Abstract
Background:
Cutaneous melanoma is the fifth most common cancer in the United States. Modifiable risk factors, with the exception of exposure to ultraviolet radiation (UVR), are poorly understood. Coffee contains numerous bioactive compounds and may be associated inversely with melanoma. However, previous epidemiological evidence is limited.
Methods:
Coffee intake was assessed at baseline with a food frequency questionnaire in the National Institutes of Health–AARP prospective cohort study. Among 447 357 non-Hispanic whites who were cancer-free at baseline, 2904 incident cases of malignant melanoma were identified during 4 329 044 person-years of follow-up, with a median of 10.5 years of follow-up. Multivariable-adjusted Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for coffee intake and subsequent melanoma risk with non–coffee drinkers as the reference group. Statistical tests were two-sided, and P values less than .05 were interpreted as statistically significant.
Results:
The highest category of coffee intake was inversely associated with malignant melanoma (≥4 cups/day: HR = 0.80, 95% CI = 0.68 to 0.93, P trend = .01). This association was statistically significant for caffeinated (≥4 cups/day: HR = 0.75, 95% CI = 0.64 to 0.89, P trend = .01) but not for decaffeinated coffee (P trend = .55).
Conclusions:
Higher coffee intake was associated with a modest decrease in risk of melanoma in this large US cohort study. Additional investigations of coffee intake and its constituents, particularly caffeine, with melanoma are warranted.
Cutaneous melanoma is the fifth most common cancer and the leading cause of skin cancer death in the United States, with an estimated 77 000 new cases and 9500 deaths in 2013 (1). Exposure to ultraviolet radiation (UVR), particularly UVB, is the only consistently associated exogenous risk factor for melanoma (2). Yet, other exposures are likely important. Experimental evidence lends biological plausibility to a possible protective role of coffee consumption in UVB-induced carcinogenesis. In vitro and animal studies have shown that coffee constituents suppress UVB-induced skin carcinogenesis (3), induce cell apoptosis (4), protect against oxidative stress and DNA damage (5), reduce inflammation in epidermal cells (6), and inhibit changes in DNA methylation (7). The protective effects of coffee constituents, especially caffeine, on UVB-induced skin cancer demonstrated by murine and cell culture models have been corroborated by epidemiological studies of coffee consumption and risk of nonmelanoma skin cancers (8–11). In contrast, the few existent epidemiological studies of coffee consumption and melanoma are marked by inconsistent results (12–17).
With few lifestyle factors as viable targets for melanoma prevention and the worldwide popularity of coffee drinking, it is important to resolve these conflicting findings. In the current study, we analyzed data from the National Institutes of Health (NIH)–AARP Study, which include nearly four times as many cases of malignant melanoma as the largest prospective study to date, to better understand the association of coffee drinking with risk of malignant melanoma, as well as risk of melanoma in situ.
Methods
Study Population
The NIH-AARP Diet and Health Study, described previously (18), commenced in 1995 to 1996 with the mailing of a self-administered questionnaire to 3.5 million AARP members age 50 to 71 years who resided in one of six US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) or two US metropolitan areas (Atlanta, Georgia and Detroit, Michigan). The baseline questionnaire queried about demographics, health-related behaviors and dietary intake. The study was approved by the Special Studies Institutional Review Board of the National Cancer Institute. Of the 566 398 participants who satisfactorily completed the questionnaire and provided informed consent, we excluded proxy-responders (n = 15 760), individuals with cancer (except nonmelanoma skin cancer) prior to baseline (n = 51 234), individuals with only a death record for cancer (n = 2354), individuals who self-identified as any race or ethnicity other than white, non-Hispanic (n = 44 600), individuals with missing information on coffee intake (n = 1781), individuals with extremely low or high caloric intake (n = 3283), defined as more than two interquartile ranges above the 75th percentile or below the 25th percentile of intake, and individuals and with zero years of follow-up (n = 29). The resulting analytic cohort consisted of 447 357 participants.
Cohort Follow-Up and Case Ascertainment
Participants were followed from baseline until the date of first melanoma or other nonepithelial skin cancer diagnosis, the date of death, the end of study follow-up (December 31, 2006), or the date the participant moved out of the registry area, whichever came first. Incident cases of cutaneous melanoma were identified by probabilistic record linkage with state cancer registries that covered the original eight states plus three additional states (Arizona, Texas, and Nevada), to which a number of participants moved during follow-up. Cutaneous melanoma was defined according to the International Classification of Disease for Oncology (ICD-O, 3rd edition) by anatomic site and histological code (C44.0-C44.9 with Histology 8720–8780). This classification includes melanoma in situ and malignant melanoma, but it does not include melanomas described as metastases. As melanoma in situ is not invasive and etiology may differ from malignant melanoma, we treated these as separate outcomes.
Assessment of Coffee Intake
Coffee intake was assessed with a self-administered 124-item food frequency questionnaire (FFQ) and calibrated using a subgroup of 1664 participants who completed two 24-hour dietary recalls on nonconsecutive days (n = 1664) (Supplementary Methods, available online). Participants reported usual coffee intake in the previous two months using ten predefined frequency categories, ranging from none to six or more cups per day. Of those who reported drinking coffee, 96.6% (n = 432 031) provided information on whether they drank caffeinated or decaffeinated coffee more than half the time. We used responses to these two FFQ items to categorize coffee drinkers into prespecified categories ranging from none to four or more cups per day for total coffee and for either caffeinated or decaffeinated coffee more than half the time. For participants with missing information on coffee type, we created a missing category. Total caffeine intake (mg/day) from beverage and food sources was estimated by assuming that “more than half the time” meant “always” and summing the product of the estimated caffeine content of each beverage or food source by the daily amount consumed for each participant (19).
Assessment of Covariables
The method for assessing erythemal UVR exposure in this cohort has been described previously (20). In brief, all available NASA Total Ozone Mapping Spectrometer (TOMS) estimates for noon-time ground-level erythemal UVR measured during the month of July, when surface UVR is strongest (21) and TOMS UVR data are in better agreement with ground-based data (22), between 1978–1993 and 1996–2005 were averaged. Participants were assigned an erythemal UVR exposure by deterministic linkage of the census tract centroid of baseline residence to the closest point on the TOMS 1° latitude by 1.25° longitude grid. The baseline questionnaire provided the data for the other covariables. Body mass index (BMI) was calculated from self-reported height and weight at baseline. Physical activity over the last 12 months was defined as frequency of activity lasting 20 minutes or longer that caused increases in breathing or heart rate or sweating. Average daily alcohol intake over the last 12 months was calculated from drinks of alcohol from beer, wine, and liquor. Smoking history was defined as never, former, or current smoker. Current and former smokers were further defined by smoking intensity, and former smokers were additionally defined by time since quitting.
Statistical Analysis
We tabulated demographic and lifestyle factors previously associated with melanoma by coffee intake. We standardized absolute risks within five-year age bands to the age distribution of the entire NIH-AARP cohort (23) and used Cox proportional hazards regression models to estimate hazard ratios (HRs) and two-sided 95% confidence intervals (CIs) separately for coffee intake with malignant melanoma and melanoma in situ. Age was used as the underlying time metric; results calculated with follow-up time as the underlying time metric were similar. We tested the proportional-hazards assumption by modeling the interaction of follow-up time with coffee intake. Consistent with proportional hazards, inclusion of the time dependent interaction did not statistically significantly improve the model fit for malignant melanoma (P likelihood ratio test (LRT) = .23) or for melanoma in situ (P LRT = .21). We used SAS software, version 9.3 (SAS Institute, Cary, NC) to conduct our analyses. Statistical tests were two-sided, and P values under .05 were interpreted as statistically significant.
In the base multivariable models, we adjusted for age and sex. We included the following additional potential confounders in the full multivariable models: tobacco smoking, alcohol drinking, education, BMI, physical activity, family history of cancer, and July erythemal UVR exposure. Only cigarette smoking, education, and alcohol drinking altered risk estimates by more than 10%. Less than 4% of the cohort lacked information on any covariable; nonetheless, we included an indicator for missing data in the regression models. We estimated hazard ratios for coffee intake using no coffee intake as the reference. We conducted tests for linear trend across categories of coffee intake by assigning participants the midpoint of their coffee intake category and entering this single variable into separate models. Participants in the four or more cups per day category were assigned a value of five cups per day. To test for smoking- (never or ever), sex-, or age-heterogeneity in the association between coffee intake and melanoma risk, we included a single cross-product term for each variable and coffee intake in three separate models. Sex and age tests for heterogeneity, which were based on LRTs, were not statistically significant (P LRT ≥ .1); therefore, we did not present sex- or age-specific results.
In secondary analyses, we calculated hazard ratios for categories of caffeinated and decaffeinated coffee intake using a single model. Participants were considered either caffeinated or decaffeinated coffee drinkers based on which type they reported drinking more than half the time; those who did not specify the type of coffee usually consumed (3.4%) were also entered into the model as unknown. We also calculated the hazard ratios for quartiles of total caffeine intake and conducted a test for linear trend by assigning participants the median of their quartile (mg) and entering this single variable into the fully adjusted model. To explore the robustness of the association between total coffee intake and malignant melanoma and to evaluate the possible impact of residual confounding or effect modification, we stratified by follow-up time (<5 or ≥5 years), cigarette smoking status (never or ever) (12,24), education (noncollege or college graduate) (12,25–27), and BMI (normal or overweight/obese) (28,29) and tested each cross-product term by the LRT. Finally, we conducted a dose-response analysis among coffee drinkers only (n = 402 783) using one or fewer cups per day as the reference category.
Results
During 4 329 044 person-years of follow-up, 2904 cases of malignant melanoma (2154 men and 750 women) and 1874 cases of melanoma in situ (1391 men, 483 women) were identified. Melanoma in situ accounted for 39% of melanoma cases in our cohort. The median age at baseline was 62.6 years, and median follow-up time was 10.5 years. The cohort was well educated (62.3% had at least some college education) and the majority male (60.5%). Most participants had a history of cigarette smoking (61.8%) and consumed one or fewer alcoholic drinks per day (76.5%). Approximately 90% reported drinking coffee, and of those 65% reported drinking two or more cups per day. At baseline, high coffee intake was associated with male sex, lower educational attainment, cigarette smoking, and alcohol drinking. Mean age, mean BMI, physical activity, and July erythemal UVR exposure did not appear to differ systematically by level of coffee intake (Table 1). Age, sex, smoking, education, physical activity, alcohol drinking, and July erythemal UVR were statistically significantly associated with malignant melanoma risk in age and sex adjusted models (Supplementary Table 1, available online). Coffee accounted for approximately 85% of dietary caffeine intake, while tea, soda, and other sources accounted for 10%, 5% and less than 1% of dietary caffeine intake, respectively.
Table 1.
NIH-AARP Diet and Health Study characteristics by level of coffee intake (n = 447 357)
| Characteristic | Coffee intake | |||
|---|---|---|---|---|
| None | ≤1 cup/d | 2–3 cup/d | ≥4 cups/d | |
| Melanoma cohort, n (%) | 44 574 (10.0) | 140 843 (31.4) | 188 020 (42.0) | 73 920 (16.5) |
| Age at entry, y | 61.3 (5.6) | 62.5 (5.4) | 62.2 (5.3) | 61.3 (5.4) |
| BMI, kg/m2 | 27.2 (5.7) | 27.1 (5.2) | 27.0 (4.7) | 27.0 (4.9) |
| Sex | ||||
| Male | 23 508 (8.7) | 80 649 (29.8) | 116 652 (43.1) | 49 866 (18.4) |
| Female | 21 066 (11.9) | 60 194 (34.1) | 71 368 (40.4) | 24 054 (13.6) |
| Cigarette smoking*† | ||||
| Never smoker | 25 408 (16.3) | 58 995 (38.0) | 56 673 (36.5) | 14 378 (9.3) |
| Former smoker | 14 822 (6.9) | 66 124 (30.7) | 98 712 (45.8) | 35 737 (16.6) |
| Current smoker | 3057 (5.0) | 10 830 (17.7) | 26 025 (42.6) | 21 182 (34.7) |
| Pipe/cigar smoking† | ||||
| Never smoker | 39 330 (10.6) | 119 113 (32.2) | 153 926 (41.6) | 57 963 (15.7) |
| Ever smoker | 3521 (5.6) | 16 753 (26.6) | 28 545 (45.4) | 14 072 (22.34) |
| Education† | ||||
| ≤11 y | 1929 (7.7) | 7783 (30.9) | 10 521 (42.0) | 4842 (19.3) |
| High school graduate | 12 557 (9.4) | 41 619 (31.3) | 56 099 (42.2) | 22 765 (17.1) |
| Some college | 10 248 (9.9) | 31 707 (30.6) | 44 220 (42.6) | 17 608 (17.0) |
| College graduate | 18 886 (10.8) | 56 390 (32.3) | 72 604 (41.5) | 26 901 (15.4) |
| Physical activity† | ||||
| Never/rarely | 8445 (10.7) | 24 843 (31.6) | 31 446 (40.0) | 13 932 (17.7) |
| 1–3/mo | 5830 (9.6) | 18 440 (30.3) | 25 535 (42.0) | 11 018 (18.1) |
| 1–2/wk | 8995 (9.2) | 30 120 (30.9) | 41 988 (43.1) | 16 237 (16.7) |
| 3–4/wk | 11 283 (9.4) | 38 680 (32.1) | 51 996 (43.2) | 18 462 (15.3) |
| 5+/wk | 9717 (11.1) | 27 688 (31.8) | 35 856 (41.2) | 13 815 (15.9) |
| Alcohol, drinks/d | ||||
| None | 19 070 (18.3) | 33 807 (32.4) | 34 628 (33.2) | 16 890 (16.2) |
| ≤1 | 20 173 (8.5) | 78 945 (33.2) | 101 246 (42.6) | 37 398 (15.7) |
| >1 and ≤3 | 3496 (5.0) | 18 992 (27.0) | 35 275 (50.1) | 12 604 (17.9) |
| >3 | 1835 (5.3) | 9099 (26.1) | 16 871 (48.4) | 7028 (20.2) |
| July erythemal UVR, J/m2‡ | ||||
| ≤186.3 | 11 282 (9.6) | 37 020 (31.4) | 49 500 (42.0) | 20 153 (17.1) |
| >186.3–236.8 | 9660 (9.2) | 33 182 (31.7) | 44 870 (42.8) | 17 126 (16.3) |
| >236.8–253.7 | 11 164 (10.6) | 32 391 (30.7) | 43 859 (41.6) | 17 955 (17.0) |
| >253.7 | 12 468 (10.5) | 38 250 (32.1) | 49 791 (41.8) | 18 686 (15.7) |
| Family history of cancer | ||||
| No | 22 570 (10.0) | 71 880 (31.8) | 94 917 (41.9) | 37 019 (16.4) |
| Yes | 22 004 (10.0) | 68 963 (31.2) | 93 103 (42.1) | 36 901 (16.7) |
* Those who quit less than one year ago were considered current smokers. Weighted mean (SD) for continuous variables and weighted row % (n) for categorical variables. NIH = National Institutes of Health; UVR = ultraviolet radiation.
† n does not total 447 357 because of missing data. Physical activity defined as frequency of activity lasting 20 minutes or more that caused increases in breathing or heart rate or sweating.
‡ July erythemal ultraviolet radiation exposure was calculated as the averaged exposure across all available measured days in the month of July between 1978 to 1993 and 1996 to 2005 and categorized as quartiles.
First, we tested the hypothesis that higher coffee intake is associated with lower risk of malignant melanoma and melanoma in situ (Table 2). We observed statistically significant trends of decreasing malignant melanoma risk with higher coffee intake in the base multivariable (ie, age- and sex-adjusted) model (P trend < .001) and in the full multivariable model (P trend = .01). The statistically significant inverse association between the highest level of coffee intake (≥4 cups/day) and malignant melanoma risk (HR = 0.72, 95% CI = 0.62 to 0.83, 55.90 vs 77.64 cases per 100 000 person-years) in the base model remained statistically significant, albeit attenuated, in the fully adjusted model (HR = 0.80, 95% CI = 0.68 to 0.93). We then tested the hypothesis that higher consumption of either caffeinated or decaffeinated coffee is associated with lower risk of malignant melanoma. We observed statistically significant trends of lower malignant melanoma risk with higher caffeinated coffee intake in the base model (P trend < .001) and in the full model (P trend = .01). The full model risk estimates were slightly attenuated; nevertheless, the inverse association between the highest level of caffeinated coffee intake (≥4 cups/day) and malignant melanoma risk (HR = 0.75, 95% CI = 0.64 to 0.89) remained statistically significant.
Table 2.
Association of daily coffee consumption with malignant melanoma and melanoma in situ in the NIH-AARP Diet and Health Study
| Melanoma type | Model Estimates | Coffee intake | ||||
|---|---|---|---|---|---|---|
| None (Ref.) | ≤1 cup/d | 2–3 cups/d | ≥4 cups/d | P trend | ||
| Malignant melanoma | No. cases/No. noncases | 310/44 264 | 942/139 901 | 1253/186 767 | 399/73 521 | — |
| Incidence rate per 100 000 person-years* | 77.64 | 69.65 | 67.76 | 55.90 | — | |
| Age & sex-adjusted hazard ratio (95% CI) | 1.00 | 0.90 (0.80 to 1.03) | 0.88 (0.78 to 1.00) | 0.72 (0.62 to 0.83) | <.001 | |
| Multivariable (fully)-adjusted hazard ratio (95% CI)† | 1.00 | 0.91 (0.80 to 1.04) | 0.90 (0.80 to 1.28) | 0.80 (0.68 to 0.93) | .01 | |
| Melanoma in situ | No. cases/No. noncases | 171/44 403 | 609/140 023 | 806/187 214 | 288/73 632 | — |
| Incidence rate per 100 000 person-years* | 44.18 | 44.46 | 43.43 | 40.11 | — | |
| Age & sex-adjusted hazard ratio (95% CI) | 1.00 | 1.05 (0.88 to 1.24) | 1.02 (0.86 to 1.20) | 0.94 (0.78 to 1.14) | .19 | |
| Multivariable (fully)-adjusted hazard ratio (95% CI)† | 1.00 | 1.06 (0.90 to 1.26) | 1.08 (0.91 to 1.28) | 1.11 (0.92 to 1.35) | .39 | |
| Caffeinated coffee intake‡ | ||||||
| Malignant melanoma | No. cases/No. noncases | 310/44 264 | 461/69 797 | 862/128 418 | 281/55 573 | — |
| Incidence rate per 100 000 person-years* | 77.64 | 68.14 | 68.08 | 52.43 | — | |
| Age & sex-adjusted hazard ratio (95% CI) | 1.00 | 0.88 (0.77 to 1.02) | 0.88 (0.78 to 1.01) | 0.67 (0.57 to 0.79) | <.001 | |
| Multivariable (fully)-adjusted hazard ratio (95% CI)† | 1.00 | 0.89 (0.77 to 1.03) | 0.91 (0.80 to 1.04) | 0.75 (0.64 to 0.89) | .01 | |
| Melanoma in situ | No. cases/No. noncases | 171/44 403 | 294/69 964 | 548/128 732 | 219/55 635 | — |
| Incidence rate per 100 000 person-years* | 44.18 | 43.20 | 43.31 | 40.68 | — | |
| Age & sex-adjusted hazard ratio (95% CI) | 1.00 | 1.01 (0.84 to 1.22) | 1.01 (0.85 to 1.20) | 0.95 (0.78 to 1.70) | .20 | |
| Multivariable (fully)-adjusted hazard ratio (95% CI)† | 1.00 | 1.04 (0.86 to 1.26) | 1.07 (0.90 to 1.28) | 1.14 (0.93 to 1.40) | .39 | |
| Decaffeinated coffee intake‡ | ||||||
| Malignant melanoma | No. cases/No. noncases | 310/44 264 | 428/62 663 | 359/52 811 | 107/15 697 | — |
| Incidence rate per 100 000 person-years* | 77.64 | 70.53 | 67.91 | 68.65 | — | |
| Age & sex-adjusted hazard ratio (95% CI) | 1.00 | 0.92 (0.79 to 1.06) | 0.88 (0.76 to 1.03) | 0.88 (0.71 to 1.10) | .16 | |
| Multivariable (fully)-adjusted hazard ratio (95% CI)† | 1.00 | 0.92 (0.79 to 1.06) | 0.90 (0.77 to 1.05) | 0.95 (0.76 to 1.18) | .55 | |
| Melanoma in situ | No. cases | 171/44 403 | 289/62 802 | 236/52 934 | 63/15 741 | — |
| Incidence rate per 100 000 person-years* | 44.18 | 46.97 | 43.96 | 39.66 | — | |
| Age & sex-adjusted hazard ratio (95% CI) | 1.00 | 1.11 (0.92 to 1.34) | 1.04 (0.85 to 1.26) | 0.93 (0.70 to 1.25) | .50 | |
| Multivariable (fully)-adjusted hazard ratio (95% CI)† | 1.00 | 1.11 (0.92 to 1.34) | 1.08 (0.89 to 1.32) | 1.06 (0.79 to 1.42) | .69 | |
* Age-standardized incidence rates adjusted for sex. — = not applicable; CI = confidence interval.
† Adjusted for age (continuous), sex, cigarette smoking (never, former [defined by time since quitting: 1–4 years, 5–9 years, ≥10 years] and smoking intensity [1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day] or current/quit less than one year ago [defined by smoking intensity 1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day]), cigar/pipe smoking (ever user or nonuser), body mass index (<25kg/m2, 25 to <30kg/m2 or ≥30kg/m2), education (less than high school, high school graduate, some college, or college graduate), average daily alcohol intake, physical activity (engaged in physical activity never or rarely, 1–3 times/month, 1–2 times per week, 3–4 times per week, or 5 or more times per week), family history of cancer (yes/no), and July erythemal exposure (quartiles).
‡ n = 15 326 individuals were missing information on caffeine type, including 96 malignant and 54 in situ melanoma cases.
The dose-response analyses among coffee drinkers (n = 402 783), suggested that higher (≥4 cups/day) versus lower (≤1 cup/day) total (HR = 0.87, 95% CI = 0.77 to 0.98, P trend = .05) and caffeinated coffee (HR = 0.82, 95% CI = 0.72 to 0.95, P trend = .03) intakes were statistically significantly associated with lower risk of malignant melanoma. Additionally, individuals in the highest compared with the lowest quartile of total caffeine intake had a 10% lower risk of malignant melanoma (HR = 0.90, 95% CI = 0.80 to 0.99, P trend = .03) (data not in table). We observed no statistically significant associations between coffee intake and risk of melanoma in situ (P trend = .39) or between decaffeinated coffee intake and malignant melanoma (P trend = .55).
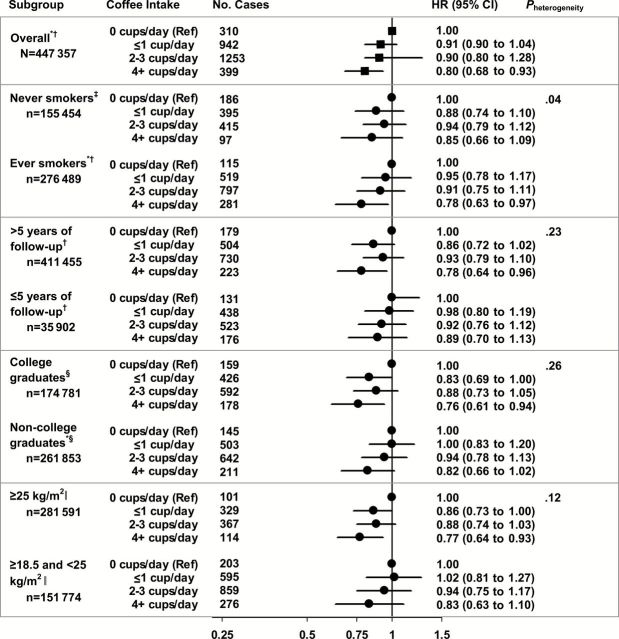
For malignant melanoma, we stratified by cigarette smoking status, follow-up time, education, and BMI (Figure 1). Evidence of a quantitative interaction between smoking status and coffee consumption was found for malignant melanoma (P heterogeneity = .04); a statistically significant inverse trend was observed among ever (P trend = .006) but not never smokers (P trend = .67). A higher percentage of ever smokers reported caffeinated coffee intake (61% vs 50%), and risk estimates comparing those in the highest level of caffeinated coffee intake with nondrinkers were similar for ever smokers (HR = 0.74, 95% CI = 0.59 to 0.94) and never smokers (HR = 0.78, 95% CI = 0.59 to 1.03) (data not in table). Risk estimates did not meaningfully differ across strata of follow-up time, education, or BMI (P heterogeneity > .10); inverse trends for coffee intake and malignant melanoma were generally non–statistically significant, driven by the reduced number of cases and subsequent loss of power in each subgroup.
Figure 1.
Association of daily coffee consumption with malignant melanoma, using one or fewer cups/day as the reference, stratified by smoking status, follow-up time and education level in the National Institutes of Health–AARP Diet and Health Study (n = 447 357). *P trend < .05. All statistical tests were two-sided. † Adjusted for age (continuous), sex, cigarette smoking (never, former [defined by time since quitting: 1–4 years, 5–9 years, ≥10 years] and smoking intensity [1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day] or current/quit less than one year ago [defined by smoking intensity 1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day]), cigar/pipe smoking (ever user or nonuser), body mass index (BMI) (<25kg/m2, 25 to <30kg/m2 or ≥30kg/m2), education (less than high school, high school graduate, some college, or college graduate), average daily alcohol intake, physical activity (engaged in physical activity never or rarely, 1–3 times/month, 1–2 times per week, 3–4 times per week, or 5 or more times per week), family history of cancer (yes/no), and July erythemal ultraviolet radiation (UVR) exposure (quartiles). ‡ Adjusted for age (continuous), cigar/pipe smoking (ever user or nonuser), BMI (<25kg/m2, 25 to <30kg/m2 or ≥30kg/m2), education (less than high school, high school graduate, some college, or college graduate), average daily alcohol intake, physical activity (engaged in physical activity never or rarely, 1–3 times/month, 1–2 times per week, 3–4 times per week, or 5 or more times per week), family history of cancer (yes/no), and July erythemal UVR exposure (quartiles). § Adjusted for age (continuous), sex, cigarette smoking (never, former [defined by time since quitting: 1–4 years, 5–9 years, ≥10 years] and smoking intensity [1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day] or current/quit less than one year ago [defined by smoking intensity 1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day]), cigar/pipe smoking (ever user or nonuser), BMI (<25kg/m2, 25 to <30kg/m2 or ≥30kg/m2), average daily alcohol intake, physical activity (engaged in physical activity never or rarely, 1–3 times/month, 1–2 times per week, 3–4 times per week, or 5 or more times per week), family history of cancer (yes/no), and July erythemal ultraviolet exposure (quartiles). ‖ Adjusted for age (continuous), sex, cigarette smoking (never, former [defined by time since quitting: 1–4 years, 5–9 years, ≥10 years] and smoking intensity [1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day] or current/quit less than one year ago [defined by smoking intensity 1–10 cigarettes/day, 11–20 cigarettes/day, 21–30 cigarettes/day, 31–40 cigarettes/day, 41–60 cigarettes/day, and ≥61 cigarettes/day]), cigar/pipe smoking (ever user or nonuser), BMI (continuous), education (less than high school, high school graduate, some college, or college graduate), average daily alcohol intake, physical activity (engaged in physical activity never or rarely, 1–3 times/month, 1–2 times per week, 3–4 times per week, or 5 or more times per week), family history of cancer (yes/no), and July erythemal UVR exposure (quartiles).
Although our cohort had information on residential UVR exposure, it lacked individual-level information of sun exposure as well as phenotypic and behavioral risk factors for melanoma. Using data from the National Health and Nutrition Examinations Survey (NHANES) (Supplementary Methods, available online), we found no statistically significant associations between phenotypic risk factors (Supplementary Table 2, available online) or behavioral risk factors (Supplementary Table 3, available online) and coffee intake among non-Hispanic white US adults (≥20 years), except for sunscreen use (P = .01). Although the Rao-Scott F adjusted Chi-Square P value was statistically significant (P = .01), there was no clear pattern for sunscreen use and coffee drinking.
Finally, we performed a calibration analysis where we used data from a subset of participants with two 24-hour dietary recalls (n = 1664) to correct daily coffee intake estimates in the entire cohort for measurement error (Supplementary Methods, available online). These measurement error–corrected intakes resulted in malignant melanoma risk estimates for total coffee that were similar in magnitude and direction to those found with uncorrected estimates (≤1 cup/day: HR = 0.97, 95% CI = 0.95 to 1.0; 2–3 cups/day: HR = 0.93, 95% CI = 0.86 to 0.99; ≥4 cups/day: HR = 0.87, 95% CI = 0.76 to 0.99; P trend < .01) (Supplementary Table 4, available online).
Discussion
In this large prospective study, coffee drinking was inversely associated with malignant melanoma. We found that as compared with non–coffee drinkers, those who drank the most coffee (≥4 cups/day) had a 20% lower risk of malignant melanoma but not of melanoma in situ, which may indicate different disease etiologies or an inhibitory role of coffee consumption in disease progression. Statistically significant inverse associations were also found for caffeinated coffee intake and malignant melanoma but not for decaffeinated coffee intake.
While our finding for total coffee intake and malignant melanoma appears robust, there is little consensus among epidemiological studies. With small case numbers, earlier studies had low power for detecting modest associations. In a prospective study of Norwegians, coffee intake was statistically significantly inversely associated with malignant melanoma in women (n = 61 cases) but not in men (n = 47 cases) (12,30). The authors used two or fewer cups per day as the reference group (12), which may have attenuated their risk estimates. Furthermore, a small number of cases in the lowest or highest intake categories reduced statistical power for the trend test. A larger prospective study of caffeine intake and skin cancer using data from the Nurses’ Health Study and the Health Professionals Follow-up Study found no associations between caffeinated or decaffeinated coffee intake or total caffeine intake and malignant melanoma risk but did observe inverse associations of caffeinated coffee and total caffeine with basal cell carcinoma risk. The authors cite lower power because of fewer melanoma cases (n = 403 female cases and n = 334 male cases), compared with basal cell carcinoma cases (n = 14 230 female cases and n = 8556 male cases), as one possible explanation for their findings (9).
Inconsistent results from case-control studies may be explained by differences in control selection or confounder adjustment. A hospital-based case-control study in Italy observed a strong protective effect of high frequency coffee drinking (>1 time/day) compared with low frequency coffee drinking (≤7 times/week) for melanoma (odds ratio [OR] = 0.46, 95% CI = 0.31 to 0.68) after adjusting for age, sex, education, sunburns in childhood, and phenotypic traits associated with increased melanoma risk (eg, hair color) (14); however, a similar study observed no association between high vs low coffee drinking (≥4 cups/day vs <1 cup/day) and risk of melanoma after adjusting for a similar set of covariables plus BMI and smoking (OR = 1.15, 95% CI = 0.68 to 1.92) (16). Advantages of our cohort design, which help to avoid selection and recall bias, include the selection of participants and recording of exposures prior to the onset of melanoma.
Several explanations for our findings are possible. As in all observational studies, associations could reflect inaccurate adjustment because of measurement error in some confounders or unmeasured risk factors that are shared by coffee drinkers and associated with melanoma risk. Known risk factors for melanoma, including individual UVR exposure, nevi, fair skin, freckling, light hair, and a family history of melanoma, were not measured in this cohort. A supplementary NHANES analysis suggested that coffee drinking was not associated with host susceptibility and UVR exposure–related behaviors, with the exception of sunscreen use, for which there was no clear pattern. Given the lack of association between these melanoma risk factors and coffee drinking, it is unlikely that our inability to adjust for these factors explains the observed inverse associations. Still, inability to assess potential confounding by these known risk factors is an important limitation of this study.
We also considered potential confounding by other risk factors. In our cohort, heavy coffee drinkers were more likely than non–coffee drinkers to be current or former smokers. Since studies have suggested that smoking is inversely associated with melanoma risk (12,24), we tested the association between coffee intake and malignant melanoma among never smokers (Figure 1). While we observed possible effect modification by smoking status, malignant melanoma risk estimates were below one for both ever and never smokers. Potential effect modification by smoking could reflect chance, residual confounding by smoking, differences in caffeine intake or an unmeasured confounding factor between never and ever smokers, or a biological interaction between smoking and coffee (eg, antioxidant activity of coffee countering oxidative stress of smoking). Higher socioeconomic status (SES), particularly education, which may be a proxy for increased exposure to sunlight and better access to healthcare, and higher BMI have also been associated with increased risk of melanoma (25–27,29,31). In stratified analyses, we found similar risk estimates, suggesting that confounding by socioeconomic status or BMI is an unlikely explanation for our findings. We also found similar, albeit non–statistically significant, risk estimates for levels of total coffee intake and malignant melanoma among those diagnosed more than five years years and five years or fewer after baseline; we cannot, however, dismiss the possibility that changes in coffee drinking may have occurred during the follow-up period.
Measurement error associated with self-reported coffee intake is another possible explanation, since its effect is unpredictable because of potential residual confounding from other confounders measured with error. To minimize measurement error, we conducted sensitivity analyses in which self-reported coffee intake was calibrated using a subgroup of participants who completed two 24-hour dietary recalls. We found that the magnitude and direction of the association between total coffee intake and malignant melanoma remained, arguing against this possibility.
Finally, the observed association between coffee drinking and malignant melanoma could reflect a true association. Experimental evidence lends biological plausibility to a protective role of coffee in UVB-induced carcinogenesis. Coffee contains numerous bioactive compounds, including polyphenols, diterpenes, trigonelline, and caffeine. The predominant chlorogenic acid in coffee, 5-O-caffeoylquinic acid, and to a greater extent its metabolite caffeic acid, have been shown to suppress UVB-induced skin carcinogenesis in mouse epidermal cells by inhibiting cyclooxygenase (COX-2) expression (3). COX-2, which is overexpressed in response to UVB exposure (32) and in human melanoma cells (33,34) compared with normal melanocytes, is thought to play a functional role in the development and progression of malignant melanoma (33,34). In vitro studies have demonstrated that the diterpenes cafestol and kahweol induce cell apoptosis (4) and protect against oxidative stress and DNA damage (5). In addition, in vivo studies have shown that topical application of diterpenes inhibits inflammation in epidermal cells (6). Coffee roasting generates nicotinic acid (vitamin B3) as well as nicotinamide (an amide form of vitamin B3) from trigonelline (35); a recent study demonstrated that nicotinamide is protective against UVB-induced skin carcinogenesis in mice and UVB-induced immunosuppression in humans and mice (36). Finally, oral and topical caffeine administrations have been shown to inhibit UVB-induced carcinogenesis by absorbing UVR (ie, functioning as a sunscreen) and enhancing UVB-induced apoptosis in mouse epidermal cells (37). Coffee may also exert anticarcinogenic effects through inhibition of DNA methylation (7) and detoxification of carcinogens (38).
In conclusion, we found a modest inverse association between higher coffee intake and melanoma risk in a large US cohort study of older non-Hispanic whites. As the results for malignant melanoma have previously been inconsistent and prior studies have not considered melanoma in situ, our findings, which suggest that consuming four or more cups per day may decrease risk of melanoma by 20%, are preliminary and require replication. Whether our findings are applicable to other populations is unclear. Because of its high disease burden (39), lifestyle modifications with even modest protective effects may have a meaningful impact on melanoma morbidity. Additional investigations of coffee intake and its constituents, particularly caffeine, in the prevention of melanoma are warranted.
Funding
This work was supported in part by the Yale–National Cancer Institute (NCI) predoctoral training grant T32 CA105666 to STM and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Supplementary Material
The study sponsor had no role in the design of the study, data collection, the analysis or interpretation of the data, the writing of the article, nor the decision to submit for publication.
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC; Miami, Florida) under contract with the Florida Department of Health (FDOH), Tallahassee, Florida. The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health, Trenton, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, State Health Division, State of Nevada Department of Health and Human Services, Las Vegas, Nevada.
We are indebted to the participants in the National Institutes of Health–AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis.
References
- 1. Howlader NNA, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975–2010. http://seer.cancer.gov/csr/1975_2010/. Accessed December 2014.
- 2. Markovic SN, Erickson LA, Rao RD, et al. Malignant melanoma in the 21st century, part 2: staging, prognosis, and treatment. Mayo Clin Proc. 2007;82(4):490–513. [DOI] [PubMed] [Google Scholar]
- 3. Kang NJ, Lee KW, Shin BJ, et al. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis. 2009;30(2):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee KA, Chae JI, Shim JH. Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma. J Biomed Sci. 2012;19:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee KJ, Jeong HG. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol Lett. 2007;173(2):80–87. [DOI] [PubMed] [Google Scholar]
- 6. Wei WC, Lin SY, Chen YJ, et al. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J Biomed Sci. 2011;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006;27(2):269–277. [DOI] [PubMed] [Google Scholar]
- 8. Abel EL, Hendrix SO, McNeeley SG, et al. Daily coffee consumption and prevalence of nonmelanoma skin cancer in Caucasian women. Eur J Cancer Prev. 2007;16(5):446–452. [DOI] [PubMed] [Google Scholar]
- 9. Song F, Qureshi AA, Han J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012;72(13):3282–3289. [DOI] [PubMed] [Google Scholar]
- 10. Jacobsen BK, Bjelke E, Kvale G, et al. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst. 1986;76(5):823–831. [PubMed] [Google Scholar]
- 11. Ferrucci LM, Cartmel B, Molinaro AM, et al. Tea, coffee, and caffeine and early-onset basal cell carcinoma in a case-control study. Eur J Cancer Prev. 2014;23(4):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71(4):600–604. [DOI] [PubMed] [Google Scholar]
- 13. Zheng W, Doyle TJ, Kushi LH, et al. Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1996;144(2):175–182. [DOI] [PubMed] [Google Scholar]
- 14. Fortes C, Mastroeni S, Boffetta P, et al. The protective effect of coffee consumption on cutaneous melanoma risk and the role of GSTM1 and GSTT1 polymorphisms. Cancer Causes Control. 2013;24(10):1779–1787. [DOI] [PubMed] [Google Scholar]
- 15. Osterlind A, Tucker MA, Stone BJ, et al. The Danish case-control study of cutaneous malignant melanoma. IV. No association with nutritional factors, alcohol, smoking or hair dyes. Int J Cancer. 1988;42(6):825–828. [DOI] [PubMed] [Google Scholar]
- 16. Naldi L, Gallus S, Tavani A, et al. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13(6):503–508. [DOI] [PubMed] [Google Scholar]
- 17. Green A, Bain C, McLennan R, et al. Risk factors for cutaneous melanoma in Queensland. Recent Results Cancer Res. 1986;102:76–97. [DOI] [PubMed] [Google Scholar]
- 18. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. [DOI] [PubMed] [Google Scholar]
- 19. Dubrow R, Darefsky AS, Freedman ND, et al. Coffee, tea, soda, and caffeine intake in relation to risk of adult glioma in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2012;23(5):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin SW, Wheeler DC, Park Y, et al. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int J Cancer. 2012;131(6):E1015–E1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziemke JR, Chandra S, Herman J, et al. Erythemally weighted UV trends over northern latitudes derived from Nimbus 7 TOMS measurements. J Geophys Research-Atmospheres. 2000;105(D6):7373–7382. [Google Scholar]
- 22. Kalliskota S, Kaurola J, Taalas P, et al. Comparison of daily UV doses estimated from Nimbus 7/TOMS measurements and ground-based spectroradiometric data. J Geophys Research-Atmospheres. 2000;105(D4):5059–5067. [Google Scholar]
- 23. Breslow NE, Day NE. Statistical Methods in Cancer Research, Vol. II-The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 24. Freedman DM, Sigurdson A, Doody MM, et al. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control. 2003;14(9):847–857. [DOI] [PubMed] [Google Scholar]
- 25. Kirkpatrick CS, Lee JA, White E. Melanoma risk by age and socio-economic status. Int J Cancer. 1990;46(1):1–4. [DOI] [PubMed] [Google Scholar]
- 26. MacKie RM, Hole DJ. Incidence and thickness of primary tumours and survival of patients with cutaneous malignant melanoma in relation to socioeconomic status. BMJ. 1996;312(7039):1125–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams RR, Horm JW. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: interview study from the Third National Cancer Survey. J Natl Cancer Inst. 1977;58(3):525–547. [DOI] [PubMed] [Google Scholar]
- 28. Sergentanis TN, Antoniadis AG, Gogas HJ, et al. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer. 2013;49(3):642–657. [DOI] [PubMed] [Google Scholar]
- 29. Shors AR, Solomon C, McTiernan A, et al. Melanoma risk in relation to height, weight, and exercise (United States). Cancer Causes Control. 2001;12(7):599–606. [DOI] [PubMed] [Google Scholar]
- 30. Stensvold I, Jacobsen BK. Coffee and Cancer - a Prospective-Study of 43,000 Norwegian Men and Women. Cancer Causes Control. 1994;5(5):401–408. [DOI] [PubMed] [Google Scholar]
- 31. Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am J Epidemiol. 1994;139(9):869–880. [DOI] [PubMed] [Google Scholar]
- 32. Buckman SY, Gresham A, Hale P, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19(5):723–729. [DOI] [PubMed] [Google Scholar]
- 33. Denkert C, Kobel M, Berger S, et al. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61(1):303–308. [PubMed] [Google Scholar]
- 34. Goulet AC, Einsphar JG, Alberts DS, et al. Analysis of cyclooxygenase 2 (COX-2) expression during malignant melanoma progression. Cancer Biol Ther. 2003;2(6):713–718. [PubMed] [Google Scholar]
- 35. Lang R, Yagar EF, Eggers R, et al. Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis. J Agric Food Chem. 2008;56(23):11114–11121. [DOI] [PubMed] [Google Scholar]
- 36. Surjana D, Halliday GM, Damian DL. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis. 2013;34(5):1144–1149. [DOI] [PubMed] [Google Scholar]
- 37. Conney AH, Lu YP, Lou YR, et al. Mechanisms of Caffeine-Induced Inhibition of UVB Carcinogenesis. Front Oncol. 2013;3:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huber WW, Parzefall W. Modification of N-acetyltransferases and glutathione S-transferases by coffee components: possible relevance for cancer risk. Methods Enzymol. 2005;401:307–341. [DOI] [PubMed] [Google Scholar]
- 39. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.