Abstract
Heat shock protein 70 (HSP70) protects cells from accumulating damaged proteins and age-related functional decline. We studied plasma and skeletal muscle (SkM) HSP70 levels in adult vervet monkeys (life span ≈ 25 years) at baseline and after 4 years (≈10 human years). Insulin, glucose, homeostasis model assessment scores, triglycerides, high-density lipoprotein and total plasma cholesterol, body weight, body mass index, and waist circumference were measured repeatedly, with change over time estimated by individual regression slopes. Low baseline SkM HSP70 was a proximal marker for developing insulin resistance and was seen in monkeys whose insulin and homeostasis model assessment increased more rapidly over time. Changes in SkM HSP70 inversely correlated with insulin and homeostasis model assessment trajectories such that a positive change in SkM level was beneficial. The strength of the relationship between changes in SkM HSP70 and insulin remained unchanged after adjustment for all covariates. Younger monkeys drove these relationships, with HSP70 alone being predictive of insulin changes with aging. Plasma and SkM HSP70 were unrelated and HSP70 release from peripheral blood mononuclear cells was positively associated with insulin concentrations in contrast to SkM. Results from aged humans confirmed this positive association of plasma HSP70 and insulin. In conclusion, higher levels of SkM HSP70 protect against insulin resistance development during healthy aging.
Key Words: Heat shock protein 70, Insulin sensitivity, Aging, Monkey.
The cytoplasmic chaperone, heat shock protein 70 (HSP70), has a fundamental role in maintenance of cellular homeostasis through its chaperone actions (1).One of the hypotheses of cellular aging is the accumulation of oxidation damage or mishandling of cellular components in which the chaperone system is critically important (2). Inadequacy of chaperone capacity can both lead to, and result from, endoplasmic reticular stress that leads to activation of c-jun N-terminal kinases and serine phosphorylation of insulin receptor substrates with reduced efficacy of insulin signaling. As such, the development of insulin resistance with aging has been considered a normal process (3–6).
Genetic modification of both the insulin sensitivity and chaperone protein systems has resulted in improved longevity in rodent and invertebrate research models (7,8). Augmenting HSP70 has been shown to improve insulin sensitivity in mice, monkeys, and people (9–11). Further tissue levels of HSP70 positively associate with life span across a wide range of vertebrate species (12) but understanding how inherent HSP70 levels affect future health span and life span is currently unknown. It is known that HSP70 is a highly heritable trait in monkeys (13) and that similar HSP70 levels are observed in human siblings despite discordant metabolic status (14). Extreme longevity in people is also heritable (15,16) and is consistently observed with preserved insulin sensitivity (17,18). We hypothesized that inherent HSP70 levels in tissues may contribute to the individual’s potential for maintaining insulin sensitivity and thus contribute to their health span and by extension, perhaps life span.
The aim of the current study was to understand whether HSP70 is a proximal risk factor in the development of insulin resistance and type 2 diabetes (T2DM) and how tissue and plasma HSP70 change with aging. This would extend our prior findings that increasing HSP70 can improve insulin sensitivity in monkeys (10) and that HSP70 is reduced in tissues of diabetic and insulin resistant monkeys (13,19). Such prospective studies evaluating insulin resistance development are difficult to achieve in clinical situations with variability in environmental influences, and so an aging nonhuman primate model allows investigations regarding healthy aging on a compressed timescale.
Methods
Participants
Animals.—
Forty-one female African green monkeys (Chlorocebus aethiops; Table 1) ranging in age from 7 to 18 years at study start (maximum life span ≈ 25 years) were sampled and evaluated at multiple time points over a 4-year period, which is the human equivalent of more than 10 years. Animals were housed in large indoor/outdoor enclosures that provide elevated perches and climbing structures within multigenerational social groups and ad libitum opportunities to socialize, eat, and exercise. A commercial laboratory primate chow (Laboratory Diet 5038; LabDiet, St. Louis, MO) with supplemental fresh fruits and vegetables was fed once per day. All animal procedures were performed on a protocol approved by the Wake Forest University Institutional Animal Care and Use Committee according to recommendations in the Guide for Care and Use of Laboratory Animals (Institute for Laboratory Animal Research) and in compliance with the USDA Animal Welfare Act and Animal Welfare Regulations (Animal Welfare Act as Amended; Animal Welfare Regulations).
Table 1.
Mean (±SEM) Baseline Metabolic and Morphometric Characteristics of the Monkeys Observed Over the 4-Year Experimental Period
| Young | Old | p Value ANOVA | |
|---|---|---|---|
| N | 24 | 17 | |
| Age (y) | 8.8 (0.23) | 15 (0.45) | <.001 |
| Body weight (kg) | 5.4 (0.14) | 5.0 (0.13) | .10 |
| BMI (kg/m2) | 26 (0.57) | 26 (0.65) | .54 |
| Waist circumference (cm) | 34 (0.68) | 34 (0.97) | .96 |
| Glucose (mg/dL) | 56 (2.1) | 68 (6.3) | .04 |
| Insulin (U/L) | 13 (4.4) | 18 (5.8) | .46 |
| HOMA (AU) | 2.2 (0.93) | 4.0 (1.7) | .31 |
| TG (mg/dL) | 33 (1.6) | 41 (4.2) | .04 |
| TPC (mg/dL) | 134 (3.3) | 143 (5.5) | .14 |
| HDL-C (mg/dL) | 79 (2.7) | 82 (3.6) | .45 |
| SBP (mmHg) | 121 (3.8) | 125 (4.4) | .53 |
| DBP (mmHg) | 68 (1.8) | 68 (2.4) | .97 |
Notes: ANOVA = analysis of variance; BMI = body mass index; DBP = diastolic blood pressure; HDL-C = high-density lipoprotein cholesterol; HOMA = homeostasis model assessment; SBP = systolic blood pressure; TG = triglyceride; TPC = total plasma cholesterol. Young monkey’s were defined as <12 years old at baseline (range 7–11 years old) and old monkey’s were >12 years old at baseline (range 12–17 years old).
Humans.—
Fifteen elderly human participants (Table 2), eight male and seven female, ranging in age from 56 to 77 years were recruited for blood sampling and glucose tolerance testing. Patients consented to inclusion in the research study and were covered under a protocol approved by the Wake Forest University Institutional Review Board.
Table 2.
Average (±SEM) Clinical Characteristics of the Human Participants Evaluated
| N | 15 |
|---|---|
| Gender (M/F) | 8/7 |
| Age (y) | 66.3 (1.61) |
| BMI (kg/m2) | 27.2 (0.93) |
| Glucose (mg/dL) | 93.7 (2.57) |
| Insulin (U/L) | 6.9 (1.27) |
| HOMA (AU) | 2.00 (0.25) |
| Glucose AUC (mg/dL/h) | 180,841 (1,011) |
| Plasma HSP70 (ng/mL) | 5.12 (0.83) |
Notes: AUC = area under the curve; BMI = body mass index; HOMA = homeostasis model assessment; HSP70 = heat shock protein 70.
Sample Collection and Analysis
In vivo.—
Animals were fasted overnight and anesthetized with intramuscular ketamine (10–15mg/kg) to allow for sample and morphometric data collection. Each animal was weighed and abdominal circumference measured with a flexible tape measure at the level of the umbilicus. Body length was measured from the crown of the head to the caudal aspect of the pubic bone, which is the equivalent to sitting height. A body mass index was calculated by dividing the weight (kg) by the body length (m) squared. Blood pressure (systolic and diastolic blood pressure) was measured indirectly by high definition oscillometry at the tail base. Blood samples were obtained by venipuncture of the femoral vein after an overnight fast and collected into ethylenediaminetetraacetic acid and serum separator blood tubes. The blood was held on ice until it could be processed. After processing the plasma and whole blood, samples were stored at −80°C until analysis. Fasting blood glucose was determined by the glucose oxidase method, and fasting plasma insulin concentration was determined by enzyme-linked immunosorbent assay (ELISA; Mercodia, Uppsala, Sweden) from the plasma sample. Homeostasis model assessment (HOMA) was determined by the product of glucose and insulin divided by 22.5 and used to evaluate insulin resistance (20). Triglyceride, high-density lipoprotein cholesterol, and total plasma cholesterol concentrations were measured enzymatically.
During anesthesia, a surgical skeletal muscle (SkM) biopsy was taken from the vastus lateralis muscle. The isolated section of SkM was excised, placed in a cryovial and snap frozen in liquid nitrogen and stored at −80°C until processing, or used promptly for in vitro studies.
HSP70 protein levels in SkM and plasma were measured at baseline and after 4 years; the metabolic end points measured three times over the 4-year period. Plasma HSP70 was measured by ELISA (Stressmarq Biosciences Inc., Victoria, BC, Canada). SkM tissue was ground in a crucible cooled by liquid nitrogen and the resulting sample homogenized in cell extraction buffer (Invitrogen, Grand Island, NY) by a PT 2100 Polytron electric blender (Kinematica, Littau-Lucerne, Switzerland) with phenylmethanesulfonyl fluoride (Sigma-Aldrich, St. Louis, MO) in dimethyl sulfoxide (Sigma-Aldrich) and a protease inhibitor cocktail (Sigma-Aldrich) solution. The homogenate was centrifuged at for 10 minutes at 15,300g and the supernate collected for analysis. Protein content was determined by bicinchoninic acid reaction (Pierce, Rockford, IL). Equivalent amounts of protein were assayed for HSP70 by ELISA (Stressmarq Biosciences Inc.).
In vitro.—
During the third year of study, peripheral blood mononuclear cells (PBMNCs) were collected from the monkeys for ex vivo assessment of HSP70 responsivity to a standard stressor. PBMNCs were isolated from ethylenediaminetetraacetic acid anticoagulated whole blood by lymphocyte separating media (Mediatech, Herndon, VA) centrifuged at 500g for 30 minutes. The white blood cell layer was suspended in phosphate buffered saline and centrifuged for 15 minutes at 500g. The white cell layer was resuspended in a solution of RPMI 1640 cell culture media (Sigma-Aldrich) to a concentration of 1 million cells per milliliter, as determined by a hemocytometer. Media was supplemented with L-glutamine (Invitrogen) and 40% fetal calf serum. Whole blood also had a white cell differential count performed at a veterinary diagnostic laboratory. Equal volumes of suspended PBMNCs supplemented with 95% oxygen and 5% carbon dioxide were then incubated in water baths held either at 37°C for 4 hours (basal) or 42°C for 1 hour and 37°C for 3 hours (stress). After treatment, the cell suspension was centrifuged for 15 minutes at 500g and media was collected following the incubation for the analysis of HSP70 concentrations (Stressmarq) by ELISA. Media was also evaluated for cell death and necrosis after the incubations by measuring lactate dehydrogenase using a lactate dehydrogenase cytotoxicity assay (Cayman Chemical, Ann Arbor, MI). Results for PBMNCs were corrected for neutrophil percentage of the PBMNCs isolated and for lactate dehydrogenase levels. HSP70 levels were quantified as described above and results are reported as the HSP70 ratio of stress to basal conditions.
Human participants.—
Baseline ethylenediaminetetraacetic acid anticoagulated blood samples from 15 older human participants who underwent standard oral glucose tolerance testing as part of a study of diet intervention were recruited (Table 2) (21). Plasma HSP70 determinations were made by ELISA as previously described from baseline samples, and insulin and glucose measured at 0-, 30-, 50-, and 120-minute time points after dextrose injection. Areas under the curve of a glucose and insulin were calculated using the trapezoidal method.
Data Analysis
Statistical analyses were performed using Statistica v.10 (StatSoft, Tulsa, OK) and p values of <.05 were considered significant. Logarithmic transformation was applied to end points not meeting normality assumptions. Rates of change over time were calculated as regression slopes for insulin, glucose, triglyceride, high-density lipoprotein cholesterol, total plasma cholesterol, body weight, waist circumference, blood pressure, HOMA score, and body mass index. Percent change from baseline HSP70 was calculated for both plasma and SkM. Animals were separated into groups by being below or above the group mean for baseline HSP70 level. Baseline age as a determining factor was investigated by examining differences in monkeys above or below the group mean (mean age 9 [n = 24] vs 15 years [n = 17]). Correlations and partial correlations were assessed by calculating Pearson’s R coefficients. Metabolic and phenotypic variables were analyzed for differences by analysis of variance, and multiple regression modeling to identify predictive variables was performed with backward stepwise removal of model end points.
Results
Nonhuman primates maintained under optimal nutritional environments demonstrate healthy aging (Table 1) with few observable metabolic changes. Only slightly greater than 20% increases in fasting glucose and triglyceride with the transition from mid-age to aged status were seen. It is worth noting that as age increases, that variability around these measures of metabolic health increases, indicating that some individuals age more healthily than others. When individual slope for change over time was estimated by regression, a significant difference was present in monkeys with low or high baseline SkM HSP70 for insulin and HOMA values, but not glucose (Figure 1). In individuals with low SkM HSP70 levels, insulin rates of change were greater than 11-fold more rapid, and drove a more than sevenfold difference in HOMA rates of change over the 10-year human equivalent period, compared with monkeys with higher baseline muscle HSP70 levels (Figure 1, p = .02 for both end points). HSP70 levels in SkM were specifically related to insulin and insulin sensitivity and not other metabolic syndrome components (Table 3). These increases in insulin over time are most apparent in the transition from the younger middle-age monkeys to aged monkey; younger animals demonstrated increased insulin with aging, whereas monkeys who were older at baseline had stable insulin levels (Figure 2; p = .02 for the difference in trajectory of insulin over time). The relationship between changes in insulin and SkM HSP70 was strong within this cohort (r = −.62, p = .001). Diabetic monkeys were not included in study; thus, this difference by age may underestimate insulin changes in the old group where T2DM and hyperinsulinemia have higher prevalence (10).
Figure 1.
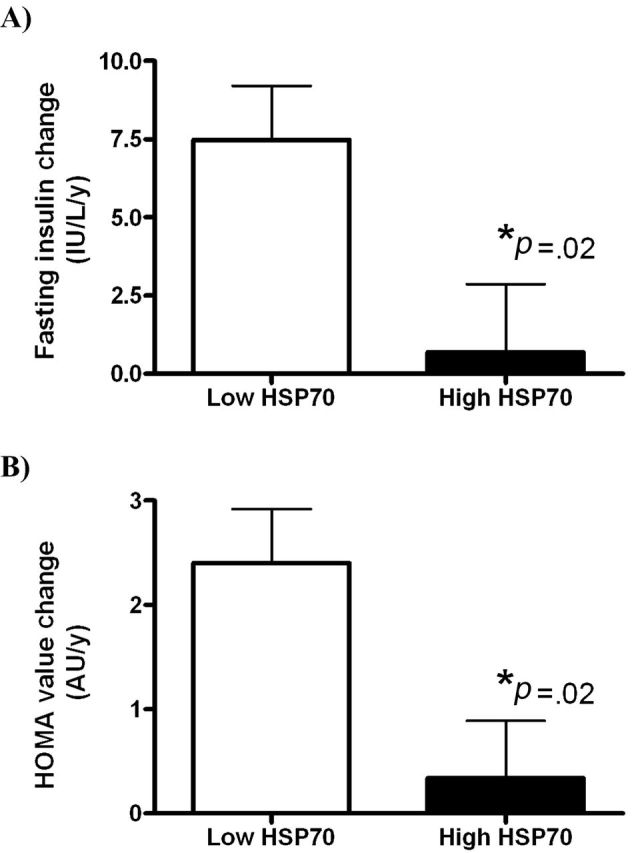
(A) Rates of change in fasted insulin concentrations from monkeys with low and high skeletal muscle (SkM) heat shock protein 70 (HSP70). Elevated SkM HSP70 protects from developing hyperinsulinemia. (B) Rates of change homeostasis model assessment (HOMA) index from monkeys with low and high SkM HSP70. Elevated SkM HSP70 protects from developing insulin resistance.
Table 3.
Average (±SEM) Calculated Rates of Change in Metabolic Syndrome Parameters Measured in Monkeys Based on Baseline SkM Tissue HSP70 Levels
| Rate of Change | Low Baseline SkM HSP70 | High Baseline SkM HSP70 | p Value ANOVA |
|---|---|---|---|
| N | 28 | 13 | |
| Age at baseline (y) | 11.1 (0.62) | 11.5 (0.90) | .73 |
| Body weight (kg/y) | 0.10 (0.042) | 0.081 (0.055) | .78 |
| BMI (kg/m2/y) | 0.78 (0.23) | 0.72 (0.32) | .88 |
| Waist circumference (cm/y) | 0.49 (0.21) | 0.32 (0.34) | .64 |
| Glucose (mg/dL/y) | 13 (1.9) | 10 (1.7) | .37 |
| TG (mg/dL/y) | 9.9 (1.2) | 8.0 (1.2) | .31 |
| TPC (mg/dL/y) | 6.5 (0.87) | 3.2 (2.8) | .15 |
| HDL-C (mg/dL/y) | −6.38 (0.52) | −7.6 (1.1) | .28 |
| SBP (mmHg/y) | −2.8 (3.2) | −7.4 (3.2) | .39 |
| DBP (mmHg/y) | 13 (8.0) | −1.8 (1.4) | .23 |
Notes: ANOVA = analysis of variance; BMI = body mass index; DBP = diastolic blood pressure; HDL-C = high-density lipoprotein cholesterol; HSP70 = heat shock protein 70; SBP = systolic blood pressure; SkM = skeletal muscle; TG = triglyceride; TPC = total plasma cholesterol. High and low baseline SkM HSP70 was defined as being above or below the mean HSP70 measurement.
Figure 2.
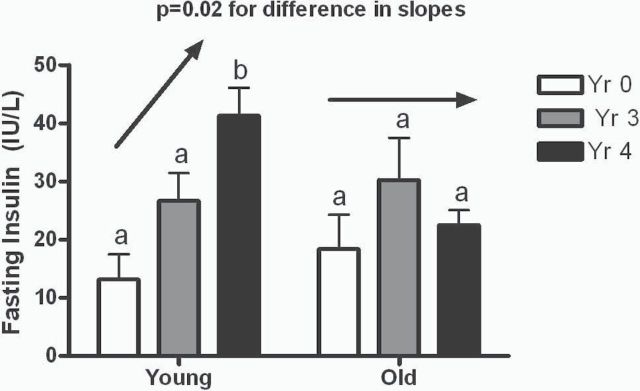
Fasting insulin concentrations in young (7–11 years) and old (12–17 years) monkeys over the course of study. Insulin levels increased significantly in the young animals as they aged but were stable at older life stages. Unlike letters indicate p < .05.
We examined the contributions of metabolic syndrome components to the trajectory of insulin sensitivity by looking at partial correlation coefficients between the change in SkM HSP70 over the 10-year equivalent study when adjusted by these potentially mediating factors (change in age, waist, triglyceride, high-density lipoprotein cholesterol, and systolic blood pressure; Table 4). Importantly, the relationship between SkM HSP70 and insulin remains as highly associated and significant as all potential metabolic covariates were accounted for. HOMA values follow a similar pattern and the specificity of the relationship between HSP70 and insulin is supported by the lack of an association with glucose. In predictive multiple regression models containing all variables, only HSP70 change over the study period predicted rates of insulin increase over time (β = −.42, p = .007) in the younger cohort. No variable predicted insulin change in the old monkeys.
Table 4.
Correlation Coefficients for Change in SkM HSP70 Levels Over the 4-Year Study and Individual Rate of Change in Insulin Sensitivity Biomarkers, With Models Additionally Adjusted for Baseline Metabolic Syndrome-Related Parameters
| Insulin (U/L/y) | HOMA (AU/y) | Glucose (mg/dL/y) | |
|---|---|---|---|
| % Change in SkM HSP70 | R = −.43 | R = −.36 | R = −.25 |
| p = .007 | p = .023 | p = .12 | |
| % Change in SkM HSP70; adjusted for age | R = −.50 | R = −.43 | R = −.26 |
| p = .001 | p = .006 | p = .12 | |
| % Change in SkM HSP70; adjusted for age, waist | R = −.49 | R = −.43 | R = −.27 |
| p = .002 | p = .008 | p = .11 | |
| % Change in SkM HSP70; adjusted for age, waist, TG | R = −.49 | R = −.43 | R = −.26 |
| p = .002 | p = .001 | p = .12 | |
| % Change in SkM HSP70; adjusted for age, waist, TG, HDL-C | R = −.46 | R = −.40 | R = −.28 |
| p = .006 | p = .016 | p = .10 | |
| % Change in SkM HSP70; adjusted for age, waist, TG, HDL-C, SBP | R = −.44 | R = −.37 | R = −.23 |
| p = .02 | p = .05 | p = .23 |
Notes: HDL-C = high-density lipoprotein cholesterol; HOMA = homeostasis model assessment; HSP70 = heat shock protein 70; SBP = systolic blood pressure; SkM = skeletal muscle; TG = triglyceride.
The relationship between plasma and SkM tissue levels of HSP70 was examined (Table 5). We saw that plasma HSP70 increased over time; however, SkM levels increased nonsignificantly and did not correlate with plasma levels at any time point. We conclude that plasma HSP70 cannot be used as a surrogate for tissue levels. In contrast to high or increasing SkM HSP70 levels being related to smaller insulin changes, changes in plasma HSP70 had no relationship with the rate of change in insulin (R = −.16, p = .40) or HOMA (R = −.10, p = .62). HSP70 release from PBMNCs with a standard heat stressor showed a strong positive relationship with fasting insulin (R = .66, p < .001; Figure 3A) such that higher plasma HSP70 suggests less insulin sensitivity. This relationship persisted even after removal of insulin values greater than 100 µIU/mL. This positive association with greater extracellular HSP70 and lower insulin sensitivity was substantiated by a significant positive relationship between lower insulin sensitivity (as reflected by higher insulin area under the curve during oral glucose tolerance testing) and plasma HSP70 in older humans (Figure 3B; R = .50, p = .03). Plasma HSP70 does not relate to tissue levels, and it has an opposing relationship with insulin sensitivity.
Table 5.
Levels of Tissue HSP70 in SkM and Extracellular Plasma HSP70 Measured From Monkeys at the Beginning of Study and After 4 Years (>10 Years Human Equivalent)
| Baseline | Study End | ANOVA p Value | |
|---|---|---|---|
| SkM HSP70 (ng HSP70/mg tissue) | 0.32 (0.05) | 0.50 (0.10) | .11 |
| Plasma HSP70 (ng/mL) | 4.74 (0.69) | 7.3 (0.88) | .02 |
| Pearson’s R | .13 | −.19 | |
| p value | .43 | .25 |
Notes: ANOVA = analysis of variance; HSP70 = heat shock protein 70; SkM = skeletal muscle. The significance of differences related to time is shown by the p value in the right column, and the relationship at between measures at each point in time is shown by correlational analyses in the rows at the bottom of the table.
Figure 3.
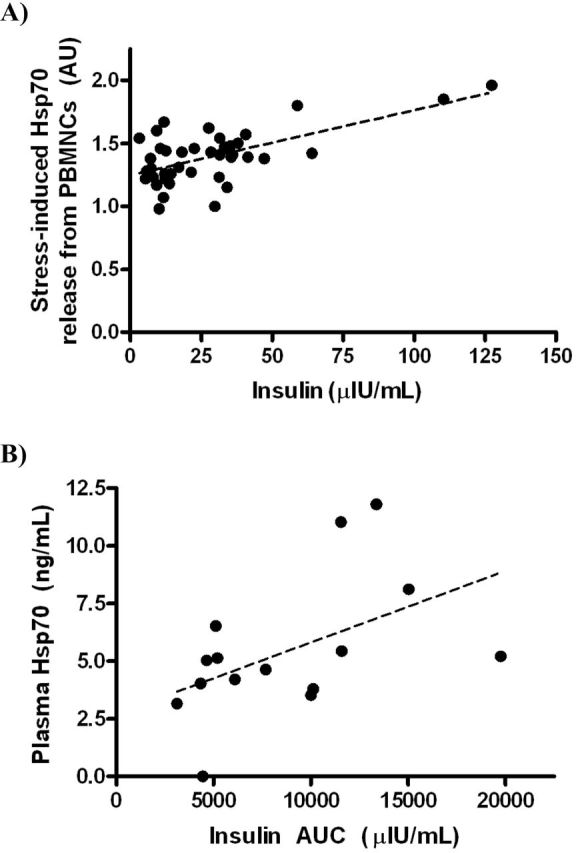
(A) Association between heat shock protein 70 (HSP70) release from peripheral blood mononuclear cells (PBMNCs) with heat stress and fasting plasma insulin concentrations in 40 monkeys (R = .66, p < .001). (B) Association between plasma levels of HSP70 and insulin area under the curve (AUC) during glucose tolerance testing in 15 aged human participants (R = .50, p = .03).
Discussion
This is the first study to the author’s knowledge that evaluates the related changes in tissue levels of HSP70 and with insulin sensitivity in healthy aging. This study is also the first to compare tissue and plasma levels within the same cohort over a significant period of time. We find that in SkM of aging monkeys, high baseline levels and increasing levels of HSP70 over time were associated with better insulin sensitivity measures. Additionally, we found that plasma (extracellular) HSP70 cannot be used as a biomarker for tissue levels and in direct contrast to tissue, higher levels signal worsened insulin sensitivity. This study validates that low muscle HSPs in youth may be a proximal event to the development of insulin resistance later in life. Our findings support the potential protective effects of new therapies and interventions purported to improve insulin sensitivity through modification of HSP70 (9,10,22) and thus may improve health span.
Insulin sensitivity, and its role in longevity, has been studied in a number of animal model systems. Caloric restriction in nonhuman primates improves life span and notably results in the reduction of incident diabetes with aging (23,24). This finding is recapitulated in centenarian human populations, which have exceedingly low prevalence of diabetes (17,18), and points to the importance of insulin resistance as a driver of age-related comorbidities. The importance of insulin sensitivity in longevity is seen both in centenarians and their offspring (17,25). The human aging phenotype includes increased adipose tissue, decreased lean body mass, insulin resistance, and cardiovascular disease. Insulin sensitivity as a central aging mechanism is supported by the longest lived mouse models, which have high insulin sensitivity through genetic modification of growth hormone biology (26). The decrease in insulin sensitivity with age in people is associated with increased morbidity and mortality (7) and has been correlated to decreased cytoplasmic HSP70 (27). Correspondingly, cytoplasmic HSP70 levels have been examined across many different species, and higher levels confer longer maximum life spans (7,12). For example, in invertebrates, overexpression of HSP70 confers a more than 40% extension in life span (28). Modulating cytoplasmic HSP70 to improve insulin sensitivity appears to be a possible therapeutic modality and our current study supports early intervention to shift the metabolic trajectory of an individual.
Studies in humans with insulin resistance demonstrate a 75%–90% lower intracellular HSP70 gene expression in SkM. In nonhuman primates, heat shock factor 1 and HSP70 are reduced by half with hyperglycemia (10,19). Monozygotic twins, with differing glucose tolerance, have low SkM HSP70 present in both individuals, which supports HSP70 as being involved in the familial risk known for metabolic disease (14,27,29). Our prior nonhuman primate studies indicate that both chronic high glucose and insulin resistance impair tissue HSP levels (10,13), and higher glucose and insulin concentrations are both common phenotypes seen with aging. Taken together with prior studies, the present results suggest that low HSP70 may also be a proximal event that permits future development of insulin resistance. Additionally, interventional studies (9,10,30) support the premise that modulation of HSP70 potentially can be both primary and secondary prevention therapeutic strategy for managing metabolic health. In both human and nonhuman primates, SkM HSP70 has been found to be highly heritable (13,31), which means populations at risk for unhealthy aging are identifiable early in life. The heritability of this “healthy” aging phenotype is supported by cohort studies of people (17,18).
Our data demonstrate that changes in HSP70 had a unique relationship to insulin concentrations, which was independent of other metabolic parameters such as glucose or plasma lipids. As differential insulin changes were related to HSP70 levels observed at baseline, we believe that HSP70 influences tissue insulin sensitivity, rather than insulin depressing HSP70. Interplay between these factors has been appreciated in invertebrate models and drive longevity phenotypes (32–34). From a clinical perspective, caloric restriction replicates this kind of interaction where metabolism and HSPs changes are coordinated by sirtuins (35). In Caenorhabditis elegans, the transcription factor that regulates HSPs, heat shock factor, is required for daf-2 mutants to express their longevity phenotype. These mutants have reduced insulin-like growth factor receptor function and double the expected life span (33). Increasing the expression of heat shock factor 1 in this model promotes longevity, in part through reduced insulin signaling and through increased resistance to environmental stressors (34). Transcription of HSPs is required for insulin signaling pathway mutants such as daf-2 and its mediator, daf-16 (Forkhead box O family transcription factor) to be long lived (33,34). Thus, simple and mutated organisms support our finding that HSP levels are very important for maintaining improved insulin signaling with aging. The heat shock chaperone system has been proposed more recently to have negative feedback regulation from insulin signaling (32), which allows a vicious cycle whereby low HSPs permit hyperinsulinemia development which then further reduces the transcriptional efficiency of heat shock factor 1. This modulation of HSPs was through actions of genes known to mediate longevity in the daf-16 mutant. HSP induction is generally believed to be impaired with aging and insulin resistance (1,35); however, it is often difficult to separate these processes. From our prior and current studies of nonhuman primates (13), we find that high HSP70 confers insulin sensitivity despite aging and that metabolic derangement must be chronic before suppressing HSP70 levels (10,13). Thus, the inhibitory effect of insulin resistance is observed in this relevant animal model; however, it does not appear to be driving the primary relationship between HSP70 and insulin sensitivity.
HSP70’s divergent role based on cellular location has given rise to the apt term “chaperokine” to describe its role as a chaperone in the cytoplasm versus a proinflammatory cytokine in the extracellular space (36). HSP70 has two modes of release into the extracellular space: passively during cell necrosis and actively by PBMNCs during stress (37). Extracellular HSP70 activates CD14 and the Toll-like receptor family members (36,38) and is considered a “danger” signal inciting inflammation. As a cytokine, HSP70 produces a pronounced proinflammatory response in mononuclear cells stimulating release of interleukin-1β, interleukin-6, and tumor necrosis factor-α in vitro (38). In vivo, extracellular HSP70 causes obese rat macrophages to have exaggerated release of interleukin-6; however, exercise-induced HSP70 release into the circulation shifts the cytokine response of obese animals toward that of lean animals (22,39,40). This dysregulation of cytokine production seen in obesity by PBMNCs, particularly the elevated interleukin-6 release, has been associated with elevated PBMNC HSP70 in unhealthy aging (40). Several clinical studies have found elevated plasma HSP70 with prolonged T2DM (27,41) and conversely, a strong association between low plasma HSP70 and healthy aging has been reported (31,42). The greater release of HSP70 with insulin resistance could be an early compensatory mechanism to stimulate tissue macrophages that have deficient activity in the diabetic state (43). Some investigators have sought to use circulatory HSP70 levels as a biomarker for T2DM because inflammation is an important component of this disease (41,44). However, we have seen depressed circulatory HSP70 with T2DM (19), and controversy exists as to whether extracellular HSP70 increases or decreases with aging (45,46). We found that plasma HSP70 concentrations rose with aging but were not predictive of a metabolic phenotype. Further, we see active secretion of HSP70 from circulatory leukocytes that intensified relative to the level of hyperinsulinemia. This mechanism could support a vicious cycle whereby insulin resistant individuals continue to have increased inflammatory stimulus with augmented extracellular HSP70 signaling. We have confidence in this biological signal between insulin and extracellular HSP70 as a strong association was seen with insulin sensitivity in an aged human cohort, and this further verifies the use of nonhuman primates as a model of healthy aging.
The use of the nonhuman primate model has proven robust in biogerontologic studies of neurologic function, skeletal changes, reproduction, cardiovascular function, and diabetes (47,48). Although the rodent model has traditionally been favored due to cost and time, the nonhuman primate model affords phylogenetic proximity, a similar life course, known diet, well-controlled environment, and physiologic similarity at the expense of using an outbred population. In our study, the nonhuman primate provides an opportunity to examine basic mechanisms of aging with invasive and noninvasive sampling within a condensed time span (relative to humans), which may not have been possible in humans. The primary limitations of this study include the relatively small, single-sex cohort, although previous studies have shown that vervet monkeys do not exhibit sex differences in cytoplasmic HSP70 (13). Menstrual phase during sampling was not known. HOMA scores may vary up to 18% on average over the menstrual cycle in women (49). This variance is relatively small compared with the annualized rate differences seen in monkeys with low and high SkM HSP70 (>1,000% difference). Female vervets enter menopause only in their terminal years, and so insulin sensitivity changes coincident with the menopausal change were unlikely (50). We acknowledge that a healthy participant selection bias may exist in the older monkeys, as diabetic and disabled monkeys are removed from the breeding colony. We feel this was a strength of the study design, as diabetes is known to further suppress HSP70 levels in tissues (10,13,19), and so we can be confident that our associations with insulin across time were not driven by the development of these diabetic phenotypes.
In conclusion, we were able to show prospectively that elevated SkM HSP70 is protective against insulin resistance and these protective effects were most prominent in younger animals. Plasma HSP70 levels were not associated with a metabolic phenotype, but did rise over the significant duration of the study period, and release by PBMNCs was increased in hyperinsulinemic states. Similar relationships between HSP70 and insulin levels were seen in human patients. Based on patterns of observed insulin changes, interventions to increase tissue HSP70 and optimize health span may be more effective if introduced before becoming geriatric. This study shows that SkM HSP70 levels play an important role determining insulin sensitivity preservation with healthy aging.
Funding
This work was supported by the National Institutes of Health (K01 AG-033641 to K.K. and R37 AG-10880 to S.C.) and the Roena B. Kulynych Center for Cognition and Memory Research of Wake Forest School of Medicine.
References
- 1. Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging–a mini-review. Gerontology. 2009;55:550–558. 10.1159/000225957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linton S, Davies MJ, Dean RT. Protein oxidation and ageing. Exp Gerontol. 2001;36:1503–1518. S0531-5565(01)00136-X [pii] [DOI] [PubMed] [Google Scholar]
- 3. Einstein FH, Huffman DM, Fishman S, et al. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J Gerontol A Biol Sci Med Sci. 2010;65:800–808. 10.1093/gerona/glq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes. 2004;53:441–446. [DOI] [PubMed] [Google Scholar]
- 5. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. [DOI] [PubMed] [Google Scholar]
- 6. Tigno XT, Gerzanich G, Hansen BC. Age-related changes in metabolic parameters of nonhuman primates. J Gerontol A Biol Sci Med Sci. 2004;59:1081–1088. 59/11/1081 [pii] [DOI] [PubMed] [Google Scholar]
- 7. Rincon M, Rudin E, Barzilai N. The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp Gerontol. 2005;40:873–877. 10.1016/j.exger.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 8. Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. [DOI] [PubMed] [Google Scholar]
- 9. Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. 10.1073/pnas.0705799105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–E901. 10.1152/ajpendo.00699.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Literáti-Nagy Z, Tory K, Literáti-Nagy B, et al. The HSP co-inducer BGP-15 can prevent the metabolic side effects of the atypical antipsychotics. Cell Stress Chaperones. 2012;17:517–521. 10.1007/s12192-012-0327-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salway KD, Gallagher EJ, Page MM, Stuart JA. Higher levels of heat shock proteins in longer-lived mammals and birds. Mech Ageing Dev. 2011;132:287–297. 10.1016/j.mad.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 13. Kavanagh K, Wylie AT, Chavanne TJ, et al. Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol A Biol Sci Med Sci. 2012;67:1014–1021. 10.1093/gerona/gls008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurucz I, Morva A, Vaag A, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. [DOI] [PubMed] [Google Scholar]
- 15. Kerber RA, O’Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. J Gerontol A Biol Sci Med Sci. 2001;56:B130–B139. [DOI] [PubMed] [Google Scholar]
- 16. Sebastiani P, Solovieff N, Dewan AT, et al. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7:e29848. 10.1371/journal.pone.0029848PONE-D-11-23542 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. 10.1111/j.1532-5415.2004.52068.x [DOI] [PubMed] [Google Scholar]
- 18. Collino S, Montoliu I, Martin FP, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8:e56564. 10.1371/journal.pone.0056564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14:291–299. 10.1007/s12192-008-0084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. [DOI] [PubMed] [Google Scholar]
- 21. Bayer-Carter JL, Green PS, Montine TJ, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743–752. 10.1001/archneurol.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skidmore R, Gutierrez JA, Guerriero V, Jr, Kregel KC. HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol. 1995;268(1 Pt 2):R92–R97. [DOI] [PubMed] [Google Scholar]
- 23. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. 10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. 10.1038/nature11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adams ER, Nolan VG, Andersen SL, Perls TT, Terry DF. Centenarian offspring: start healthier and stay healthier. J Am Geriatr Soc. 2008;56:2089–2092. 10.1111/j.1532-5415.2008.01949.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. 10.1093/gerona/gls086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodrigues-Krause J, Krause M, O’Hagan C, et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. 10.1007/s12192-011-0319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokoyama K, Fukumoto K, Murakami T, et al. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516:53–57. S0014579302024705 [pii] [DOI] [PubMed] [Google Scholar]
- 29. Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. [DOI] [PubMed] [Google Scholar]
- 30. Heydari AR, You S, Takahashi R, Gutsmann A, Sarge KD, Richardson A. Effect of caloric restriction on the expression of heat shock protein 70 and the activation of heat shock transcription factor 1. Dev Genet. 1996;18:114–124. 10.1002/(SICI)1520-6408(1996)182<114AID-DVG4>3.0.CO;2-C[pii] [DOI] [PubMed] [Google Scholar]
- 31. Terry DF, McCormick M, Andersen S, et al. Cardiovascular disease delay in centenarian offspring: role of heat shock proteins. Ann N Y Acad Sci. 2004;1019:502–505. 10.1196/annals.1297.0921019/1/502 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–334. 10.1016/j.cell.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. 10.1126/science.1083701300/5622/1142 [pii] [DOI] [PubMed] [Google Scholar]
- 34. Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saunders LR, Verdin E. Cell biology. Stress response and aging. Science. 2009;323:1021–1022. 10.1126/science.1170007 [DOI] [PubMed] [Google Scholar]
- 36. Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. 10.1074/jbc.M200497200 [DOI] [PubMed] [Google Scholar]
- 37. Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. [DOI] [PubMed] [Google Scholar]
- 38. Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 39. Garcia JJ, Martin-Cordero L, Hinchado MD, Bote ME, Ortega E. Effects of habitual exercise on the eHsp72-induced release of inflammatory cytokines by macrophages from obese Zucker rats. Int J Sports Med. 2013;34:559–564. 10.1055/s-0032-1327650 [DOI] [PubMed] [Google Scholar]
- 40. Njemini R, Bautmans I, Onyema OO, Van Puyvelde K, Demanet C, Mets T. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol. 2011;12:24. 10.1186/1471-2172-12-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakhjavani M, Morteza A, Khajeali L, et al. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15:959–964. 10.1007/s12192-010-0204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Terry DF, Wyszynski DF, Nolan VG, et al. Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mech Ageing Dev. 2006;127:862–868. 10.1016/j.mad.2006.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vega VL, Charles W, Crotty Alexander LE, Alexander LE. Rescuing of deficient killing and phagocytic activities of macrophages derived from non-obese diabetic mice by treatment with geldanamycin or heat shock: potential clinical implications. Cell Stress Chaperones. 2011;16:573–581. 10.1007/s12192-011-0268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. 10.1089/ars.2007.1595 [DOI] [PubMed] [Google Scholar]
- 45. Njemini R, Lambert M, Demanet C, Kooijman R, Mets T. Basal and infection-induced levels of heat shock proteins in human aging. Biogerontology. 2007;8:353–364. 10.1007/s10522-006-9078-y [DOI] [PubMed] [Google Scholar]
- 46. Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. S0531-5565(00)00215-1 [pii] [DOI] [PubMed] [Google Scholar]
- 47. Lane MA. Nonhuman primate models in biogerontology. Exp Gerontol. 2000;35:533–541. S0531-5565(00)00102-9 [pii] [DOI] [PubMed] [Google Scholar]
- 48. Nadon NL. Of mice and monkeys: National Institute on Aging resources supporting the use of animal models in biogerontology research. J Gerontol A Biol Sci Med Sci. 2006;61:813–815. 61/8/813[pii] [DOI] [PubMed] [Google Scholar]
- 49. Yeung EH, Zhang C, Mumford SL, et al. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95:5435–5442. 10.1210/jc.2010-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod. 2008;79:398–406. 10.1095/biolreprod.108.068536 [DOI] [PMC free article] [PubMed] [Google Scholar]


