Abstract
Background.
Coadministration of co-trimoxazole with sulfonylureas is reported to increase the risk of hypoglycemia.
Methods.
We identified a cohort of Medicare beneficiaries aged 66 years or older who took glyburide or glipizide for diabetes from a 5% national sample of Medicare Part D claims data in 2008 (n = 34,239). We tracked each participant’s claims during 2008–2010 for a co-trimoxazole prescription and subsequent emergency room visits for hypoglycemia. Descriptive statistics and logistic regression modeling were used to evaluate hypoglycemia-related emergency room visits after coadministration of co-trimoxazole with sulfonylureas and its utilization patterns in older adults with diabetes.
Results.
Sulfonylureas users prescribed co-trimoxazole had a significant higher risk of emergency room visits for hypoglycemia, compared with those prescribed noninteracting antibiotics (odds ratio = 3.89, 95% confidence interval = 2.29–6.60 for glipizide and odds ratio = 3.78, 95% confidence interval = 1.81–7.90 for glyburide with co-trimoxazole, using amoxicillin as the reference). Co-trimoxazole was prescribed to 16.9% of those taking glyburide or glipizide during 2008–2010, varying from 4.0% to 35.9% across U.S. hospital referral regions. Patients with polypharmacy and with more prescribers were more likely to receive co-trimoxazole. Patients with an identifiable primary care physician had 20% lower odds of receiving a co-trimoxazole prescription. Hospital referral regions with more PCPs had lower rates of coadministration of the two drugs (r = −.26, p < 0.001).
Conclusions.
Coadministration of co-trimoxazole with sulfonylureas is associated with increased risk of hypoglycemia, compared with noninteracting antibiotics. Such coadministration is prevalent among older diabetic patients in the United States, especially in patients without an identifiable primary care physician.
Key Words: Diabetes, Drug–drug interaction, Hypoglycemia, Medicare, Older adults.
Glyburide and glipizide are commonly used sulfonylurea antidiabetic drugs (1). However, the effect of glyburide and glipizide may be altered by drugs that inhibit CYP2C9, a cytochrome P450 enzyme involved in glyburide and glipizide metabolism in the liver (2,3). Co-trimoxazole, a combination of trimethoprim and sulfamethoxazole, was found to inhibit the activities of CYP2C9 (3). Juurlink and coworkers reported that use of co-trimoxazole is associated with a sixfold greater risk of hospitalization for hypoglycemia among older patients taking glyburide in Canada, compared with those using amoxicillin (4). Similarly, Schelleman and coworkers reported that use of co-trimoxazole is associated with 3 times higher odds of hospitalization due to hypoglycemia among Medicaid patients who took glipizide, using cephalexin as the reference (5). Patients with diabetes are more susceptible to infection because of their hyperglycemic environment and impaired immune function (6). Although co-trimoxazole is generally considered a safe broad-spectrum antibiotic, it should be avoided in diabetic patients taking glyburide or glipizide (7).
Few studies have evaluated the prevalence of exposure to potential drug interactions (8,9). For example, a recent hospital-based study found that 80% of older patients with polypharmacy (>5 drugs) had been exposed to at least one of the 238 potential cytochrome P450-mediated drug–drug interactions during their stay in a community hospital (9). Little is known about the prevalence of clinically significant drug interactions in outpatient settings. This study assessed the prevalence of coadministration of co-trimoxazole with sulfonylureas and risk of subsequent emergency room (ER) visits for hypoglycemia among older patients with diabetes. We also examined the pattern of such coadministration, associated factors, and its geographic variation across the United States. We hypothesized that patients with poor access to primary care, those with multiple prescribers and those on a greater number of medications would have higher risk of exposure to potential drug interaction.
Methods
Study Cohort
Using the 5% sample of national Medicare claims data, we identified beneficiaries aged 66 years or older as of January 1, 2008 who had continuous Medicare Parts A (hospital care) and B (physician and outpatient services) and no Health Maintenance Organization coverage during 2007–2010, and who had continuous Part D (prescription drug) coverage during 2008–2010 (n = 502,797). The information on each beneficiary’s demographics, Medicare entitlement, Parts A and B coverage, and Health Maintenance Organization enrollment was obtained from the Medicare enrollment files. We searched the 2007 claims for each patient from the Medicare Carrier files (claims for physician services), Outpatient Statistical Analysis Files (claims for outpatient services), and Medicare Provider Analysis and Review (MEDPAR) files (claims for inpatient services) for a diagnosis of diabetes (International Classification of Diseases, 9th revision, Clinical Modification [ICD-9-CM] codes of 250.x). Patients with diabetes were defined as those with at least two claims on different dates in 2007 with diabetes diagnoses (n = 122,470). From Medicare Part D (prescription) claims, we identified patients with diabetes who had any glyburide or glipizide prescription during 2008–2010, leaving 37,086 (representing 741,720 older adults with diabetes in the United States) in the study cohort.
To study hypoglycemia events after co-trimoxazole exposure, we extracted a subcohort from the main study cohort described earlier using Medicare Part D claims. The subcohort consisted of sulfonylurea (glipizide or glyburide) users with at least one episode of coadministration of co-trimoxazole, amoxicillin, cephalexin, or azithromycin. A coadministration episode was defined as at least 1 day’s overlap of the antibiotic and sulfonylurea based on their fill dates and days of supply. For example, a glyburide prescription on May 1, 2008 with 30 days of supply and also a co-trimoxazole prescription on May 20, 2008 with 10 days of supply was identified as an episode of coadministration of co-trimoxazole and glyburide. Amoxicillin, cephalexin, or azithromycin were chosen as the reference antibiotics because there is neither a plausible mechanism nor prior evidence that suggests that they interact with sulfonylurea. Episodes overlapping with other oral or injected antimicrobials in the 14-day window of prescription of the index antibiotic were excluded. The final subcohort consisted of 10,352 glipizide users with 21,473 episodes of index antibiotic coprescription and 7,836 glyburide users with 16,154 episodes of index antibiotic coprescription.
Outcome Measures
The two outcome measures were (a) coadministration of co-trimoxazole with glyburide or glipizide and (b) ER visits for hypoglycemia within 14 days after coadministration of co-trimoxazole or a reference antibiotic with glyburide or glipizide.
Coadministration of co-trimoxazole with glyburide or glipizide.
For each glyburide or glipizide user in the main study cohort, we tracked their claims for the prescription of co-trimoxazole. The Medicare Part D claims provide data on a prescription’s fill date and days of supply. Coadministration of co-trimoxazole with glyburide or glipizide was defined as mentioned earlier.
ER visits for hypoglycemia.
For each episode of coadministration of a sulfonylurea and one of the antibiotics in the subcohort, we reviewed the Carrier, Outpatient Statistical Analysis Files, and MEDPAR claims for ER visits due to hypoglycemia within 14 days after the antibiotic prescription was filled. ER visits with no hospital admission were identified from Carrier files and Outpatient Statistical Analysis Files using the Healthcare Common Procedure Coding System codes (99281–99285), revenue center codes 0450-0459 and 0981. ER visits resulting in hospital admission were identified from MEDPAR claims using the Emergency Room Charge Amount field in which the amount is more than $0. Then we used an algorithm developed and validated by Ginde and coworkers to identify ER visits due to hypoglycemia (10). Specifically, hypoglycemia was identified if a claim had (a) any of the ICD-9-CM codes 251.0–251.2, 270.3, 775.0, or 775.6 or (b) an ICD-9-CM code of 250.8 but without any codiagnosis code of 259.9, 272.7, 681.xx, 682.xx, 686.9x, 707.1–707.9, 709.3, 730.0–730.2, or 731.8. The algorithm had a sensitivity of 97% and positive predictive value of 89% for detecting hypoglycemia ER visits (10).
Explanatory Variables
Age (67–74, 75–84, and 85+ years) and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other) were obtained from the Medicare Part D denominator file. We used the ICD-9-CM coding algorithm modified by Quan and coworkers (11) to identify patient comorbid conditions from the Medicare outpatient and inpatient claims in 2007 using the Elixhauser method (12). The number of comorbidities was categorized as 0, 1, 2, 3, or more. We defined a patient as having an identifiable primary care physician (PCP) if he or she saw a physician who specialized in family medicine, general practice, internal medicine, or geriatrics on two or more occasions in an outpatient setting for evaluation and management (Current Procedural Terminology codes 99201–99205 and 99211–99215) in the year 2007 (13,14). A validation study found 82.6% concordance between PCPs identified from the claim-based algorithm and those from patient self-reports (14). We reviewed patients’ MEDPAR claims in 2007 for the number of hospitalizations (none vs 1 or more). As a measure of polypharmacy, the number of different oral medications on the first day of each month during 2008–2010 was calculated using Medicare Part D claims. The monthly totals were then converted to a yearly average. We also used Medicare Part D claims to extract the number of different prescribers (1–2, 3–6, and 7 or more) a patient used in 2008–2010.
The number of PCPs per 100,000 residents for each of the 306 hospital referral regions (HRRs) were obtained from the Dartmouth Atlas of Health Care files of hospital and physician capacity in 2006 (15). For each HRR, we calculated the percentage of oral prescriptions that were antibiotics and the percentage of antibiotic prescriptions that were co-trimoxazole in 2008–2010 for all patients with Medicare Part D data.
Statistical Analysis
Descriptive statistics were used for sample characteristics and for rates of co-trimoxazole prescription by patient characteristics.
For participants in the main study cohort, logistic regression modeling was used to assess the patient characteristics associated with co-trimoxazole prescription. We calculated the HRR-level rates of co-trimoxazole prescription and created a map to describe the variation in co-trimoxazole prescription across the United States by HRR. The Pearson correlation tests were used to assess the association between HRR characteristics and co-trimoxazole prescription. For HRR-level analyses, we deleted the 17 HRRs with fewer than 20 patients in the study cohort. The remaining HRRs (289 of 306) had an average of 117 patients (range: 20–964).
For participants in the subcohort of glipzide/glyburide users with coadministration of co-trimoxazole, amoxicillin, cephalexin, or azithromycin, we used descriptive statistics to describe pattern of use and estimate the rates of ER visits for hypoglycemia within 14 days after the index antibiotics prescription. The mixed effects logistic regression modeling was used to compare the risk of ER visits for hypoglycemia in those with co-trimoxazole prescription vs those prescribed a reference antibiotic (amoxicillin, cephalexin, or azithromycin). We adjusted for age, sex, race/ethnicity, Medicaid eligibility, comorbidity, number of hospitalizations in the previous year, and the clustering of multiple episodes within-patient.
We used SAS version 9.3 (SAS Institute, Cary, NC) for data extraction and statistical analyses and ArcGIS (ESRI, Redlands, CA) for mapping.
Results
Overall, 30.3% of the older patients with diabetes diagnosed as of December 31, 2007 were prescribed glyburide (13.9%) or glipizide (18.0%) during 2008–2010. A great majority of them were on the medicine for 6 months or longer (85.2% for glyburide users and 83.7% for glipizide users). Among those on glyburide or glipizide, 16.6% received a co-trimoxazole prescription during 2008–2010 while they were concurrently prescribed glyburide or glipizide: 16.3% for those taking glyburide and 17.1% for those taking glipizide. Table 1 shows the rates of co-trimoxazole prescription among patients taking glipizide or glyburide, by patient characteristics. The rates of co-trimoxazole prescription were higher among those aged 85 or older, women, non-Hispanic whites, those eligible for Medicaid, those with two or more comorbidities, and those with at least one hospitalization in the previous year. After controlling for other factors in the model, patients with an identifiable PCP had a lower risk of receiving co-trimoxazole, compared with those with no identifiable PCP (odds ratio: 0.88, 95% confidence interval: 0.83–0.93). Patients taking 5–9 and 10+ different oral medications had a 44% and 86% greater risk, respectively, of receiving a co-trimoxazole prescription, compared with patients taking 1–4 medications. Patients with 4–7 and 8+ different prescribers had a 34% and 93% greater risk, respectively, of receiving a co-trimoxazole prescription, compared with patients with 1–3 prescribers (Table 1).
Table 1.
Patient Characteristics of Older Diabetic Patients Taking Glyburide or Glipizide for Diabetes and Their Association With Being Prescribed Co-trimoxazole in a 5% National Sample of Medicare Recipients in 2008–2010
| Patient Characteristics | N | % (95% CI) Prescribed Co-trimoxazole | OR (95% CI) |
|---|---|---|---|
| All | 37,086 | 16.6 (16.2–17.0)* | |
| Age (y) | |||
| 66–74 | 17,378 | 16.1 (15.6–16.6) | ref |
| 75–84 | 14,771 | 16.9 (16.3–17.5) | 1.05 (0.99–1.12) |
| 85+ | 4,937 | 19.4 (18.3–20.5) | 1.27 (1.16–1.38) |
| Sex | |||
| Male | 14,133 | 13.5 (12.9–14.1) | Ref |
| Female | 22,953 | 19.0 (18.5–19.5) | 1.44 (1.35–1.53) |
| Race | |||
| Non-Hispanic white | 26,973 | 17.5 (17.0–18.0) | Ref |
| Non-Hispanic black | 4,024 | 15.5 (14.4–16.6) | 0.76 (0.69–0.83) |
| Hispanic | 3,893 | 16.2 (15.0–17.4) | 0.82 (0.74–0.90) |
| Other | 2,158 | 13.2 (11.8–14.6) | 0.72 (0.63–0.83) |
| Medicaid eligibility | |||
| Yes | 13,533 | 19.2 (18.5–19.9) | 1.21 (1.14–1.30) |
| No | 23,553 | 15.5 (15.0–16.0) | Ref |
| Number of comorbidities | |||
| 0 | 3,668 | 14.7 (13.6–15.8) | Ref |
| 1 | 12,126 | 14.5 (13.9–15.1) | 0.93 (0.83–1.03) |
| 2 | 8,558 | 16.5 (15.7–17.3) | 0.96 (0.86–1.08) |
| 3+ | 12,734 | 20.0 (19.3–20.7) | 0.98 (0.88–1.10) |
| Number of hospitalizations in 2007 | |||
| 1+ | 27,072 | 15.6 (15.2–16.0) | 1.16 (1.07–1.26) |
| 0 | 10,014 | 20.4 (19.6–21.2) | Ref |
| Having a primary care provider | |||
| No | 14,982 | 17.7 (17.1–18.3) | Ref |
| Yes | 22,104 | 16.3 (15.8–16.8) | 0.88 (0.83–0.93) |
| Average number of different oral medications† | |||
| 1–4 | 15,785 | 12.6 (12.1–13.1) | Ref |
| 5–9 | 18,875 | 19.3 (18.7–19.9) | 1.44 (1.35–1.53) |
| 10+ | 2,426 | 25.9 (24.2–27.6) | 1.86 (1.66–2.07) |
| Number of different prescribers‡ | |||
| 1–3 | 10,254 | 12.3 (11.7–12.9) | Ref |
| 4–7 | 14,679 | 15.8 (15.2–16.4) | 1.34 (1.25–1.45) |
| 8+ | 12,153 | 22.1 (21.4–22.8) | 1.93 (1.79–2.08) |
Notes: CI = confidence interval; OR = odds ratio.
*The coadministration rates of co-trimoxazole were 16.3% (95% CI: 15.7–16.8%) for glyburide users and 17.1% (95% CI: 16.6–17.6%) for glipizide users.
†The number of different oral medications on the first day of each month during 2008–2010 was calculated using Medicare Part D claims. The monthly totals were then converted to a yearly average as a measure of polypharmacy.
‡The Medicare Part D claims in 2008–2010 were used to extract the number of different prescribers.
Table 2 shows the pattern of use and ER visits for hypoglycemia within 14 days after coadministration of co-trimoxazole with glyburide/glipizide. The majority (>80%) of the co-trimoxazole and glyburide/glipizide prescriptions were filled at the same pharmacy and more than half were prescribed by the same prescriber. Among patients with a coadministration of co-trimoxazole with sulfonylruea, about 87% had at least 5 days of overlap. Table 2 also shows that those prescribed co-trimoxazole had higher rates of ER visits for hypoglycemia than those prescribed reference antibiotics (1.23% for glipizide and 0.88% for glyburide users, respectively, vs 0.25%–0.44% for those prescribed amoxicilline, cephalexin, or azithromycin). After adjusting for patient demographics and comorbidities, co-trimoxazole users were 2.2–4.8 times more likely to have a hypoglycemia-related ER visit than those prescribed reference antibiotics.
Table 2.
Emergency room Visits for Hypoglycemia Within 14 Days of Antibiotic Exposure Among Glipizide/Glyburide Users (5% Medicare data, 2008–2010)
| Coadministration | Glipizide | Glyburide | ||||||
|---|---|---|---|---|---|---|---|---|
| Co-trimoxazole | Amoxicillin | Cephalexin | Azithromycin | Co-trimoxazole | Amoxicillin | Cephalexin | Azithromycin | |
| Characteristics | ||||||||
| N of episodes | 4,323 | 6,211 | 4,586 | 6,353 | 3,079 | 4,867 | 3,393 | 4,815 |
| % by the same prescriber | 59.1 | 46.5 | 49.2 | 63.0 | 56.5 | 44.2 | 47.0 | 63.1 |
| % filled at the same pharmacy | 86.3 | 82.5 | 85.0 | 82.5 | 86.8 | 81.0 | 83.8 | 82.6 |
| % by days of overlap* | ||||||||
| 1–4 days | 12.7 | — | — | — | 12.1 | — | — | — |
| 5–9 days | 42.9 | — | — | — | 43.0 | — | — | — |
| 10+ days | 44.4 | — | — | — | 44.9 | — | — | — |
| Hypoglycemia emergency room visits | ||||||||
| N (%) | 53 (1.23) | 19 (0.31) | 20 (0.44) | 16 (0.25) | 27 (0.88) | 10 (0.21) | 13 (0.38) | 12 (0.25) |
| OR† (95% CI) | ||||||||
| Reference: Amoxicillin | 3.89 (2.29–6.60) | — | — | — | 3.78 (1.81–7.90) | — | — | — |
| Reference: Cephalexin | 2.70 (1.61–4.54) | — | — | — | 2.17 (1.11–4.23) | — | — | — |
| Reference: Azithromycin | 4.79 (2.73–8.41) | — | — | — | 3.10 (1.55–6.17) | — | — | — |
*The days of overlap was based on the fill dates and days of supply of the antibiotic and sulfonylurea prescriptions. For example, there was 10 days’ overlap for a patient who filled a glyburide prescription on May 1, 2008 with 30 days of supply and also a co-trimoxazole prescription on May 20, 2008 with 10 days of supply. The information was not provided for reference antibiotics because it was not of study interest.
†Adjusted for age, sex, race/ethnicity, Medicaid eligibility, comorbidity, and number of hospitalizations in the previous year.
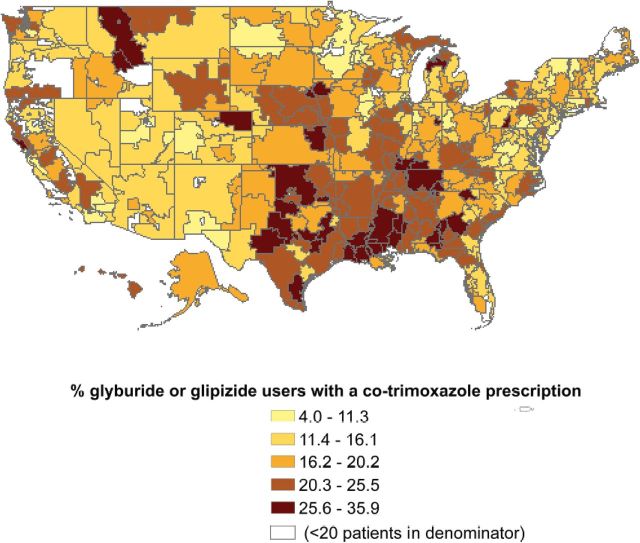
Figure 1 shows that the U.S. HRRs varied substantially (average 17.9%, range: 4.0%–35.9%) in the proportion of sulfonylurea users who were also prescribed co-trimoxazole in 2008–2010. In the 29 HRRs in the lowest decile, the average proportion of patients who received a co-trimoxazole prescription was 8.6% vs 28.8% for the 31 HRRs in the highest decile. Most HRRs with high rates of co-trimoxazole prescriptions were located in the South region.
Figure 1.
Geographic variation in the proportion of patients received a co-trimoxazole prescription in 2008–2009 for older diabetic patients taking glyburide or glipizide in the United States, by hospital referral region.
Table 3 presents the associations between HRR characteristics and risk of coprescription of co-trimoxazole for diabetic patients taking glyburide or glipizide. More PCPs per capita in the HRR was associated with a lower risk of co-trimoxazole prescription (r = −.23, p < .001). Among all oral medications prescribed during 2007–2008, an average of 4.1% were antibiotics (range: 2.8%–5.7%). A higher rate of antibiotics prescription in the HRR was associated with a higher rate of coadministration of co-trimoxazole with sulfonylureas (r = .26, p < .001). The HRR-level average rate of co-trimoxazole prescription among all oral antibiotics was 9.0% (range: 3.8%–14.2%). HRRs with a greater rate of co-trimoxazole use also had a higher rate of coadministration of co-trimoxazole with sulfonylureas (r = .46, p < .001).
Table 3.
Hospital Referral Region (HRR) Characteristics and Their Association With the Rate of Coprescription of Co-trimoxazole and Glyburide/Glipizide
| HRR-Level Characteristics* | Mean (SD) | Range | Correlation Coefficient (p Value)† |
|---|---|---|---|
| % of glyburide or glipizide users with a co-trimoxazole prescription | 17.9 (5.8) | 4.0–35.9 | ― |
| Number of primary care physicians per 100,000 residents‡ | 70.5 (12.0) | 43.9–117.0 | −0.23 (< 0.001) |
| % of all oral prescriptions that were antibiotics, 2008–2010§ | 4.1 (0.4) | 2.8–5.7 | 0.26 (< 0.001) |
| % of all antibiotics prescribed that were co-trimoxazole, 2008–2010§ | 9.0 (1.5) | 3.8–14.2 | 0.46 (< 0.001) |
*The 11 HRRs with fewer than 20 diabetic patients taking glyburide or glipizide were excluded from these analyses.
†The Pearson correlation coefficient between HRR-level characteristics and the rate of coprescription of co-trimoxazole with glyburide or glipizide.
‡The number of primary care physicians per 100,000 residents were obtained from the Dartmouth Atlas of Health Care files of hospital and physician capacity in 2006 (15).
§The percentage of oral prescriptions that were antibiotics and the percentage of antibiotic prescriptions that were co-trimoxazole were extracted from Medicare Part D data in 2008–2010.
Discussion
Hospitalizations for hypoglycemia are prevalent among older patients with diabetes on sulfonylureas, with a reported rate of 12.3 hospitalizations per 1,000 patients per year (16). Such events are associated with a number of poor outcomes, including declining cognition and death (17,18). Avoiding prescription of interacting drug is an important strategy for reducing hospitalizations for hypoglycemia. However, our study found that many older diabetic patients on sulfonylureas were exposed to one well-described drug interaction. We estimated that 32.1% of older patients with diabetes were on glyburide or glipizide. Among these glyburide or glipizide users, 16.6% also had a co-trimoxazole prescription over a 3-year period. The rate was as high as 35.9% in certain areas. In 2011, an estimated 8.7 million older adults were diagnosed with diabetes (19). Extrapolating our estimates of hypoglycemia incidence to U.S. older patients with diabetes diagnosed as of 2011, about 5,700 ER visits for hypoglycemia in the period 2011–2013 may be attributable to adverse drug interaction of co-trimoxazole and sulfonylureas.
Both polypharmacy and greater number of different prescribers were associated with greater risk for coadministration of co-trimoxazole with glyburide or glipizide (9). These associations were independent of each other and of the number of comorbidities in the multivariable adjusted model. Patients with diabetes generally take more medications than nondiabetic patients (20). In our study cohort, 57.4% of patients took on average of five or more different oral medications.
Greater access to primary care is associated with better preventive and chronic care management and better overall health (21,22). For example, individuals with a PCP are more likely to receive influenza vaccination, health promotion counseling, and cancer screening (13,23). Our study found that diabetic patients on glyburide or glipizide with a PCP had less likelihood of receiving a co-trimoxazole prescription than those with no identifiable PCP. In addition, HRRs with more PCPs per capita had significantly lower rates of concurrent use of co-trimoxazole with glyburide or glipizide. Our findings concur with those from McWilliams and coworkers (24) that a strong primary care orientation was associated with better quality of diabetes care.
Unlike the matched case–control studies by Juurlink and coworkers (4) and Schelleman and coworkers (5), we used a population-based retrospective cohort design. Our results support the previous findings of Juurlink and coworkers (4) and Schelleman and coworkers (5) that coadministration of co-trimoxazole with glyburide or glipizide was associated with significantly higher risk of severe hypoglycemia events. The association of co-trimoxazole with hypoglycemia-related ER visits or hospitalization could reflect the presence of an acute infection with resulting poor calorie intake and increased energy expenditure, rather than a drug effect (25). This was controlled for by using patients treated with other antibiotics as the reference group.
A recent systematic review concluded that current clinical standards for diabetes care do not sufficiently incorporate evidence about increased risk for hypoglycemia in vulnerable populations (26). The case of concurrent use of co-trimoxazole with glyburide or glipizide is an excellent example. The study by Juurlink and coworkers (4) was published in 2003 in a high visibility journal. However, we are not aware of any guideline (eg, clinical standards for diabetes care) (27) that warns against co-trimoxazole use in diabetic patients taking glyburide or glipizide. The Physicians’ Desk Reference states that co-trimoxazole potentiates oral hypoglycemics without specifying which ones (28).
A number of interventions have been shown to reduce inappropriate prescribing in other settings, including Electronic Medical Record-based alerts, computerized physician order entry and pharmacist interventions (29,30). Our data showed that over 80% of the prescriptions for the coadministration prescriptions were filled at the same pharmacy, suggesting the dispensing pharmacist as a potential point of intervention. For example, the use of computerized prescription entry and drug interaction screening in community pharmacies resulted in 62.8% reduction of pharmacy-dispensed prescriptions with severe drug interactions (31). A randomized trial found that 70% medication issues could be resolved if pharmacists routinely review patient prescription history and medical records (29). Such pharmacist-led interventions require communication across information technologies and can be incentivized by reimbursing pharmacist for providing such services (32).
We also found that about a half of the coadministration prescriptions were prescribed by the same prescriber, while the other half were from different prescribers. Also, patients with more prescribers had a significantly higher likelihood of receiving a co-trimoxazole prescription. Interventions to enhance physicians’ knowledge of potential drug–drug interactions, minimize the number of providers for a patient, and improve care coordination among providers are needed to reduce the coadministration of drugs with potential interactions (33). A recent published study found that displaying posters in physician offices stating that the provider was committed to following prescription guidelines for antibiotics reduced inappropriate antibiotic prescribing by 20% (34). A similar approach has been proposed to reduce potential drug–drug interactions by posting in the physician offices a short-list of high-alert drugs especially prone to drug interactions (35).
Medicare Part D was implemented in 2006. In 2012, approximately 65% of Medicare beneficiaries were enrolled in Part D plans (36). The availability of Medicare Part D claims data allows investigators to examine national patterns of drug use in Medicare recipients. Our study demonstrates that Medicare Part D data can be used to examine specific prescription issues, such as prevalence of potential drug interactions. This information can then inform decisions about interventions to improve prescribing practices.
The study has several limitations. First, claims data do not contain information on whether the patient actually takes the prescribed medication. We identified only those severe hypoglycemia events that resulted in an ER visit. Also, we could not track whether patients underwent closer monitoring during their use of co-trimoxazole concurrently with glipizide or glyburide. Claims data could not capture patients who stopped taking their medication or who took it less often while taking co-trimoxazole. In addition, we excluded patients with Health Maintenance Organization enrollment and those without Medicare Part D coverage because their medical or prescription claims were not completely captured in Medicare data.
Finally, we found substantial geographic variation in the concurrent use of co-trimoxazole with glyburide or glipizide in the United States, with HRRs in the South having higher rates. Other studies have also reported problematic prescription patterns in the U.S. South. For example, Qato and coworkers found that a greater percentage of older enrollees of the Medicare Advantage Plans in the South received high-risk medications (37). Zhang and coworkers found that antibiotic use was highest in the South, with 21.4% of Medicare Part D enrollees per quarter filling a script (38). This geographic variation may be associated with different prescription patterns among providers in different regions. Our study used a 5% sample of national Medicare data, which could not provide a sufficient number of patients per physician to study physician-level measures. However, prescriber-level measures can be estimated using 100% Medicare data. For example, a recent report from the Office of the Inspector General of the Health and Human Services Department identified 736 general-care physicians who had questionable prescribing patterns, using 100% Medicare Part D data in 2009 (39).
In conclusion, the coadministration of co-trimoxazole with sulfonylureas is associated with increased risk of hypoglycemia. Such coadministration is prevalent among older diabetic patients and varies substantially across U.S. geographic regions. Interventions to enhance physicians’ knowledge of potential drug interactions, reduce polypharmacy, increase access to PCPs, and improve care coordination among providers should be considered to reduce the risk of prescribing potentially interacting drugs.
Funding
The study was supported by the National Institutes of Health (5R01AG033134, UL1TR000071, and K23AG038476) and the Agency for Healthcare Research and Quality (1R24HS022134-01).
Conflict of Interest
None.
Acknowledgment
Sarah Toombs Smith, PhD, Science Editor and Assistant Professor in the Sealy Center on Aging, University of Texas Medical Branch at Galveston, provided editorial assistance in manuscript preparation. Dr. Toombs Smith received no compensation for her assistance apart from her employment at the institution where the study was conducted.
References
- 1. Foster PD, Mamdani MM, Juurlink DN, Shah BR, Paterson JM, Gomes T. Trends in selection and timing of first-line pharmacotherapy in older patients with Type 2 diabetes diagnosed between 1994 and 2006. Diabet Med. 2013;30:1209–1213. 10.1111/dme.12214 [DOI] [PubMed] [Google Scholar]
- 2. Hines LE, Murphy JE. Potentially harmful drug-drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011;9:364–377. 10.1016/j.amjopharm.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 3. Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos. 2002;30:631–635. [DOI] [PubMed] [Google Scholar]
- 4. Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–1658. 10.1001/jama.289.13.1652 [DOI] [PubMed] [Google Scholar]
- 5. Schelleman H, Bilker WB, Brensinger CM, Wan F, Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther. 2010;88:214–222. 10.1038/clpt.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knapp S. Diabetes and infection: is there a link?–A mini-review. Gerontology. 2013;59:99–104. 10.1159/000345107 [DOI] [PubMed] [Google Scholar]
- 7. Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183:1851–1858. 10.1503/cmaj.111152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahmood M, Malone DC, Skrepnek GH, et al. Potential drug-drug interactions within Veterans Affairs medical centers. Am J Health Syst Pharm. 2007;64:1500–1505. 0.2146/ajhp060548 [DOI] [PubMed] [Google Scholar]
- 9. Doan J, Zakrzewski-Jakubiak H, Roy J, Turgeon J, Tannenbaum C. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother. 2013;47:324–332. 10.1345/aph.1R621 [DOI] [PubMed] [Google Scholar]
- 10. Ginde AA, Blanc PG, Lieberman RM, Camargo CA., Jr Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. 00005650-200511000-00010 [pii] [DOI] [PubMed] [Google Scholar]
- 12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 13. Finison KS, Wellins CA, Wennberg DE, Lucas FL. Screening mammography rates by specialty of the usual care physician. Eff Clin Pract. 1999;2:120–125. [PubMed] [Google Scholar]
- 14. Shah BR, Hux JE, Laupacis A, Zinman B, Cauch-Dudek K, Booth GL. Administrative data algorithms can describe ambulatory physician utilization. Health Serv Res. 2007;42:1783–1796. 10.1111/j.1475-6773.2006.00681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dartmouth Atlas of Health Care. Selected Hospital and Physician Capacity Measures, and Claims-Based Reimbursement Measures. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; http://www.dartmouthatlas.org/tools/downloads.aspx#resources Accessed May 1, 2013. [Google Scholar]
- 16. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc. 1996;44:751–755. [DOI] [PubMed] [Google Scholar]
- 17. Lipska KJ, Montori VM. Glucose control in older adults with diabetes mellitus–more harm than good? JAMA Intern Med. 2013;173:1306–1307. 10.1001/jamainternmed.2013.6189 [DOI] [PubMed] [Google Scholar]
- 18. Yaffe K, Falvey CM, Hamilton N, et al. ; Health ABC Study. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173:1300–1306. 10.1001/jamainternmed.2013.6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 20. Good CB. Polypharmacy in elderly patients with diabetes. Diabetes Spectr. 2002;15:240–248. [Google Scholar]
- 21. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 22. Robert Wood Johnson Foundation and the University of Wisconsin School of Medicine and Public Health. County Health Rankings & Roadmaps http://www.countyhealthrankings.org/roadmaps Accessed July 20, 2013.
- 23. Lewis CE, Clancy C, Leake B, Schwartz JS. The counseling practices of internists. Ann Intern Med. 1991;114:54–58. [DOI] [PubMed] [Google Scholar]
- 24. McWilliams JM, Chernew ME, Zaslavsky AM, et al. Delivery system integration and health care sSpending and quality for medicare beneficiaries. JAMA Intern Med. 2013:1–9. 10.1001/jamainternmed.2013.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arinzon Z, Fidelman Z, Berner YN, Adunsky A. Infection-related hypoglycemia in institutionalized demented patients: a comparative study of diabetic and nondiabetic patients. Arch Gerontol Geriatr. 2007;45:191–200. 10.1016/j.archger.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 26. Berkowitz SA, Aragon K, Hines J, Seligman H, Lee S, Sarkar U. Do clinical standards for diabetes care address excess risk for hypoglycemia in vulnerable patients? A systematic review. Health Serv Res. 2013;48:1299–1310. 10.1111/1475-6773.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Physicians’ Desk Reference Network. Physicians’ Desk Reference 2013. Montvale, NJ: PDR Network; 2012. [Google Scholar]
- 29. Krska J, Cromarty JA, Arris F, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30:205–211. [DOI] [PubMed] [Google Scholar]
- 30. Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–1316. [DOI] [PubMed] [Google Scholar]
- 31. Halkin H, Katzir I, Kurman I, Jan J, Malkin BB. Preventing drug interactions by online prescription screening in community pharmacies and medical practices. Clin Pharmacol Ther. 2001;69:260–265. 10.1067/mcp.2001.114228 [DOI] [PubMed] [Google Scholar]
- 32. Mehuys E, Dupond L, Petrovic M, et al. Medication management among home-dwelling older patients with chronic diseases: possible roles for community pharmacists. J Nutr Health Aging. 2012;16:721–726. 10.1007/s12603-012-0028-x [DOI] [PubMed] [Google Scholar]
- 33. Wilsey BL, Fishman SM, Gilson AM, et al. An analysis of the number of multiple prescribers for opioids utilizing data from the California Prescription Monitoring Program. Pharmacoepidemiol Drug Saf. 2011;20:1262–1268. 10.1002/pds.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174:425–431. 10.1001/jamainternmed.2013.14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Juurlink D, Mamdani M, Iazzetta J, Etchells E. Avoiding drug interactions in hospitalized patients. Healthc Q. 2004;7:27–28. [DOI] [PubMed] [Google Scholar]
- 36. Medicare Payment Advisory Commission. Status Report on Part D http://www.medpac.gov/chapters/Mar13_Ch15.pdf Accessed July 20, 2013.
- 37. Qato DM, Trivedi AN. Receipt of high risk medications among elderly enrollees in Medicare Advantage plans. J Gen Intern Med. 2013;28:546–553. 10.1007/s11606-012-2244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172:1465–1471. 10.1001/archinternmed.2012.3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levinson DR. Prescribers with Questionable Patterns in Medicare Part D http://oig.hhs.gov/oei/reports/oei-02-09-00603.pdf Accessed July 10, 2013.