Abstract
Aging is associated with alterations in glucose metabolism and sarcopenia that jointly contribute to a higher risk of developing type 2 diabetes. Because aging is considered as a state of low-grade inflammation, in this study we examined whether older, healthy (lean, community-dwelling) participants have altered signaling flux through toll-like receptor 4 (TLR4), a key mediator of innate and adaptive immune responses. We also examined whether a 4-month aerobic exercise program would have an anti-inflammatory effect by reducing TLR4 expression and signaling. At baseline, muscle TLR4, nuclear factor κB p50 and nuclear factor κB p65 protein content, and c-Jun N-terminal kinase phosphorylation were significantly elevated in older versus young participants. The plasma concentration of the TLR4 agonist lipopolysaccharide and its binding protein also were significantly elevated in older participants, indicative of metabolic endotoxemia, which is a recently described phenomenon of increased plasma endotoxin level in metabolic disease. These alterations in older participants were accompanied by decreased insulin sensitivity, quadriceps muscle volume, and muscle strength. The exercise training program increased insulin sensitivity, without affecting quadriceps muscle volume or strength. Muscle TLR4, nuclear factor κB, and c-Jun N-terminal kinase, and plasma lipopolysaccharide and lipopolysaccharide binding protein were not changed by exercise. In conclusion, insulin resistance and sarcopenia of aging are associated with increased TLR4 expression/signaling, which may be secondary to metabolic endotoxemia.
Key Words: Aging, LPS, TLR4, NFκB, JNK, Sarcopenia
Insulin resistance is associated with a clustering of metabolic disorders including type 2 diabetes mellitus (T2DM), sarcopenia, obesity, hypertension, and atherosclerotic vascular disease. Aging is associated with low-grade systemic inflammation that may contribute to insulin resistance (1–3). Skeletal muscle is an important target for the prevention and management of metabolic disorders because it is the predominant site of glucose uptake in the postprandial state (4). However, as people age, there is a significant decline in muscle mass. Thus, developing strategies that prevent/reverse insulin resistance and sarcopenia in older participants would have a major impact on their health.
There is growing recognition that insulin resistance in muscle is, in part, a result of an inflammatory response mediated via toll-like receptor 4 (TLR4) (5,6). TLR4 is a member of the TLR family of pattern recognition receptors that generate innate immune responses to pathogens by activating a cascade of proinflammatory events. Different ligands of TLR4 have been described, including lipopolysaccharide (LPS) (7,8) and saturated free fatty acids (FFA) (9). Following ligand binding, TLR4 and its coreceptors, CD14 and MD2, interact with adaptor proteins (Myd88) that facilitate downstream signaling through the IκB kinase–nuclear factor κB (NFκB) complex and the mitogen-activated kinase (MAPK) pathways. The activation of IκB kinase, MAPKs, extracellular signal-regulated kinase (ERK) (10), c-Jun N-terminal kinase (JNK) (11–13), and p38 (14) impair insulin receptor signaling.
Studies in rodents (15) and humans (16–18) have demonstrated an association between advancing age and elevated NFκB activity/content in muscle. Increased JNK phosphorylation/activity also has been reported in aged rodents and human participants (19–21). Even though these inflammatory pathways have been associated with the loss of skeletal muscle mass and function in frail elderly individuals (22), it is not clear whether increased signaling through the TLR4–NFκB–MAPK network contributes to aging-related skeletal muscle insulin resistance in otherwise healthy older individuals.
Aerobic exercise is an effective intervention for the prevention and treatment of insulin resistance in older individuals (23). Yet, the mechanisms by which physical activity improves glucose metabolism are not fully understood. Regular physical activity induces adaptive changes in skeletal muscle through alterations in metabolic gene expression. Such changes include increases in mitochondria number and function, changes of muscle fiber type distribution, and increased muscle mass, which untimely helps to decrease insulin resistance and T2DM risk (23–26). Accumulating evidence suggests that regular exercise may provide additional health benefits against insulin resistance, acting as an anti-inflammatory therapy (27). Of particular importance, exercise training reduces signaling through the NFκB signaling pathway in obese rodents (28) and humans with T2DM (29,30). However, it is not known whether exercise training inhibits the TLR4–NFκB–MAPK network in healthy older participants and whether this is associated with improvements in glucose metabolism.
The goal of this study was to evaluate whether increased signaling through the TLR4–NFκB–MAPK network occurs in association with impaired insulin signaling/sensitivity in muscle of older individuals. We also examined whether an exercise training program designed to enhance insulin sensitivity could reverse abnormalities in the TLR4–NFκB–MAPK pathway in older participants. We hypothesized that muscle of older individuals would have increased signaling through TLR4–NFκB–MAPK, and that reduced signaling through this inflammatory network is a mechanism by which exercise training improves insulin sensitivity in these participants.
Methods
Participants
Thirteen young (6 men/7 women) and 12 older (8 men/4 women) healthy, lean, nonsmoking, community-dwelling participants, without a family history of diabetes mellitus (first-degree relatives), were studied. Each participant underwent a medical history, physical examination, screening laboratory tests, and a 75 g oral glucose tolerance test. Participants were sedentary (≤1 exercise session per week) and their body weight was stable (±1kg) for 3 months or more prior to enrollment. The study was approved by the Institutional Review Board of The University of Texas Health Science Center at San Antonio, and all participants gave written voluntary consent.
Oral Glucose Tolerance Test
After an overnight fast, participants ingested a 75g glucose solution. Plasma glucose, insulin, and FFA concentrations were measured at baseline and every 15 minutes for 2 hours. The HOMA-IR and Matsuda indexes were calculated as described previously (31,32). The glucose and FFA area under the curves were calculated using the trapezoidal rule.
Dual X-Ray Absorptiometry
Dual x-ray absorptiometry (Hologic, Bedford, MA) was used to measure fat and fat-free mass in young and older participants.
Magnetic Resonance Imaging
The volume of the quadriceps muscle was measured with a 3T magnetic resonance imaging scanner (Siemens TIM Trio, Siemens Medical, Erlangen, Germany) in the leg of young and older (pre- and postexercise) participants. Leg muscle volume measurements were acquired by body phased array and spine phased array coils with flash sequences. A series of continuous muscle measurements along the thigh (45 slices with 5.5-mm slice thickness and 1.56 × 1.56mm in plane resolution) were made. All magnetic resonance imaging data were analyzed using Mango software (http://ric.uthscsa.edu/mango/).
Muscle Biopsy and Insulin Clamp
After an overnight fast, participants underwent a vastus lateralis muscle biopsy using a modified Bersgtrom technique (30). The muscle was debrided of adipose and connective tissue and immediately (within 5–7 seconds after the biopsy) frozen in liquid nitrogen and stored at −80°C. Thirty minutes after the muscle biopsy, a 120-minute euglycemic, hyperinsulinemic (40 mU/m2.min) insulin clamp was started. At the end of the clamp, a second muscle biopsy was performed in the contralateral leg. Insulin-stimulated glucose metabolism (M) was determined based on the average glucose infusion rate and the plasma glucose concentrations during the last 30 minutes of the clamp (33) and adjusted to plasma insulin concentrations (M/I).
Exercise Training Protocol
Within 1 week after the first muscle biopsy, 11 older participants started a 16-week aerobic exercise training program (34) on a stationary bicycle under supervision. For the first 4 weeks, participants exercised for three sessions per week at 65% VO2max for 20min/session. During weeks 5–8, the intensity, duration, and number of sessions were gradually increased so that during weeks 9–16 participants exercised four sessions per week for 45min/session at 80% VO2max. VO2peak was tested every 4 weeks to adjust exercise intensity. Participants maintained their usual dietary intake throughout the 16-week training period and were asked to increase their caloric intake to avoid weight loss, if needed. After an overnight fast, and 48–72h after the last exercise session, a second biopsy and insulin clamp were performed.
Laboratory Analyses
Plasma insulin concentration was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA), plasma glucose was measured using the glucose oxidase method (Beckman glucose analyzer, Fullerton, CA), and hemoglobin A1c was measured using a DCA 2000 analyzer (Bayer Corporation, Tarrytown, NY). Plasma FFA concentration was determined by an enzymatic colorimetric method (Wako Chemicals, Nuess, Germany). Plasma interleukin-6 and tumor necrosis factor-α concentrations were measured using an enzyme-linked immunosorbent assay(R&D Systems Inc., Minneapolis, MN). Plasma LPS concentration was determined using a Limulus Amoebocyte Lysate assay kit (Lonza, Walkersville, MD). Plasma lipopolysaccharide binding protein (LBP) concentration was measured using an enzyme-linked immunosorbent assay kit from Cell Sciences (Canton, MA). All materials were endotoxin-free.
Exercise Testing
VO2max was determined using a cycle ergometer and a Metabolic Measurement System (Sensormedics, Savi Park, CA) as described (35,36). Briefly, participants warmed-up and performed exercise in a ramped fashion increasing at a rate of 8–10W/min to exhaustion and until at least two of the following criteria for a valid test were obtained: a leveling of VO2, respiratory exchange ratio >1.1, and a maximal heart rate within 15 beats of age-predicted maximal heart rate.
Muscle Strength Assessment
Peak force generation was assessed with a manual muscle testing system (Model 01163, Lafayette Instruments, IN) (37). Peak force for knee extension was tested at a knee angle of 25°. Participants were positioned sitting upright, with no back support and with the hips in 90° flexion. The patient stabilized the trunk by grasping the table. The thigh of the patient was stabilized by the examiner’s hand. The test was performed using the “make technique” where the participants perform a maximal isometric contraction, whereas the examiner holds the dynamometer in a fixed position. The patient was encouraged by means of standardized, verbal instructions during the tests. After technique familiarization, participants performed four consecutive repetitions, and the rest interval between the test repetitions was approximately 30 seconds. The three highest peak force values were averaged. All measurements were performed by the same examiner.
Quantitative Reverse Transcription–PCR
Total RNA was isolated from muscle tissue using TRIZOL reagent (Sigma, St. Louis, MO). One-step quantitative reverse transcription–PCR was carried on an ABI-Prism 7900HT System (Applied Biosystems, Foster City, CA). Each sample was run in duplicate and the messenger RNA expression of the genes of interest was normalized to that of 18S ribosomal RNA, using the following assay-on-demand primers/probes, TLR4: Hs00152939_m1 and 18S: Hs99999901_s1.
Western Blot Analysis
Western blotting was performed as described previously (24). Primary antibodies against the following proteins were used: TLR4 and NFκB p65 (Santa Cruz Biotechnology, Santa Cruz, CA); NFκB p50, JNK, phosphor-JNK, ERK, phosphor-ERK, p38, phosphor-p38, AKT, phosphor-Akt, AS160, phosphor-Akt substrate, GSK3α/β, and phosphor- GSK3α/β (all from Cell Signaling Technology, Beverly, MA); phosphor IRS1 (pTyr612; Sigma–Aldrich Corp, St. Louis, MO); and IRS1 (Invitrogen, Grand Island, NY). Equal sample loading was confirmed by Ponceau red staining. Bands were quantitated with ImageQuant software (Sunnyvale, CA).
Statistical Analysis
All data are expressed as means ± SE. Differences between groups were analyzed using unpaired t test. The effects of exercise within each group were analyzed using paired t test. Pearson correlation was utilized to determine relationship between variables. Differences with p < .05 were considered statistically significant.
Results
Participants’ Characteristics
Body mass index was similar in young and older participants (Table 1). Older participants had elevated fasting plasma glucose, blood hemoglobin A1c, and plasma interleukin-6 concentrations. Consistent with a very sedentary lifestyle, both groups had poor cardiorespiratory fitness levels, and the VO2max was significantly reduced in older participants by approximately 40%. Older participants were more insulin resistant, as evidenced by a reduced Matsuda index (56%; Figure 1A), M/I (31%; Figure 1B), and elevated HOMA-IR (46%; Table 1; p < .05 for all variables). The FFA area under the curve during the oral glucose tolerance test tended to be elevated in older participants (24%, p = .05; Table 1). Quadriceps muscle volume and muscle strength were reduced in older participants by 20% (p < .01; Figure 1D) and 16%, respectively (Figure 1F).
Table 1.
Baseline Characteristics
| Young NGT (n = 13) | Older NGT (n = 12) | p Value | |
|---|---|---|---|
| Age (y) | 25.5±1.0 | 73.8±2.1 | .00006 |
| Gender (male/female) | 6/7 | 8/4 | .32 |
| Body mass index (kg/m2) | 23.5±0.7 | 24.1±1.0 | .60 |
| OGTT fasting glucose (mg/dL) | 77.6±3.6 | 88.4±2.0 | .01 |
| OGTT fasting insulin (mU/mL) | 4.4±0.7 | 6.9±1.1 | .06 |
| OGTT fasting free fatty acids (mmol/L) | 0.41±0.05 | 0.53±0.05 | .40 |
| OGTT free fatty acids AUC (μmol/L/(120min)) | 28.2±2.17 | 35.2±2.6 | .05 |
| HbA1C (%) | 5.0±0.1 | 5.5±0.1 | .0005 |
| Plasma interleukin-6 (pg/mL) | 1.24±0.2 | 2.1±0.2 | .03 |
| Plasma tumor necrosis factor-α (pg/mL) | 1.14±0.2 | 1.7±0.3 | .20 |
| HOMA-IR | 0.97±0.2 | 1.80±0.3 | .02 |
| VO2 max (mL/kg.min) | 27.6±2.4 | 16.5±0.7 | .003 |
| Fat mass % | 24.0±2.5 | 28.1±1.9 | .22 |
| Fat-free mass % | 71.9±2.5 | 68.6±1.8 | .29 |
Note: AUC = area under the curve; NGT= normal glucose tolerant; OGTT = oral glucose tolerance test.
Figure 1.
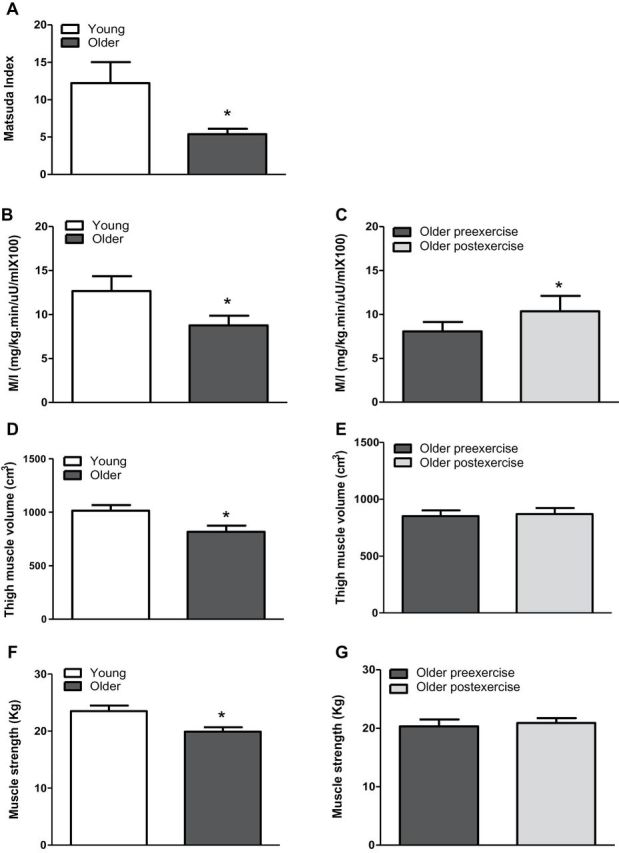
Metabolic characteristics and quadriceps muscle volume. Matsuda index was calculated at baseline in young versus older participants (A), and M/I (B) was calculated in young and older participants and (C) in older participants before and after exercise. Quadriceps muscle volume was measured at baseline in (D) young versus older participants and (E) in older participants before and after exercise. Muscle strength was measured at baseline in (F) young versus older participants and (G) in older participants before and after exercise. Data are means ± SE; n = 11–13 per group. *p < .05.
Metabolic Effects of Exercise
Older participants underwent a supervised aerobic exercise training program. Exercise increased VO2 max by 16% (p < .05) and insulin sensitivity (M/I) by approcimately 21% (p < .05; Figure 1C). Body mass index, fat-free mass, fat mass, quadriceps muscle volume, muscle strength, hemoglobin A1c, plasma concentrations of glucose, FFA, tumor necrosis factor-α, and interleukin-6 were unaffected by aerobic exercise.
Inflammatory Signaling in Muscle
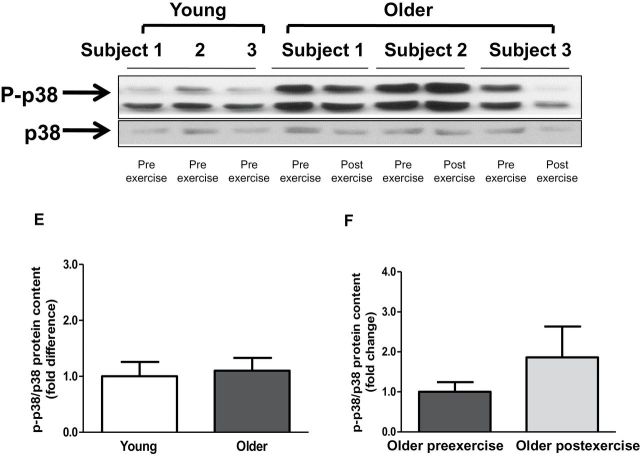
Baseline TLR4 protein content and gene expression were significantly elevated in older participants by 2.1- and 3.8-fold, respectively (Figure 2A and C). Exercise training had no effect on TLR4 protein content and gene expression (Figure 2B and D). Compared with young participants, older participants had increased NFκB p65 (1.6-fold; p < .05) and NFκB p50 (1.5-fold, p < .05) protein content (Figure 3A and C). Exercise training had no effect on NFκB p65 or NFκB p50 protein (Figure 3B and D). Compared with young participants, JNK phosphorylation was significantly elevated in older participants (2.4-fold; p < .05; Figure 4A). In contrast, the phosphorylation of ERK and p38 was not different between the two groups (Figure 4C and E). Exercise training had no effect on JNK, ERK, or p38 phosphorylation (Figure 4B, D, and F).
Figure 2.
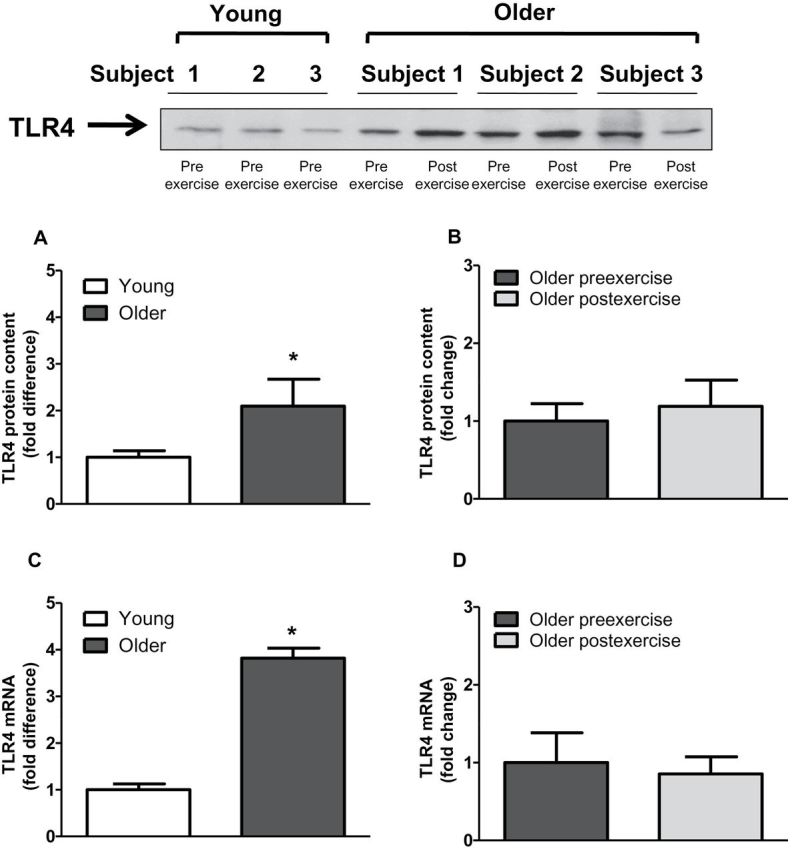
Toll-like receptor 4 (TLR4) protein content and gene expression in human skeletal muscle. TLR4 protein content and messenger RNA levels was measured at baseline (A and C) in young versus older participants and (B and D) in older participants before and after exercise. Data are means ± SE; n = 9–13 per group. *p < .05 versus young group.
Figure 3.
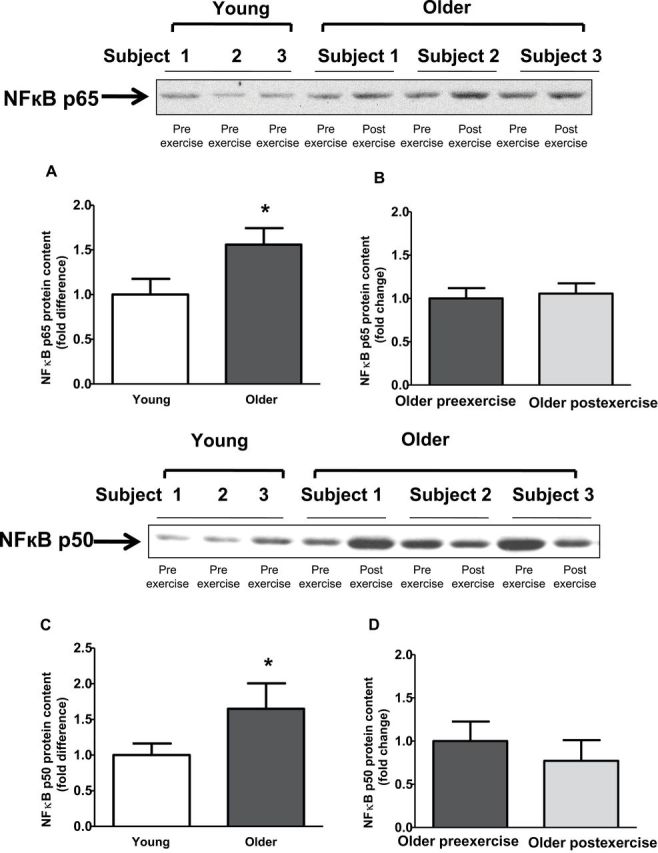
Protein content of nuclear factor κB (NFκB) p50 and NFκB p65 in human skeletal muscle. NFκB p65 (A and B) and NFκB p50 (C and D) protein content was measured at baseline in (A and C) young versus older participants and (B and D) in older participants before and after exercise. Data are means ± SE; n = 11–13 per group. *p < .05 versus young group.
Figure 4.
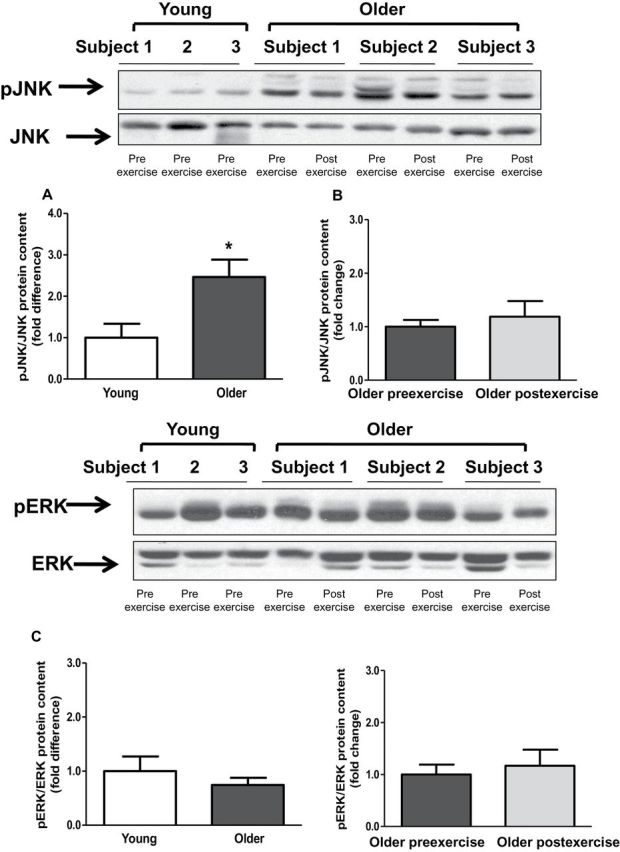
Phosphorylation levels of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38. Phosphorylation of JNK (A and B), ERK (C and D), p38 (E and F) was measured at baseline in (A, C, and E) young versus older participants and (B, D, and F) in older participants before and after exercise. Phosphorylation of JNK, ERK and p38 was normalized to total JNK, ERK and p38 content, respectively. Data are means ± SE; n = 11–13 per group. *p < .05 versus young group.
Plasma LPS and LBP
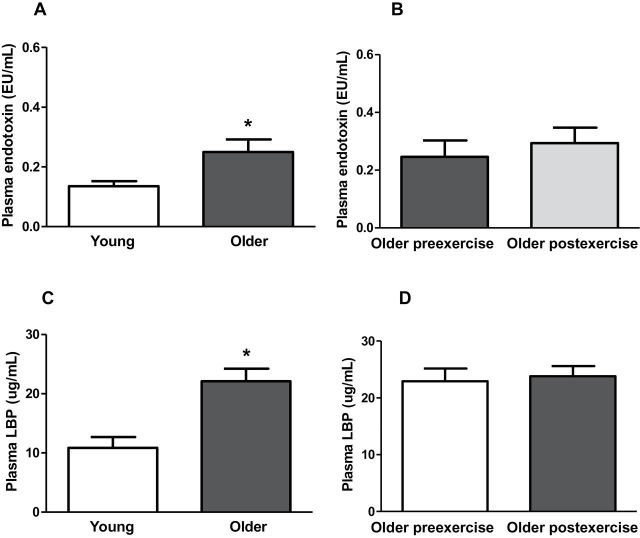
To identify factors that could be responsible for the proinflammatory state present in older participants, we measured plasma LPS and LBP concentrations in both young and older participants. Older participants had higher LPS (1.8-fold; p < .05) and LBP (1.9-fold; p < .05; Figure 5A and C) plasma level, and these were not affected by exercise (Figure 5B and D). Both plasma LPS (r = 0.470, p = .03) and LBP (r = 0.637, p = .003) concentrations positively correlated with age. A positive correlation between blood HbA1C concentration and LPS (r = 0.616, p = .01) and LBP (r = 0.562, p = .01) also was observed.
Figure 5.
Plasma endotoxin and plasma lipopolysaccharide binding protein levels. Plasma endotoxin (A and B) and lipopolysaccharide binding protein (C and D) content was measured at baseline in (A and C) young versus older participants and (B and D) in older participants before and after exercise. Data are means ± SE; n = 11–13 per group. *p < .05 versus young group.
Insulin Signaling
We measured insulin-stimulated IRS1, Akt, GSK3, and AS160 phosphorylation to determine whether upregulation of the TLR4 signaling pathway is associated with impaired insulin action. The phosphorylation state of IRS1 pTyr612, GSK3, and AS160 was similar between young and older participants (Figure 6). In contrast, Akt phosphorylation was approximately 50% lower in muscle of older individuals (p < .05; Figure 6C). Exercise training had no effect on the phosphorylation state of IRS1, AKT, GSK3, or AS160 (Figure 6B, D, F, and H).
Figure 6.
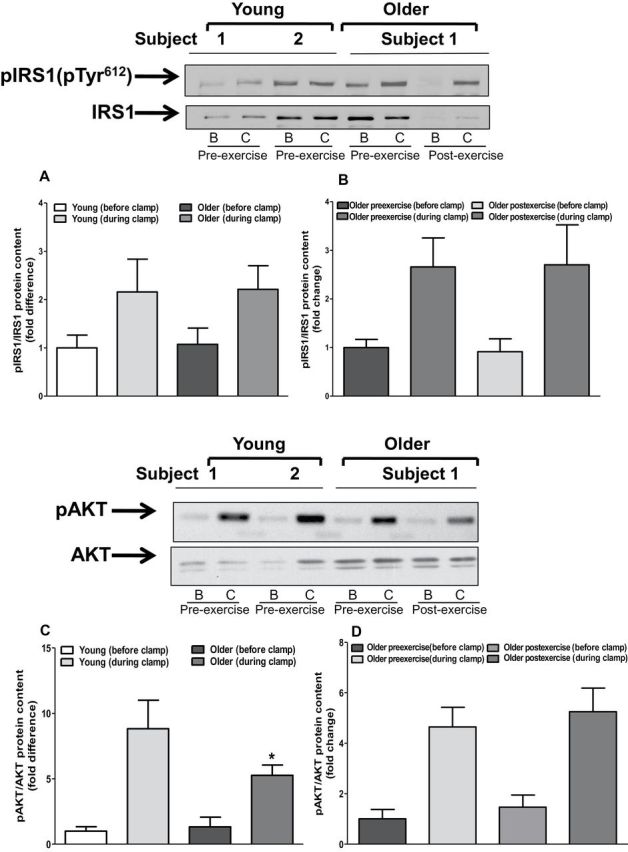
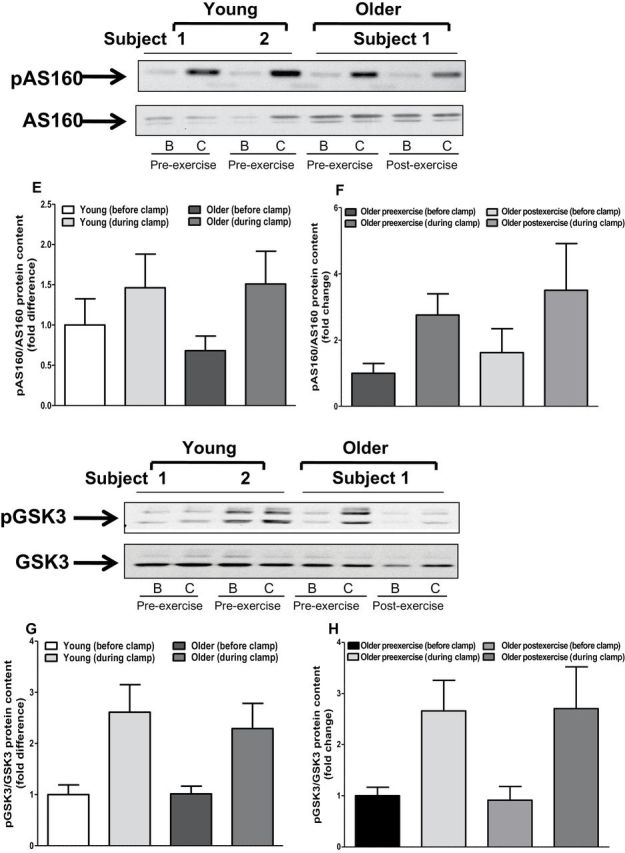
Protein content of insulin signaling intermediates. Muscle biopsies obtained from both young and older participants (before and after exercise) before and during clamp were lysed. Phosphorylation of IRS1-tyrosine (A and B), AKT (C and D), AS160 (E and F), and GSK3 (G and H), respectively, was measured at baseline in (A, C, D, and G) young versus older participants and (B, D, F, and H) in older participants before and after exercise. Phosphorylation of IRS1-tyrosine, AKT, AS160, and GSK3 was normalized to total IRS1, AKT, AS160, and GSK3 content, respectively. Data are means ± SE; n = 11–13 per group. *p < .05 versus young group. Note: B = before clamp; C = during clamp.
Discussion
Chronic systemic inflammation is a common feature in the normal aging process and also is involved in the pathogenesis of several age-related diseases (38). Prior studies have reported increased inflammatory signaling through NFκB (18,39) and MAPKs (19) in muscle of older individuals. This phenomenon has been described in the context of muscle mass loss (40) and weakness in frail elderly individuals (41,42). Here, we were able to examine whether there is an association between TLR4-mediated inflammation and aging per se (in the absence of frailty) because participants were free of chronic age-related diseases and physical disabilities. We demonstrate that human aging is associated with increased TLR4 expression and downstream (NFκB and MAPK) signaling.
Here we demonstrate that human aging is associated with an increase in circulating plasma LPS concentration. In line with this finding, the acute phase reactant LBP was elevated in the older group. LPS induces hepatic LBP synthesis, and serum LBP level is a reliable integrated measure of serum LPS concentration (43,44). These data suggest that increased circulating LPS could play a causal role in the proinflammatory state present in older individuals.
Our laboratory (8) and others (45,46) have demonstrated elevated plasma LPS concentration in middle-aged, obese nondiabetic, and T2DM participants. This phenomenon of increased circulating LPS concentration in metabolic disease was termed “metabolic endotoxemia” by Cani and coworkers (7). The basis for the elevation in circulating LPS in obese nondiabetic and T2DM participants is not clear; hypothesized mechanisms include (i) changes in the composition of the microbiome, which favor LPS production (47); (ii) LPS transfer through chylomicrons (47,48); and (iii) lipid- and/or microbial-induced damage of the intestinal epithelial barrier (ie, increased permeability). Studies in rodents (49) and humans (50,51) have shown alterations in intestinal microbiota composition with age. Age-associated remodeling of the intestinal epithelial barrier has also been demonstrated in nonhuman primates (52). These findings are consistent with the metabolic endotoxemia phenomenon observed in this study. Future research will be needed to test whether human aging is associated with alterations in intestinal microbiome composition and intestinal barrier integrity/function.
There is evidence suggesting that NFκB may play a role in the pathophysiology of several age-related diseases (38). In old rodents, increased NFκB abundance and activity have been shown in numerous tissues including skeletal muscle, liver, kidney, cardiac muscle, and gastric mucosa (17,38,53–55). In older human participants, elevated protein abundance of NFκB has been reported in vascular endothelial cells (56) and skeletal muscle (15,16). In line with these findings, NFκB p65 protein was elevated in skeletal muscle of older participants studied here. It is noteworthy that a recent study reported similar NFκB p65 abundance in older and young human participants (57). The reason for these discrepant study outcomes is not clear but may be explained by differences in participant cohorts (ie, fitness level and mean age of the older and young groups) and different timing of the muscle biopsies. Also, we were unable to measure NFκB p50-p65 DNA binding because, for several participants, there was not sufficient tissue to perform this assay. It will be important to measure NFκB p50-p50 DNA binding in future studies with larger populations.
Similar to NFκB, activation of MAPKs has been associated with aging-related inflammation in several tissues from different species (58,59). Few studies have investigated the independent effect of age on MAPK activation in human muscle. Williamson and coworkers (19) demonstrated increased phosphorylation of JNK, p38, and ERK in muscle of frail, elderly individuals. In this study, JNK phosphorylation was increased in the older participants. This suggests that increased JNK phosphorylation may be an early event in the aging process contributing to reduced insulin sensitivity and muscle mass/volume in otherwise healthy older individuals.
In this study, elevated TLR4 expression and signaling was associated with reduced insulin signaling and peripheral (muscle) glucose disposal in older participants. Previously, we demonstrated increased TLR4 gene expression and signaling in muscle of middle-aged individuals with obesity and T2DM (6,29). Elevation in JNK phosphorylation also has been shown in insulin resistance participants (12–14,60). Therefore, flux through TLR4 might play a role in the alterations in glucose metabolism that occur as people age. In vitro studies have shown that phosphorylation of IκB kinase (61) and JNK (13,62) promotes insulin resistance by increasing serine phosphorylation of IRS1. Serine phosphorylation on IRS1 impairs its phosphorylation on tyrosine residues and downstream activation of Akt (63,64). In this study, increased TLR4 expression and signaling were associated with impaired insulin-stimulated Akt phosphorylation. Notably, IRS1 phosphorylation on tyrosine 612 was not affected in older participants, suggesting that the TLR4–NFκB–MAPK signaling network may act on a different IRS1 phosphorylation site or downstream of IRS1 to reduce insulin signaling. It is also possible that, under the experimental conditions studied, subtle alterations in IRS1 function may not be detected. Dissociations in the activation of proximal and more distal steps within the insulin pathway have been noted previously (65).
Elevated plasma LPS concentration was observed in older participants, which presumably is the cause of the increases in TLR4 expression and signaling. However, there are other factors, not directly evaluated in this study that can also stimulate TLR4 and downstream signaling pathways. For example, lipids can activate NFκB and JNK (6,66), and older participants have increased levels of intramyocellular lipids (67,68). Unfortunately, we did not have sufficient muscle tissue left for measurements of intramyocellular lipid content. Reactive oxygen species also can activate the NFκB and MAPK pathways (69,70) although previously we did not observe increased mitochondrial reactive oxygen species production in muscle from older participants (24). Elevated plasma FFA level, due to reduced fat oxidation and increased lipolysis, is a common feature associated with human aging (68,71,72). We (6,66) and others (9,73) have demonstrated that FFA can activate TLR4 receptors, consequently activating inflammation pathways and impair insulin action in muscle cells (6,66). In this study, fasting plasma FFA were not significantly different between the young and older participants. However, older participants had an increase in FFA area under the curves during the oral glucose tolerance test, which nearly reached statistical significance (p = .05). This finding indicates insulin resistance at the level of the adipose tissue resulting in increased lipolysis and suggests that an elevation in circulating FFA may contribute to the proinflammatory state (ie, increased TLR4 expression and signaling) observed in aging.
Rodent studies have demonstrated that stimulation of TLR4 with LPS reduces muscle mass and impairs muscle function (74). Constitutive activation of IκB kinase β and NFκB also induces muscle wasting in mice (75). In line with these findings, the older participants studied here had increased plasma LPS level, together with decreased quadriceps muscle volume and strength, in comparison with young participants. Although our findings do not prove cause and effect, they provide additional evidence that circulating LPS and subsequent increased flux through TLR4 could play a role in aging-related sarcopenia. Studies in aged rodents with genetic blockade of TLR4 signaling will be useful to evaluate the role of TLR4 and metabolic endotoxemia on sarcopenia of aging.
Previously, we showed that an 8-week aerobic exercise program increased the protein content of the NFκB inhibitory proteins, IκBα and IκBβ, in muscle from middle-aged participants with T2DM (30). Lambert and coworkers (23) reported that 12 weeks of combined (aerobic and resistance) exercise reduced muscle TLR4 messenger RNA and plasma cytokine levels in frail obese elderly participants. Based upon these findings, we anticipated that exercise would have an anti-inflammatory effect, manifested by a reduction in TLR4 expression and signaling (NFκB, MAPK), that may help to explain the insulin-sensitizing effect of exercise. Contrary to our hypothesis, 16 weeks of aerobic exercise training program had no effect on muscle TLR4 expression/signaling or circulating LPS/LBP levels. Although the basis for these discrepant findings is not clear, it is possible that a protective adaption (downregulation) in TLR4 after exercise may occur only under situations of pronounced inflammation and insulin resistance, such as those present in participants with obese, T2DM, and severe muscle wasting. Future studies with larger cohorts likely will be needed to determine whether a more prolonged or versatile (also incorporating resistance training) exercise regime is sufficient to reverse the inflammatory profile (elevated LPS levels and TLR4 signaling) observed in older individuals.
In summary, human aging is associated with metabolic endotoxemia and elevated signaling through the TLR4–NFκB–MAPK network in muscle that may play a role in aging-mediated insulin resistance and muscle loss. Future studies with larger populations are needed to establish the relationship between human aging phenotypes, metabolic endotoxemia, microbiome composition, and environmental factors such as dietary habits.
Funding
This study was supported by grants from the American Diabetes Association (N.M.), the National Institutes of Health (DK-80157 and DK-089229 to N.M.), the San Antonio Nathan Shock Center (AG-013319). N.M. was the recipient of a Paul B. Beeson Career Development Award (AG-030979) from the American Federation for Aging Research and the National Institute on Aging. S.G. was supported by an Institutional Ruth L. Kirchstein National research Service Award (NRSA) (T32-HL-04776).
Conflict of Interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We thank all the volunteers who participated in the study.
References
- 1. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R; Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. 10.2337/diabetes.52.6.1475 [DOI] [PubMed] [Google Scholar]
- 2. Brüünsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39 Retrieved from http://dx.doi.org/10.1016/S0889-8561(02)00056-5 [DOI] [PubMed] [Google Scholar]
- 3. Lechleitner M. Obesity and the metabolic syndrome in the elderly–a mini-review. Gerontology. 2008;54:253–259. 10.1159/000161734 [DOI] [PubMed] [Google Scholar]
- 4. Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85 Retrieved from http://dx.doi.org/10.1016/0026-0495(88)90033-9 [DOI] [PubMed] [Google Scholar]
- 5. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. 10.2337/db06-1595 [DOI] [PubMed] [Google Scholar]
- 6. Reyna SM, Ghosh S, Tantiwong P, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. 10.2337/db08-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 8. Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013;8:e63983. 10.1371/journal.pone.0063983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwang SL, Jeong YT, Li X, et al. Inhibitory cross-talk between the AMPK and ERK pathways mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Br J Pharmacol. 2013;169:69–81. 10.1111/bph.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xi L, Xiao C, Bandsma RH, Naples M, Adeli K, Lewis GF. C-reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: role of mitogen-activated protein kinases. Hepatology. 2011;53:127–135. 10.1002/hep.24011 [DOI] [PubMed] [Google Scholar]
- 12. Masharani UB, Maddux BA, Li X, et al. Insulin resistance in non-obese subjects is associated with activation of the JNK pathway and impaired insulin signaling in skeletal muscle. PLoS One. 2011;6:e19878. 10.1371/journal.pone.0019878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–9054. 10.1074/jbc.275.12.9047 [DOI] [PubMed] [Google Scholar]
- 14. Hemi R, Yochananov Y, Barhod E, et al. p38 mitogen-activated protein kinase-dependent transactivation of ErbB receptor family: a novel common mechanism for stress-induced IRS-1 serine phosphorylation and insulin resistance. Diabetes. 2011;60:1134–1145. 10.2337/db09-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thalacker-Mercer AE, Dell’Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics. 2010;40:141–149. 10.1152/physiolgenomics.00151.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci. 2010;65:532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Broome CS, Kayani AC, Palomero J, et al. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549–1551. 10.1093/gerona/glp196 [DOI] [PubMed] [Google Scholar]
- 18. Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1485–R1495. 10.1152/ajpregu.00467.2009 [DOI] [PubMed] [Google Scholar]
- 19. Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547(Pt 3):977–987. 10.1113/jphysiol.2002.036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol (1985). 2008;105:839–848. 10.1152/japplphysiol.00148.2008 [DOI] [PubMed] [Google Scholar]
- 21. Pauli JR, Ropelle ER, Cintra DE, et al. Acute exercise reverses aged-induced impairments in insulin signaling in rodent skeletal muscle. Mech Ageing Dev. 2010;131:323–329 Retrieved from http://dx.doi.org/10.1016/j.mad.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 22. Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. 10.1111/j.1532-5415.2004.52308.x [DOI] [PubMed] [Google Scholar]
- 23. Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol (1985). 2008;105:473–478. 10.1152/japplphysiol.00006.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060. 10.2337/db11-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3:130–140 Retrieved from http://dx.doi.org/10.1016/j.ncl.2006.03.008 [PMC free article] [PubMed] [Google Scholar]
- 26. Forbes SC, Little JP, Candow DG. Exercise and nutritional interventions for improving aging muscle health. Endocrine. 2012;42:29–38. 10.1007/s12020-012-9676-1 [DOI] [PubMed] [Google Scholar]
- 27. Nimmo MA, Leggate M, Viana JL, King JA. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. 2013;15(suppl 3):51–60. 10.1111/dom.12156 [DOI] [PubMed] [Google Scholar]
- 28. Oliveira AG, Carvalho BM, Tobar N, et al. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60:784–796. 10.2337/db09-1907 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Sriwijitkamol A, Christ-Roberts C, Berria R, et al. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–767. 10.2337/diabetes.55.03.06.db05-0677 [DOI] [PubMed] [Google Scholar]
- 30. Tantiwong P, Shanmugasundaram K, Monroy A, et al. NF-κB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab. 2010;299:E794–E801. 10.1152/ajpendo.00776.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192 Retrieved from http://dx.doi.org/10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 32. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 33. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. 10.2337/diab.30.12.1000 [DOI] [PubMed] [Google Scholar]
- 34. Coker RH, Hays NP, Williams RH, et al. Exercise-induced changes in insulin action and glycogen metabolism in elderly adults. Med Sci Sports Exerc. 2006;38:433–438. 10.1249/01.mss.0000191417.48710.11 [DOI] [PubMed] [Google Scholar]
- 35. DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981;68:1468–1474. 10.1172/JCI110399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sriwijitkamol A, Coletta DK, Wajcberg E, et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56:836–848. 10.2337/db06-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sisto SA, Dyson-Hudson T. Dynamometry testing in spinal cord injury. J Rehabil Res Dev. 2007;44:123–136. 10.1682/JRRD.2005.11.0172 [DOI] [PubMed] [Google Scholar]
- 38. Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-κB in Aging and Disease. Aging Dis. 2011;2:449–465. [PMC free article] [PubMed] [Google Scholar]
- 39. Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. 10.1096/fj.04-2640fje [DOI] [PubMed] [Google Scholar]
- 40. Flach RJ, Bennett AM. MAP kinase phosphatase-1–a new player at the nexus between sarcopenia and metabolic disease. Aging (Albany NY). 2010;2:170–176. 10.1096/fj.09-150045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22. 10.1096/fj.04-2640fje [DOI] [PubMed] [Google Scholar]
- 42. Beyer I, Njemini R, Bautmans I, Demanet C, Bergmann P, Mets T. Inflammation-related muscle weakness and fatigue in geriatric patients. Exp Gerontol. 2012;47:52–59 Retrieved from http://dx.doi.org/10.1016/j.exger.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 43. Katsargyris A, Theocharis SE, Tsiodras S, Giaginis K, Bastounis E, Klonaris C. Enhanced TLR4 endothelial cell immunohistochemical expression in symptomatic carotid atherosclerotic plaques. Expert Opin Ther Targets. 2010;14:1–10. 10.1517/14728220903401294 [DOI] [PubMed] [Google Scholar]
- 44. Sun R, Zhu Z, Su Q, Li T, Song Q. Toll-like receptor 4 is involved in bacterial endotoxin-induced endothelial cell injury and SOC-mediated calcium regulation. Cell Biol Int. 2012;36:475–481. 10.1042/CBI20110535 [DOI] [PubMed] [Google Scholar]
- 45. Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. 10.1152/ajpendo.00302.2006 [DOI] [PubMed] [Google Scholar]
- 46. Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. 10.2337/dc09-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–844 Retrieved from http://dx.doi.org/10.1210/er.2009-0030 [DOI] [PubMed] [Google Scholar]
- 48. Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. 10.1194/jlr.M800156-JLR200 [DOI] [PubMed] [Google Scholar]
- 49. Brasili E, Mengheri E, Tomassini A, et al. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 induce different age-related metabolic profiles revealed by 1H-NMR spectroscopy in urine and feces of mice. J Nutr. 2013;143:1549–1557. 10.3945/jn.113.177105 [DOI] [PubMed] [Google Scholar]
- 50. Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. [DOI] [PubMed] [Google Scholar]
- 51. Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70:6113–6122. 10.1128/AEM.70.10.6113-6122.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68:1045–1056. 10.1093/gerona/glt106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Helenius M, Hänninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318 (Pt 2):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brégégère F, Milner Y, Friguet B. The ubiquitin-proteasome system at the crossroads of stress-response and ageing pathways: a handle for skin care? Ageing Res Rev. 2006;5:60–90 Retrieved from http://dx.doi.org/10.1016/j.arr.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 55. Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. 10.1096/fj.04-2870fje [DOI] [PubMed] [Google Scholar]
- 56. Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. 10.1161/01.RES.0000269183.13937.e8 [DOI] [PubMed] [Google Scholar]
- 57. Caldow MK, Cameron-Smith D, Levinger P, McKenna MJ, Levinger I. Inflammatory markers in skeletal muscle of older adults. Eur J Appl Physiol. 2013;113:509–517. 10.1007/s00421-012-2458-x [DOI] [PubMed] [Google Scholar]
- 58. Wu J, Mei C, Vlassara H, Striker GE, Zheng F. Oxidative stress-induced JNK activation contributes to proinflammatory phenotype of aging diabetic mesangial cells. Am J Physiol Renal Physiol. 2009;297:F1622–F1631. 10.1152/ajprenal.00078.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li Z, Li J, Bu X, et al. Age-induced augmentation of p38 MAPK phosphorylation in mouse lung. Exp Gerontol. 2011;46:694–702. 10.1016/j.exger.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 60. Andreasen AS, Kelly M, Berg RM, Møller K, Pedersen BK. Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PLoS One. 2011;6:e23999. 10.1371/journal.pone.0023999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Alvaro C, Teruel T, Hernandez R, Lorenzo M. Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem. 2004;279:17070–17078. 10.1074/jbc.M312021200 [DOI] [PubMed] [Google Scholar]
- 62. Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. 10.1038/nature01137 [DOI] [PubMed] [Google Scholar]
- 63. Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. 10.1172/JCI25151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S157–S163. 10.2337/dc09-S302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. 10.1172/JCI6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hussey SE, Liang H, Costford SR, et al. TAK-242, a small-molecule inhibitor of Toll-like receptor 4 signalling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci Rep. 2013;33:37–47. 10.1042/BSR20120098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. 10.1126/science.1082889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dubé J, Goodpaster BH. Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr Opin Clin Nutr Metab Care. 2006;9:553–559. 10.1097/01.mco.0000241664.38385.12 [DOI] [PubMed] [Google Scholar]
- 69. Silveira LR, Fiamoncini J, Hirabara SM, et al. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008;217:1–12. 10.1002/jcp.21514 [DOI] [PubMed] [Google Scholar]
- 70. Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–593 Retrieved from http://dx.doi.org/10.1016/j.exger.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 71. Bonadonna RC, Groop LC, Simonson DC, DeFronzo RA. Free fatty acid and glucose metabolism in human aging: evidence for operation of the Randle cycle. Am J Physiol. 1994;266(3 Pt 1):E501–E509. [DOI] [PubMed] [Google Scholar]
- 72. Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab. 2012;97:3302–3309 Retrieved from http://dx.doi.org/10.1210/jc.2012-1428 [DOI] [PubMed] [Google Scholar]
- 73. Radin MS, Sinha S, Bhatt BA, Dedousis N, O’Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336–346. 10.1007/s00125-007-0861-3 [DOI] [PubMed] [Google Scholar]
- 74. Choo JJ, Horan MA, Little RA, Rothwell NJ. Muscle wasting associated with endotoxemia in the rat: modification by the beta 2-adrenoceptor agonist clenbuterol. Biosci Rep. 1989;9:615–621 Retrieved from http://dx.doi.org/10.1007/bf01119805 [DOI] [PubMed] [Google Scholar]
- 75. Cai D, Frantz JD, Tawa NE, Jr, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298 Retrieved from http://dx.doi.org/10.1016/j.cell.2004.09.027 [DOI] [PubMed] [Google Scholar]