7), the relevant immunosuppression may primarily involve T-cell deficiency. There may also be an additional role for B-cell deficiency, which is prominent in another predisposing condition, chronic lymphocytic leukemia (8). Presumably, long-term immune deficits in transplant recipients allow for expression of the MCV viral proteins required for carcinogenesis.
Nonetheless, it is also likely that UVR works in concert with immunosuppression to cause MCC. UVR exposure is a key contributor to MCC and other skin cancers in light-skinned populations (4), with the presumed mechanism involving direct DNA damage and mutation, especially with cumulative, prolonged exposure to ionizing ultraviolet wavelengths in sunlight. Supporting the relevance of UVR exposure in transplant recipients, we found greater risk in whites than in other groups, and most cases occurred on sun-exposed sites. Also, incidence increased steeply with age, as is seen in the general population (2,3), likely reflecting chronic skin damage over many years of sunlight exposure.
While the mechanism by which UVR contributes to DNA damage is well-understood (25), there are practical difficulties in measuring lifetime cumulative UVR exposure in an epidemiologic study. Thus, we used residential latitude and AVGLO as broad, ecologic surrogates of individual UVR exposure. These measures did not associate meaningfully with MCC incidence across the whole cohort, but we did observe interesting associations among a subset, specifically white recipients who received cyclosporine and azathioprine. In basic science settings, azathioprine has been shown to increase the DNA mutation frequency associated with a given level of UVR exposure (13,16,18,24). Cyclosporine is thought to reduce capacity for DNA repair (14,15,17). Although our analyses for cyclosporine and azathioprine were prespecified, we point out that they involve a test for interaction in a cohort subgroup and should be considered somewhat exploratory. Nonetheless, our study provides the first epidemiologic evidence suggesting a synergistic effect of these medications with UVR exposure in the development of MCC. Although patterns of medication use have changed over time, we consider it unlikely that our observed associations are confounded by attained year or calendar year of transplant, as inclusion of these variables in sensitivity analyses did not alter the multivariable associations with any medication regimen (not shown).
We also found statistically significantly higher risk of MCC associated with use of mTOR inhibitors for maintenance, which was unexpected, because these medications decrease risk of cutaneous squamous cell carcinoma (26). Interestingly, however, the MCV small T antigen interacts with downstream proteins in the AKT pathway, bypassing mTOR itself, and, perhaps as a result, mTOR inhibitors do not inhibit MCC tumor cell growth (28). One possible explanation for the adverse association we observed with MCC could be that mTOR inhibitors were preferentially prescribed as part of an initial regimen to recipients perceived by clinicians to be at risk of UV-related skin cancers. Of note, mTOR inhibitors are also often prescribed following initial discharge from the hospital after transplantation because of their negative effects on wound healing, but we did not capture this pattern of use. We also found significantly reduced risk of MCC with use of monoclonal, T-cell depleting antibodies but not with polyclonal antibodies.
Our estimate of 23.8-fold higher risk of MCC in transplant recipients relative to the general population is substantially higher than previous estimates for immunosuppressed populations. To our knowledge, the only other published estimate for transplant-related MCC was based on the SEER-Medicare database, a linkage of the US cancer registry and Medicare claims data (11). That study, which included only elderly white adults, reported about five-fold increased risk following organ transplantation (odds ratio = 4.95, 95% CI = 2.62 to 9.3) (11), about half the risk we found for recipients over age 65 years (SIR = 9.9, 95% CI = 5.6 to 16.0). Our estimate is also higher than the two published estimates of MCC risk among persons with HIV/AIDS. A population-based linkage of US AIDS and cancer registries (7) identified six cases of AIDS-related MCC, corresponding to a relative risk of 13.4 (95% CI = 4.9 to 29.1) compared with the general population. The SEER-Medicare analysis reported a two-fold elevated risk of MCC (odds ratio = 2.30, 95% CI = 0.94 to 5.67) associated with HIV among older, white adults (11). We found higher MCC incidence in male recipients, consistent with the studies reporting higher MCC rates in males in the general population (2,4) but different than others finding higher rates in females (3).
This study has several important strengths. It is the first cohort study of transplant recipients with a sufficient number of incident MCC case patients to allow assessment of risk in subgroups defined by age, race, and transplanted organ. Prior efforts to quantify these risks were limited by small size or restriction to specific subpopulations, eg, Medicare beneficiaries over age 65 years (11). Our cohort included a well-defined, population-based sample representing nearly half of US transplants, and linkage with corresponding state-mandated, population-based cancer registries allowed for complete ascertainment of MCC in transplant recipients. The population-based cancer registries also provided a large and well-standardized resource for accurate estimation of expected cases and the SIR.
Among the study’s limitations, we note that MCC was identified from cancer registry abstractions of medical records, and pathology laboratories may not have utilized standardized diagnostic protocols. We observed a substantial increase in transplant-related MCC over time (as was noted in the general population [3]), perhaps because of changes in dermatological or pathologic practices (including growth in the use of CK20 staining as a diagnostic tool in the 1990s) or in registration following the introduction of the distinct ICD code for MCC in 1986. Risks reported here may be underestimated if MCCs were diagnosed in transplant recipients after they had migrated out of the cancer registry catchment area, although this was likely infrequent (20). Surveillance bias is another possible limitation of studying cancer in a population receiving intensive medical attention; however, it is unlikely that MCC frequently escapes diagnosis, even in the general population, because of its aggressive behavior. Available transplant medication data represent those prescribed at time of transplant. Regimens are somewhat stable for most patients (29,30), but we could not account for changes in medications over time. Finally, despite the large size of our study, statistical power for evaluating risks in patient subgroups was still limited.
In summary, we found strikingly high risks of MCC among transplant recipients as compared with the general population. We interpret these patterns as indicating important roles in MCC development for sustained, long-term immunosuppression, UVR, and photosensitizing effects of some maintenance medications. Future studies should explore the specific interplay between carcinogenic viruses, immune dysfunction, and UVR as they relate to skin cancer etiology. Transplant recipients should be encouraged to adopt sun safety practices and limit UVR exposure to reduce their likelihood of developing MCC and other skin cancers for which they are at exceedingly high risk (31).
Funding
This work was supported in part by the Stanford Cancer Institute and the National Cancer Institute of the National Institutes of Health.
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the Scientific Registry of Transplant Recipients (SRTR) (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado, Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii, Iowa, Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington. We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors. This research was supported in part by Stanford Cancer Institute and the Intramural Research Program of the National Cancer Institute.
During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (5U58/DP003931-02), New York (U58DP003879), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14–2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, WA.
The authors have no conflicts of interest to declare.
References
- 1. Reichgelt BA, Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993–2007. Eur J Cancer. 2011;47(4):579–585. [DOI] [PubMed] [Google Scholar]
- 2. Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832–841. [DOI] [PubMed] [Google Scholar]
- 3. Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102(11):793–801. [DOI] [PubMed] [Google Scholar]
- 4. Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8(2):153–158. [PubMed] [Google Scholar]
- 5. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. An KP, Ratner D. Merkel cell carcinoma in the setting of HIV infection. J Am Acad Dermatol. 2001;45(2):309–312. [DOI] [PubMed] [Google Scholar]
- 7. Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–498. [DOI] [PubMed] [Google Scholar]
- 8. Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1545–1549. [DOI] [PubMed] [Google Scholar]
- 9. Eftekhari F, Wallace S, Silva EG, Lenzi R. Merkel cell carcinoma of the skin: imaging and clinical features in 93 cases. Br J Radiol. 1996;69(819):226–233. [DOI] [PubMed] [Google Scholar]
- 10. Gooptu C, Woollons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137(4):637–641. [DOI] [PubMed] [Google Scholar]
- 11. Lanoy E, Costagliola D, Engels EA, et al. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer. 2010;126(7):1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68(11):1717–1721. [DOI] [PubMed] [Google Scholar]
- 13. Attard NR, Karran P. UVA photosensitization of thiopurines and skin cancer in organ transplant recipients. Photochem Photobiol Sci. 2012;11(1):62–68. [DOI] [PubMed] [Google Scholar]
- 14. Han W, Soltani K, Ming M, He YY. Deregulation of XPC and CypA by cyclosporin A: an immunosuppression-independent mechanism of skin carcinogenesis. Cancer Prev Res (Phila). 2012;5(9):1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herman M, Weinstein T, Korzets A, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001;137(1):14–20. [DOI] [PubMed] [Google Scholar]
- 16. Hofbauer GF, Attard NR, Harwood CA, et al. Reversal of UVA skin photosensitivity and DNA damage in kidney transplant recipients by replacing azathioprine. Am J Transplant. 2012;12(1):218–225. [DOI] [PubMed] [Google Scholar]
- 17. Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125(5):1020–1025. [DOI] [PubMed] [Google Scholar]
- 18. O’Donovan P, Perrett CM, Zhang X, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309(5742):1871–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer. 2013;132(6):1429–1438. [DOI] [PubMed] [Google Scholar]
- 20. Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. CivicSpace Labs. CivicSpace US ZIP Code Database. 2004 [3/30/2014]; Available at: http://www.boutell.com/zipcodes/.
- 22. Tatalovich Z, Wilson JP, Cockburn MG. A Comparison of Thiessen Polygon, Kriging, and Spline Models of Potential UV Exposure. Cartography and Geographic Information Science. 2006;33(3):217–237. [Google Scholar]
- 23. Tatalovich Z, Wilson JP, Mack T, Yan Y, Cockburn M. The objective assessment of lifetime cumulative ultraviolet exposure for determining melanoma risk. J Photochem Photobiol B. 2006;85(3):198–204. [DOI] [PubMed] [Google Scholar]
- 24. Perrett CM, Walker SL, O’Donovan P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol. 2008;159(1):198–204. [DOI] [PubMed] [Google Scholar]
- 25. Rass K, Reichrath J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:162–178. [DOI] [PubMed] [Google Scholar]
- 26. Balagula Y, Kang S, Patel MJ. Synergism between mTOR pathway and ultraviolet radiation in the pathogenesis of squamous cell carcinoma and its implication for solid-organ transplant recipients. Photodermatol Photoimmunol Photomed. 2014; In press. [DOI] [PubMed] [Google Scholar]
- 27. Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121(9):3623–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4(133):133ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meier-Kriesche HU, Li S, Gruessner RW, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6(5 Pt 2):1111–1131. [DOI] [PubMed] [Google Scholar]
- 30. Stirnemann PM, Takemoto SK, Schnitzler MA, et al. Agreement of immunosuppression regimens described in Medicare pharmacy claims with the Organ Procurement and Transplantation Network survey. J Am Soc Nephrol. 2006;17(8):2299–2306. [DOI] [PubMed] [Google Scholar]
- 31. Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients--where do we stand today? Am J Transplant. 2008;8(11):2192–2198. [DOI] [PubMed] [Google Scholar]


