In this assessment of volatile Aspergillus metabolites in the breath of 64 patients with suspected fungal pneumonia, a secondary metabolite signature of α-trans-bergamotene, β-trans-bergamotene, a β-vatirenene–like sesquiterpene, or trans-geranylacetone identified patients with invasive aspergillosis with 94% sensitivity and 93% specificity.
Keywords: aspergillosis, diagnostics, volatile organic compounds, breath, fungal infection
Abstract
Background. Invasive aspergillosis (IA) remains a leading cause of mortality in immunocompromised patients, in part due to the difficulty of diagnosing this infection.
Methods. Using thermal desorption-gas chromatography/mass spectrometry, we characterized the in vitro volatile metabolite profile of Aspergillus fumigatus, the most common cause of IA, and other pathogenic aspergilli. We prospectively collected breath samples from patients with suspected invasive fungal pneumonia from 2011 to 2013, and assessed whether we could discriminate patients with proven or probable IA from patients without aspergillosis, as determined by European Organization for Research and Treatment of Cancer/Mycoses Study Group consensus definitions, by direct detection of fungal volatile metabolites in these breath samples.
Results. The monoterpenes camphene, α- and β-pinene, and limonene, and the sesquiterpene compounds α- and β-trans-bergamotene were distinctive volatile metabolites of A. fumigatus in vitro, distinguishing it from other pathogenic aspergilli. Of 64 patients with suspected invasive fungal pneumonia based on host risk factors, clinical symptoms, and radiologic findings, 34 were diagnosed with IA, whereas 30 were ultimately diagnosed with other causes of pneumonia, including other invasive mycoses. Detection of α-trans-bergamotene, β-trans-bergamotene, a β-vatirenene–like sesquiterpene, or trans-geranylacetone identified IA patients with 94% sensitivity (95% confidence interval [CI], 81%–98%) and 93% specificity (95% CI, 79%–98%).
Conclusions. In patients with suspected fungal pneumonia, an Aspergillus secondary metabolite signature in breath can identify individuals with IA. These results provide proof-of-concept that direct detection of exogenous fungal metabolites in breath can be used as a novel, noninvasive, pathogen-specific approach to identifying the precise microbial cause of pneumonia.
Invasive aspergillosis (IA) is a major cause of morbidity and mortality in immunocompromised patients, particularly patients with hematologic malignancy [1] or recipients of hematopoietic stem-cell transplant [2, 3] or solid organ transplant [3, 4]. Despite advances in antifungal therapy, IA remains associated with mortality rates of 25%–58% [2, 3, 5, 6], in part due to the difficulty of diagnosing IA in its early stages.
Symptoms of IA are nonspecific [7], and the radiologic findings associated with IA often represent other invasive fungal disease (IFD) such as mucormycosis, or nonfungal processes such as bacterial pneumonia, organizing pneumonitis, or hemorrhage [8]. Respiratory tract cultures, even when obtained by bronchoalveolar lavage (BAL), lack sensitivity, may reflect airway colonization, and, even when informative, usually require a few days of incubation before yielding diagnostic information [9, 10]. Detection of the fungal antigens galactomannan and (1→3)-β-d-glucan in serum and galactomannan in BAL fluid have limited sensitivity and specificity for IA [10–12]. Lack of standardized methods and potential for contamination due to the environmental ubiquity of fungal nucleic acids have hindered clinical implementation of blood and BAL Aspergillus polymerase chain reaction assays [10, 13], which also have suboptimal diagnostic performance for IA [14]. Definitive diagnosis often relies on biopsy, which can be challenging in the debilitated patients at risk for IFD.
Filamentous fungi can produce a vast array of secondary metabolites, many of which are volatile [15, 16]. Aspergillus fumigatus, the most common cause of IA, contains numerous biosynthetic clusters that enable it to synthesize at least 226 secondary metabolite products [17, 18]. Although not required for primary growth, these metabolites, which often have antibiotic, cytotoxic, and phytotoxic properties, likely influence interactions between fungi and their ecological niche [19].
Whereas Aspergillus volatile organic compounds (VOCs) have been characterized under growth conditions that promote sporulation [20–23], with identification of small alcohols, ketones, and furans, little is known about VOC production in vivo in patients with IA. We therefore assessed VOC profiles of pathogenic Aspergillus species in vitro in conditions approximating in vivo growth during IA, then assessed whether we could identify evidence of Aspergillus metabolism in the breath of patients undergoing evaluation for IA.
METHODS
Aspergillus Isolates
We characterized the in vitro VOC profile of reference and clinical strains of A. fumigatus, the most common cause of IA. For comparison, we investigated the in vitro VOC profiles of Aspergillus terreus, Aspergillus flavus, Aspergillus niger, and an emerging species with in vitro resistance to triazole antifungal drugs, Aspergillus calidoustus [24, 25]. Specific isolates are listed in the Supplementary Methods.
Fungal Culture and Headspace Extraction Conditions
We inoculated 104 A. fumigatus conidia into 5 mL of various liquid media in a 20-mL glass vial sealed with an airtight cap incorporating a silicone septum (Restek Corporation, Bellefonte, Pennsylvania), with concurrent media controls. Given the potential for substrate-dependent secondary metabolite production [26], we used a range of liquid media—yeast extract peptone-dextrose (YPD) broth (Teknova, Hollister, California), Aspergillus minimal media [27], and culture conditions that generate A. fumigatus transcriptomes in vitro that overlap with its transcriptome in a murine lung infection model, including iron-limited, nitrogen-depleted, and alkaline stress conditions [18]—in sets of 4 technical replicates for each A. fumigatus isolate. Vials were incubated at 37°C for 96 hours in an orbital shaker at 250 rpm to promote hyphal growth and prevent conidiation. Headspace gas in each vial was adsorbed over 2 minutes per sample onto thermal desorption tubes containing tandem beds of Tenax TA (200 mg), Carbograph 1 TD (100 mg), and Carboxen 1003 (100 mg) (Markes International, Llantrisant, United Kingdom), to retain polar and nonpolar VOCs over a wide range of boiling points.
We assessed whether we could modulate the A. fumigatus VOC metabolome with voriconazole, micafungin, and amphotericin exposure, as detailed in the Supplementary Methods.
Headspace volatile metabolites of A. terreus, A. flavus, A. niger, and A. calidoustus were characterized as outlined above, in YPD broth at 96 hours.
Patients and Study Procedures
Patients at Brigham and Women's Hospital and Dana-Farber Cancer Institute with suspected pulmonary IA, based on host risk factors, clinical symptoms, and radiologic findings suggestive of IFD, were eligible for this breath collection study from November 2011 through September 2013. We were notified of patients with suspected IFD by inpatient and ambulatory oncology, transplant, and immunocompromised host infectious disease care teams. Exclusion criteria were technical inability to provide a tidal breath sample and receipt of mechanical ventilation. Sixty-five of 67 consecutive individuals approached for this study provided written informed consent. One patient developed delirium shortly after providing informed consent and was unable to participate. This study was approved by the Partners Human Research Committee and the Office for Human Research Studies at the Dana-Farber Cancer Institute.
We prospectively collected breath samples using a programmable breath sampler (Gruppo Loccioni, Ancona, Italy) that displays real-time measurements of carbon dioxide and mouth pressure, allowing reproducibility of breathing patterns in each patient. We sampled 4 minutes of tidal breathing, with adsorption of VOCs using an air sampling pump calibrated to 900 mL per minute over the 4-minute period. Breath VOCs were adsorbed onto 2 parallel thermal desorption tubes made to the same specifications as the tubes we used for the in vitro experiments. We sampled breath in each patient's inpatient or ambulatory room and concurrently collected ambient air controls using identical air sampling and thermal desorption parameters, to assess environmental volatiles.
In addition to prospective collection of data on patient demographics and host, clinical, and mycology data required for an assessment of the likelihood of IFD in each patient, we recorded factors that might potentially cause spurious signals in each patient's breath VOC profile, including the time and contents of the last meal prior to breath sampling, tobacco use, and concurrent medication exposure.
Patients were classified as having “proven,” “probable,” or “possible” IFD independently by 2 investigators (S. K. and F. M. M.) blinded to the volatile assessment, according to the revised European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) consensus criteria [28], the current standard for diagnostic classification of patients with IFD. This assessment was performed ≥1 month after initial breath collection. Patients with proven or probable aspergillosis were considered true IA cases for the reference standard, whereas patients with possible IFD or other fungal causes of proven or probable IFD were considered non-IA cases.
Thermal Desorption/Gas Chromatography–Mass Spectrometry
For both in vitro culture headspace extractions and patient breath samples, VOCs were thermally desorbed onto an automated thermal desorption unit (TD-100, Markes International) and gas chromatography (GC) unit interfaced to a single quadrupole mass spectrometry (MS) detector (Agilent 5975, Agilent Technologies, Santa Clara, California), as outlined in the Supplementary Methods.
Spectral Data Analysis and Confirmation of Metabolite Identity
Two investigators (H. R. T. and S. D. D.) blinded to patient IA status analyzed fungal culture and patient breath VOCs, with provisional identification of GC–MS peaks in each sample against the National Institute of Standards and Technology (NIST) 11 Mass Spectral Library (Scientific Instrument Services, Ringoes, New Jersey) and assessment of the integrated area of each peak, a unitless value with a linear relationship to the absolute quantity of each metabolite in each sample. We verified the identity of key metabolites with pure chemical standards or essential oils, as detailed in the Supplementary Methods.
Statistical Analysis
We used a Bayesian approach to the analysis of patient breath data, focusing on distinctive A. fumigatus terpene volatile metabolites identified in vitro and their derivatives. As we hypothesized a priori that these distinctive A. fumigatus VOCs would be entirely absent in individuals without IA, we assessed for the qualitative presence or absence of any of these volatile elements in each individual breath sample. We used the heatmap.2 function in the R gplots package [29] to plot the relative abundance of monoterpene and sesquiterpene metabolites and related compounds in the first breath of each study patient. We used Mann–Whitney and Fisher exact tests to assess the null hypothesis of no difference in clinical covariates between patients with IA and patients without IA and calculated 2-tailed P values. We calculated the sensitivity and specificity of the A. fumigatus VOC metabolite signature for IA with exact binomial 95% confidence intervals (CIs). We calculated positive and negative likelihood ratios and corresponding 95% CIs [30]. We used Stata 11 (StataCorp, College Station, Texas) for these analyses.
RESULTS
In Vitro VOC Profile of A. fumigatus
The monoterpenes camphene, α-pinene, β-pinene, and limonene, and the sesquiterpenes α-trans-bergamotene and β-trans-bergamotene were distinctive and prominent in vitro features of A. fumigatus (Figure 1A; Supplementary Figure 1), consistent in all A. fumigatus biologic replicates. Growth in Aspergillus minimal media or under iron-limited, nitrogen-depleted, or alkaline stress conditions did not induce the production of any additional VOCs. Iron-limited conditions attenuated monoterpene and sesquiterpene production, whereas nitrogen starvation and alkaline stress enhanced β-trans-bergamotene production (Supplementary Figure 2).
Figure 1.
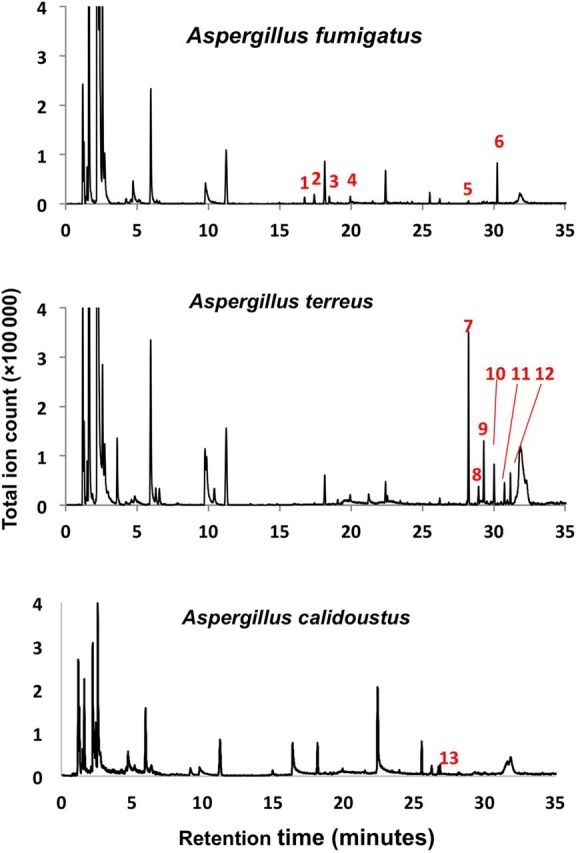
In vitro volatile organic compound profiles of Aspergillus fumigatus, Aspergillus terreus, and Aspergillus calidoustus. Aspergillus species have distinctive volatile organic compound profiles, with particular interspecies heterogeneity in monoterpene and sesquiterpene metabolites. The following peaks were identified as (1) α-pinene; (2) β-pinene; (3) camphene; (4) limonene; (5) α-trans-bergamotene; (6) β-trans-bergamotene; (7) elixene; (8) santalene; (9) elemene; (10) acoradien; (11) 1,5,9-trimethyl-1,5,9-cyclododecatriene; (12) chamigrene; and (13) β-sesquiphellandrene.
Exposure of A. fumigatus hyphae to antifungal drugs modulated VOC production, particularly sesquiterpenes: β-trans-bergamotene increased 10-fold from baseline with 12 hours of micafungin exposure and 3-fold with 12 hours of amphotericin exposure, followed by near-complete attenuation of all volatile metabolites 36 hours later. In vitro voriconazole exposure, in contrast, reduced primary metabolite, monoterpene, and sesquiterpene production at 12 hours, with attenuation of all volatile metabolites 36 hours later (Supplementary Figure 3).
Distinct VOC Profiles in Other Aspergilli
Each Aspergillus species we assessed had a distinct VOC profile, consistent within biological replicates of each species and distinct between species, with particular interspecies heterogeneity in monoterpene and sesquiterpene metabolites (Figure 1). Aspergillus terreus had a particularly rich and abundant sesquiterpene secondary metabolite profile, and A. calidoustus consistently produced β-sesquiphellandrene. Under these culture conditions, A. flavus and A. niger produced alcohols and ketones in abundance, but no volatile secondary metabolites other than limonene (Supplementary Figure 4). Beyond limonene, there was no terpene overlap between A. fumigatus and any of the other Aspergillus species assessed.
An Aspergillus Secondary Metabolite Signature in Invasive Aspergillosis
Of 64 consecutive patients with suspected IFD, comprising a heterogeneous group of patients with hematologic malignancy, allogeneic stem-cell transplant, and solid organ transplant (Table 1), 34 were ultimately diagnosed with IA and 30 with other pneumonia, including other IFD (Table 2). Most patients had received empiric or prophylactic mold-active antifungal therapy for a median of 2 days prior to breath sampling (Table 1). There were no adverse events related to the breath collection procedure; it was well tolerated, even by patients who were dyspneic or requiring supplemental oxygen.
Table 1.
Patient Characteristics
| Clinical Variable | Invasive Aspergillosis (n = 34) |
Other Pneumonia (n = 30) |
P Value |
|---|---|---|---|
| Age, y, median (IQR; range) | 55 (47–62; 22–79) | 54 (44–63; 28–87) | .92 |
| Female sex | 17 (50) | 8 (27) | .07 |
| Hematologic malignancy | 29 (85) | 24 (80) | .74 |
| Allogeneic hematopoietic stem-cell transplant | 18 (53) | 7 (27) | .02 |
| Solid organ transplant | 3 (9) | 5 (17) | .46 |
| Recent neutropeniaa | 13 (38) | 15 (50) | .45 |
| T-cell immunosuppressantsb | 29 (85) | 26 (87) | .58 |
| Prolonged corticosteroid exposurec | 7 (21) | 5 (17) | .45 |
| Exposure to mold-active antifungal therapy on date of breath samplingd | 25 (74) | 26 (87) | .23 |
| Duration of mold-active antifungal exposure prior to breath sampling, d, median (IQR;range) | 2 (2–11; 1–205) | 2 (1–13; 1–345) | .52 |
Data are presented as No. (%) unless otherwise specified.
Abbreviation: IQR, interquartile range.
a Less than 500 neutrophils/µL for >10 days [28].
b Treatment with recognized T-cell immunosuppressants, such as cyclosporine, tumor necrosis factor–α blockers, specific immunomodulating antibodies, or nucleoside analogues during the prior 90 days [28].
c Exposure to corticosteroids at a mean minimum dose of 0.3 mg/kg/day of prednisone equivalent for >3 weeks [28].
d Specific antifungal agents included voriconazole (n = 17), micafungin (n = 15), liposomal amphotericin B (n = 11), terbinafine (n = 1), isavuconazole (n = 1), voriconazole and micafungin (n = 2), voriconazole and terbinafine (n = 2), posaconazole and liposomal amphotericin B (n = 1), and fluconazole (n = 1) in a patient with suspected cryptococcal pneumonia.
Table 2.
Invasive Fungal Disease Classification
| Invasive aspergillosis (n = 34) |
| 5 proven invasive aspergillosisa |
29 probable invasive aspergillosisb
|
| Other pneumonia (n = 30) |
8 proven invasive fungal disease
|
a Aspergillus fumigatus was identified as the cause of pneumonia in all patients with proven invasive aspergillosis.
b One patient had concurrent probable invasive aspergillosis and Pneumocystis jirovecii pneumonia.
c Four A. fumigatus.
d Three A. fumigatus, 1 Aspergillus niger.
e Streptococcus pneumoniae pneumonia (n = 1), Stenotrophomonas maltophilia pneumonia (n = 1), methicillin-resistant Staphylococcus aureus septic pulmonary emboli (n = 1), coagulase-negative Staphylococcus septic pulmonary emboli (n = 1), and Enterococcus faecalis septic pulmonary emboli (n = 1). The specific underlying cause of pneumonia in the remaining 15 patients was not identified.
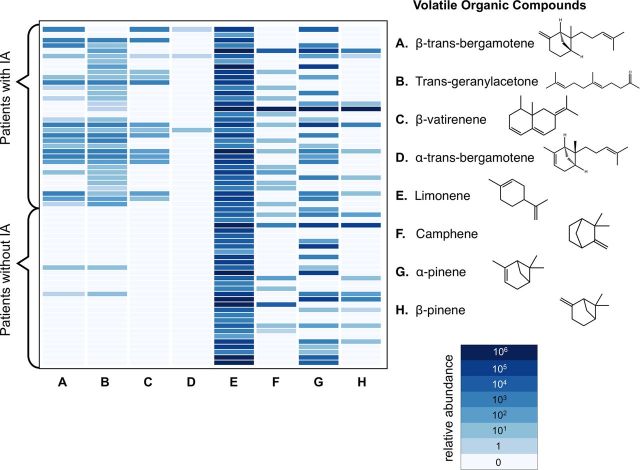
Although monoterpenes produced in vitro by A. fumigatus (camphene, α-pinene, β-pinene, and limonene) were equally present in the breath of patients with or without IA, the presence of any of the elements of a 4-metabolite signature—the volatile sesquiterpenes β-trans-bergamotene and α-trans-bergamotene and 2 closely related metabolites that we did not observe in vitro, the terpenoid ketone trans-geranylacetone and a β-vatirenene–like sesquiterpene—distinguished the breath of patients with IA from patients without IA (Figure 2). There were no sesquiterpenes in any ambient air control samples. The detection of any of the elements of this Aspergillus secondary metabolite signature distinguished patients with IA from patients with other IFD or other pneumonia with 94% (95% CI, 81%–98%) sensitivity and 93% (95% CI, 79%–98%) specificity against the reference standard of proven or probable IA by EORTC/MSG consensus criteria (Table 3).
Figure 2.
Relative abundance of Aspergillus terpene metabolites in breath samples. This heatmap shows the average integrated area of terpene metabolites (columns A–H) in the breath of 64 patients with and without invasive aspergillosis (IA).
Table 3.
Breath Aspergillus Metabolite Signature by the Reference Standard and Test Parameters
| Parameter | Invasive Aspergillosisa | Other Pneumonia | Total Patients |
|---|---|---|---|
| Aspergillus metabolite signatureb + | 32 | 2 | 34 |
| Aspergillus metabolite signature − | 2c | 28 | 30 |
| Total patients | 34 | 30 | 64 |
| Test parameters | |||
| Sensitivity (95% CI) | 0.94 (.81–.98) | ||
| Specificity (95% CI) | 0.93 (.79–.98) | ||
| Positive likelihood ratio (95% CI) | 14.1 (3.69–54.0) | ||
| Negative likelihood ratio (95% CI) | 0.063 (.02–.24) | ||
Abbreviation: CI, confidence interval.
a Proven or probable invasive aspergillosis, according to the revised European Organization for Research and Treatment of Cancer/Mycoses Study Group consensus criteria [28].
b Defined as the presence of any of the 4 elements of a metabolite volatile signature, comprised of β-trans-bergamotene, α-trans-bergamotene, trans-geranylacetone, and a β-vatirenene-like sesquiterpene, in the patient's breath.
c Aspergillus niger was identified as the etiology of invasive aspergillosis in 1 of these 2 patients.
This breath metabolite signature correctly identified all 5 patients with proven IA, all of whom also grew A. fumigatus in culture, 6 of 7 patients (86%) who met mycologic criteria for probable IA by growth of A. fumigatus in their respiratory tract cultures, and all 21 patients who met mycologic criteria for probable IA by serum galactomannan ≥0.5 or BAL galactomannan >1.0. Aspergillus niger, which emits a distinct VOC profile from A. fumigatus in vitro, was the cause of pneumonia in 1 patient with A. niger in multiple respiratory tract cultures and an elevated serum galactomannan. Instead, we detected a novel sesquiterpene in this patient's breath that we did not detect among in vitro cultures of this patient's fungal isolate.
There are limitations of the EORTC/MSG reference standard, which relies on the sensitivity of fungal antigens and cultures for the classification of probable IA [28]. One patient with breath β-trans-bergamotene and trans-geranylacetone was classified as having possible IFD during his lifetime, with pulmonary nodules but unrevealing respiratory tract cultures and negative fungal antigen testing. On autopsy, however, these pulmonary nodules contained invasive septate hyphae, identified as Aspergillus by immunohistochemical staining by the CDC Infectious Diseases Pathology Branch.
We found no association between contents of the meal prior to breath sampling, tobacco use, or concurrently administered inhaled, oral, or topical medications and detection of these secondary metabolites.
Although we did not discern a clear association between relative abundance of Aspergillus breath metabolites and the size or number of lung lesions on chest imaging, the smallest IA lesion detected through breath metabolite analysis was 0.88 cm3.
DISCUSSION
We identified distinctive terpene secondary metabolites in the volatile metabolome of the most common pathogenic Aspergillus species in vitro, and found that β-trans-bergamotene, α-trans-bergamotene, a β-vatirenene–like sesquiterpene, and trans-geranylacetone comprise an Aspergillus metabolic signature that can accurately discriminate patients with IA from patients with other pneumonia, in a heterogeneous population at risk for IA. Our results suggest that direct detection of exogenous fungal metabolites in breath can be used as a novel, noninvasive, species-specific approach to identify patients with IA, potentially allowing more precise targeting of antifungal therapy and fewer invasive diagnostic procedures.
Although microbial VOC detection in the breath has been suggested for the diagnosis of pneumonia, including IA [31], these studies have proposed biomarkers that are either primary metabolites or catabolic products ubiquitous in many species or compounds that lack biologically plausible synthetic pathways [32]. For example, 2-pentylfuran, a linoleic acid breakdown product, was proposed as an IA breath biomarker [23, 33] but has since been shown to be widely present in food products and ambient air [33], and not detected in other series [34] or in any breath samples in our study. Other studies have taken hypothesis-free feature classification approaches without identifying the biologic components distinguishing infected and noninfected patients, increasing the risk of identifying signal in noise and limited reproducibility [31, 35].
In contrast, we took a biologically guided approach to biomarker identification. We identified terpenes released during A. fumigatus growth and modulated these metabolites with various stress conditions and antifungal exposures, suggesting a biologic relationship between the metabolically active organism and production of these compounds. Knowledge of these unique metabolites informed identification of these fungal metabolites and closely related sesquiterpenes in breath.
Others have previously described sesquiterpenes in the in vitro volatome of A. fumigatus [20, 34, 36], and one group identified a sesquiterpene in the breath of 8 IA patients [34], although the dominant metabolite, β-trans-bergamotene, was misidentified as β-farnesene in these studies, given the similarity in fragmentation patterns and the absence of β-trans-bergamotene from the NIST library. In our study, and in an independent assessment of the in vitro A. fumigatus volatome [37], the NIST library initially identified β-trans-bergamotene as β-farnesene, but we observed perfect retention time and spectral alignment between this endogenous sesquiterpene and β-trans-bergamotene. β-trans-bergamotene is putatively a precursor of fumagillin, a secondary meroterpenoid with antibiotic and anti-angiogenic properties [17, 38, 39].
The biologic significance of these sesquiterpenes, whether as end products or as precursors to other secondary metabolites, and their role in fungal pathogenesis are yet undefined. While not required for primary growth of the organism, a substantial diversion of resources from primary metabolism is required to synthesize these metabolites, and many fungi have evolved unique terpene cyclases with distinctive suites of sesquiterpene products of great structural and stereochemical diversity [15, 16, 40]. These products are believed to have roles in inter- and intraspecies communication, deterring competing microorganisms in the environment and potentially contributing to survival of the organism in its hosts [15, 18, 19].
Based on the marked interspecies diversity of sesquiterpene production in vitro, we believe the secondary metabolite signature identified in IA patients in this study is likely specific for A. fumigatus, the dominant cause of IA [12]. Other Aspergillus species likely have their own distinctive volatile secondary metabolite signatures in vivo. With the advent of galactomannan testing, proven IA cases and cases with species-level Aspergillus identification are increasingly rare, but the breath metabolite signature identified all of the galactomannan-positive probable IA cases, suggesting A. fumigatus as the causative species. Aspergillus flavus, A. terreus, or A. calidoustus were not identified as the causal species of any IA cases at our institution during the study period.
These findings provide proof-of-concept that direct detection of exogenous fungal metabolites in breath can be used to identify the underlying microbial etiology of pneumonia; however, this signature needs additional validation and refinement before clinical use. We measured and reported the integrated area of each terpene GC–MS peak to provide a sense of the relative abundance of these metabolites in each breath sample. We did not formally convert these values to absolute quantities because of the potential for drift of our GC–MS over time and the lack of pure chemical standards for some of these metabolites. β-trans-bergamotene and trans-geranylacetone, for which we had pure chemical standards, were highly abundant in each breath sample, at >2 parts per million.
Further research should focus on a more systematic assessment of the relationship between lesion size and the intensity of this A. fumigatus secondary metabolite signature, response of this signature to antifungal therapy in vivo, and characterization of breath VOC profiles of patients who are colonized, rather than infected, with Aspergillus species. From the data collected for this study, we did not discern a clear relationship between nodule size and VOC abundance, although radiologic appearance is often a complex function of the host immune response and hyphal burden. Although we did not systematically collect breath samples during antifungal therapy, it appears from the few patients who we did sample over time (Supplementary Figure 5) that the abundance of the VOC signature declined with effective antifungal therapy, disappearing a few weeks into treatment. We assessed the breath of patients with suspected pulmonary IA in this study, not patients with chronic noninvasive Aspergillus colonization; this will be an important focus of future investigations.
Additional work will also involve identification of distinguishing VOC features of other less common but clinically important fungal species, including other pathogenic Aspergillus species and Mucorales, and accumulation of fungal breath metabolite signatures in patients infected with these species, as the host–pathogen interaction appears to activate secondary metabolite clusters that are silent in vitro.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge Drs Jean-Paul Latgé, Christoph Heddergott, E. Francis Cook, Paul Vouros, and Terence Risby for insightful discussions and advice. We are also thankful to Drs Jean-Paul Latgé, Josep Guarro, Tom Patterson, Benjamin Park, and TRANSNET for providing several of the Aspergillus strains assessed in the in vitro experiments. We are indebted to Drs Hsiao-Ching Lin and Yi Tang for providing the β-trans-bergamotene standard.
Financial support. This work was supported by the Harvard Catalyst Pilot Grant Program (grant number UL1 RR 025758) and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant numbers K23 AI097225 to S. K. and R21 AI085454).
Potential conflicts of interest. Brigham and Women's Hospital and the Charles Stark Draper Laboratory have filed a patent based in part on this work (patent WO2014039856 A1, filed 6 September 2013) listing S. K., P. R., J. C. C., L. R. B., F. M. M., and H. R. T. as inventors. F. M. M. has received research grant support from Astellas and WHISCON; has received consulting honoraria from Astellas, Merck, and WHISCON; and has served as an ad hoc advisory board member for Astellas. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hammond SP, Marty FM, Bryar JM, DeAngelo DJ, Baden LR. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am J Hematol. 2010;85:695–9. doi: 10.1002/ajh.21776. [DOI] [PubMed] [Google Scholar]
- 2.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 5.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–73. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 6.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358–66. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 7.Segal BH. Aspergillosis. N Engl J Med. 2009;360:1870–84. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 8.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–9. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 9.Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;33:1824–33. doi: 10.1086/323900. [DOI] [PubMed] [Google Scholar]
- 10.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 11.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–27. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 12.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. Diagnostic performance of the (1→3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis. 2009;49:1650–9. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]
- 13.Harrison E, Stalhberger T, Whelan R, et al. Aspergillus DNA contamination in blood collection tubes. Diagn Microbiol Infect Dis. 2010;67:392–4. doi: 10.1016/j.diagmicrobio.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mengoli C, Cruciani M, Barnes RA, Loeffler J, Donnelly JP. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:89–96. doi: 10.1016/S1473-3099(09)70019-2. [DOI] [PubMed] [Google Scholar]
- 15.Keller NP, Turner G, Bennett JW. Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol. 2005;3:937–47. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 16.Kramer R, Abraham W-R. Volatile sesquiterpenes from fungi: what are they good for? Phytochem Rev. 2012;11:15–37. [Google Scholar]
- 17.Frisvad JC, Rank C, Nielsen KF, Larsen TO. Metabolomics of Aspergillus fumigatus. Med Mycol. 2009;((suppl 1)):S53–71. doi: 10.1080/13693780802307720. [DOI] [PubMed] [Google Scholar]
- 18.McDonagh A, Fedorova ND, Crabtree J, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154. doi: 10.1371/journal.ppat.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–14. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 20.Fiedler K, Schutz E, Geh S. Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int J Hyg Environ Health. 2001;204:111–21. doi: 10.1078/1438-4639-00094. [DOI] [PubMed] [Google Scholar]
- 21.Perl T, Jünger M, Vautz W, et al. Detection of characteristic metabolites of Aspergillus fumigatus and Candida species using ion mobility spectrometry–metabolic profiling by volatile organic compounds. Mycoses. 2011;54:e828–37. doi: 10.1111/j.1439-0507.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao P, Korley F, Martin J, Chen BT. Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. AIHA J (Fairfax, Va) 2002;63:135–40. doi: 10.1080/15428110208984696. [DOI] [PubMed] [Google Scholar]
- 23.Syhre M, Scotter JM, Chambers ST. Investigation into the production of 2-pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med Mycol. 2008;46:209–15. doi: 10.1080/13693780701753800. [DOI] [PubMed] [Google Scholar]
- 24.Balajee SA, Kano R, Baddley JW, et al. Molecular identification of Aspergillus species collected for the Transplant-Associated Infection Surveillance Network. J Clin Microbiol. 2009;47:3138–41. doi: 10.1128/JCM.01070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol. 2009;47:3271–5. doi: 10.1128/JCM.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brakhage AA. Regulation of fungal secondary metabolism. Nat Rev Microbiol. 2013;11:21–32. doi: 10.1038/nrmicro2916. [DOI] [PubMed] [Google Scholar]
- 27.Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AWJ. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- 28.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warnes G, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T. The Comprehensive R Archive Network; 2009. gplots: various R programming tools for plotting data. Available at: http://cran.r-project.org/package=gplots . Accessed September 2014. [Google Scholar]
- 30.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 31.Sethi S, Nanda R, Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. 2013;26:462–75. doi: 10.1128/CMR.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak J, Preti G. Volatile disease biomarkers in breath: a critique. Curr Pharm Biotechnol. 2011;12:1067–74. doi: 10.2174/138920111795909050. [DOI] [PubMed] [Google Scholar]
- 33.Chambers ST, Bhandari S, Scott-Thomas A, Syhre M. Novel diagnostics: progress toward a breath test for invasive Aspergillus fumigatus. Med Mycol. 2011;49:S54–61. doi: 10.3109/13693786.2010.508187. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Li M, Xu W, et al. Techniques in infectious diseases: identification of unique volatile compounds of Aspergillus fumigatus for potential diagnostic breath test by HS-SPME and GC-MS. J Immunol Tech Infect Dis. 2013;2:3. [Google Scholar]
- 35.Baggerly KA, Morris JS, Edmonson SR, Coombes KR. Signal in noise: evaluating reported reproducibility of serum proteomic tests for ovarian cancer. J Natl Cancer Inst. 2005;97:307–9. doi: 10.1093/jnci/dji008. [DOI] [PubMed] [Google Scholar]
- 36.Bazemore RA, Feng J, Cseke L, Podila GK. Biomedically important pathogenic fungi detection with volatile biomarkers. J Breath Res. 2012;6:016002. doi: 10.1088/1752-7155/6/1/016002. [DOI] [PubMed] [Google Scholar]
- 37.Heddergott C, Calvo AM, Latgé JP. The volatome of Aspergillus fumigatus. Eukaryot Cell. 2014;13:1014–25. doi: 10.1128/EC.00074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozoe S, Kobayashi H, Morisaki N. Isolation of b-trans-bergamotene from Aspergillus fumigatus, a fumagillin producing fungi. Tetrahedron Lett. 1976;50:4625–6. [Google Scholar]
- 39.Lin H-C, Chooi Y-H, Dhingra S, Xu W, Calvo AM, Tang Y. The fumagillin biosynthetic gene cluster in Aspergillus fumigatus encodes a cryptic terpene cyclase involved in the formation of β-trans-bergamotene. J Am Chem Soc. 2013;135:4616–9. doi: 10.1021/ja312503y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christianson DW. Chemistry. Roots of biosynthetic diversity. Science. 2007;316:60–1. doi: 10.1126/science.1141630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.