Abstract
Background.
The frailty syndrome is as a well-established condition of risk for disability. Aim of the study is to explore whether a physical activity (PA) intervention can reduce prevalence and severity of frailty in a community-dwelling elders at risk of disability.
Methods.
Exploratory analyses from the Lifestyle Interventions and Independence for Elders pilot, a randomized controlled trial enrolling 424 community-dwelling persons (mean age=76.8 years) with sedentary lifestyle and at risk of mobility disability. Participants were randomized to a 12-month PA intervention versus a successful aging education group. The frailty phenotype (ie, ≥3 of the following defining criteria: involuntary weight loss, exhaustion, sedentary behavior, slow gait speed, poor handgrip strength) was measured at baseline, 6 months, and 12 months. Repeated measures generalized linear models were conducted.
Results.
A significant (p = .01) difference in frailty prevalence was observed at 12 months in the PA intervention group (10.0%; 95% confidence interval = 6.5%, 15.1%), relative to the successful aging group (19.1%; 95% confidence interval = 13.9%,15.6%). Over follow-up, in comparison to successful aging participants, the mean number of frailty criteria in the PA group was notably reduced for younger subjects, blacks, participants with frailty, and those with multimorbidity. Among the frailty criteria, the sedentary behavior was the one most affected by the intervention.
Conclusions.
Regular PA may reduce frailty, especially in individuals at higher risk of disability. Future studies should be aimed at testing the possible benefits produced by multidomain interventions on frailty.
Key Words: Frailty, Physical activity, Physical function, Successful aging, Clinical trials.
The steady and rapid increase of the absolute and relative number of older persons is a global phenomenon of Western countries. Such demographical changes require immediate actions to render the healthcare systems capable of sustaining the growing number of individuals with multiple age-related conditions. In fact, the prevention of disabling conditions is important because disability severely impacts the quality of life of the individual (1) and is economically burdening for public health (2). Therefore, preventive interventions able to modify the natural history of age-related conditions are urgently needed.
During the last two decades, special interest has been devoted by the scientific community to the study of the frailty, a syndrome characterized by reduced homeostatic reserves and resistance to endogenous and exogenous stressors (3). Frailty represents a major risk factor for negative health-related events in the elderly, including disability, falls, hospitalizations, and mortality (4). Consequently, the clinical identification of frailty may play an important role in the development of preventive strategies against age-related conditions.
To transfer the theoretical concept of frailty into clinical practice, several operational definitions have been proposed (4). The best known and most commonly used is the one proposed by Fried and colleagues (5) and validated in the Cardiovascular Health Study. It defines the presence of a frailty phenotype when three or more of the following features are simultaneously present: exhaustion, involuntary weight loss, sedentary behavior, slow gait speed, poor muscle strength.
Of the most promising preventive interventions for disability, physical activity (PA) is surely one of the most studied and promising (6). Recent findings from the Lifestyle Interventions and Independence for Elders pilot (LIFE-P) study showed the capacity of a PA intervention to improve physical performance in sedentary older persons (7).
To our knowledge, no study has yet explored whether PA can modify the frailty status of elders. In this work, we hypothesize that the beneficial effects of PA may positively influence the frailty syndrome, allowing a reduction of its prevalence and severity toward a status of restored robustness. Thus, we conducted analyses aimed at exploring the effects of PA on frailty status in a sample of community-dwelling sedentary elders at initial risk of mobility disability.
Methods
Data are from the LIFE-P study (7). This is a two-arm, single blind, multicenter, randomized controlled trial comparing a PA intervention versus a successful aging (SA) health education program. The study was conducted from 2004 (first randomization) to 2006 at four clinical sites: Wake Forest University-School of Medicine (Winston Salem, NC), University of Pittsburgh (Pittsburgh, PA), Cooper Institute (Dallas, TX), and Stanford University-School of Medicine (Palo Alto, CA). The study was approved by the local institutional review boards.
The eligibility criteria were aimed at recruiting sedentary persons, aged 70–89 years, having a sedentary lifestyle (<20 min/wk spent in structured PA during the past month) and at increased risk of mobility disability (incapacity to walk 400 m at usual pace without any assistive device). Exclusion criteria were based on the presence of clinical conditions rendering the study intervention unsafe (eg, symptomatic coronary artery disease) or infeasible (eg, severe illnesses, cognitive disorders). Individuals recovering from acute conditions, surgery, rehabilitation, or those with conditions amenable to medical intervention were temporarily excluded and rescreened at a later time. The full list of inclusion and exclusion criteria as well as details about the design/methods of the trial have been previously published (7,8).
Randomization groups
PA group. The PA intervention included aerobic, strength, flexibility, and balance training. The intervention was organized into the following three phases:
- Adoption (weeks 1–8): three center-based exercise sessions (40–60 minutes) per week conducted under supervision;
- Transition (weeks 9–24): two center-based exercise sessions per week and home-based endurance, strengthening, and flexibility exercises (at least three times per week);
- Maintenance (week 25 to the end of the study): home-based intervention with optional once-to-twice per week center-based sessions and monthly phone contacts.
The intervention was specifically focused on walking, with the aim of helping participants reach at least 150 min/wk (9). Other forms of endurance activity (eg, stationary cycling) were utilized when regular walking was contraindicated. Each session was preceded by a brief warm-up and followed by a brief cool-down period. Participants were instructed to complete flexibility exercises following each bout of walking. Following the bouts of walking each week, participants were instructed to complete a 10-minute routine primarily focused on strengthening exercises. Strength training was focused on lower extremity physical activities by using variable weight ankle weights. Balance training was added during the adoption phase of the program. Participants were encouraged to increase all forms of PA throughout the day.
The intensity of training was gradually increased over the first 2–3 weeks. Using Borg’s scale (range from 6 [ie, 20% effort] to 20 [ie, exhaustion]) (10), participants were asked to walk at an intensity of 13 (ie, 70% effort, “somewhat hard”). They were discouraged from exercising at levels ≥15 (ie, 80% effort, “hard”) or ≤11 (ie, 60% effort, “fairly light”). Lower extremity strengthening exercises were performed at an intensity of 15–16 on the Borg’s scale.
SA health education group. A SA intervention served as an active control group. In this group, participants were invited to meet once a week in small groups for the first 26 weeks of the study, and subsequently on a monthly basis. The topics presented at the meetings were relevant to older persons’ health, including education on nutrition, medications, foot care, recommended preventive services. At the end of each meeting, a bout of gentle upper extremity stretching was conducted.
As previously reported (7), the attendance rate for follow-up assessments was particularly high (ie, 94.8% and 94.0% at 6 and 12 months, respectively). Attendance rates were also high in the PA (ie, 70.7% and 60.9% at 6 and 12 months, respectively) as well as in the SA (ie, 70.0% and 73.0% at 6 and 12 months, respectively) groups.
Frailty
Frailty phenotype (5) was measured at each clinic assessment (ie, baseline, 6 months, and 12 months). This frailty definition is based on the assessment of five signs/symptoms, which are computed as follows in these analyses:
- Exhaustion. As in the Cardiovascular Health Study (5), exhaustion was defined from two questions included in the Center for Epidemiologic Studies-Depression scale (11).
- Involuntary weight loss. In the LIFE-P database, no information is available about weight prior to the beginning of the study. For this reason, at the baseline visit, the criterion was considered present if a loss of appetite was reported by the participant on the Center for Epidemiologic Studies-Depression scale (11). The objective evaluation of changes in body weight was available at the 6- and 12-month clinic assessments. For these two visits, commensurate with the original definition (5), the criterion was considered as present if the participant had experienced a weight loss ≥2.275kg or ≥2.5% of body weight during the last 6 months of follow-up, or ≥4.55kg or ≥5% of body weight during the last 12 months of follow-up.
- Sedentary behavior. Self-reported level of PA was assessed using the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (12). The gender-specific cut-points (ie, men <383 kcal/wk; women <270 kcal/wk) proposed by Fried and colleagues (5) were applied to categorize the participants’ weekly caloric expenditure in moderate-intensity exercise-related activities.
- Slow gait speed. The gender- and height-specific cut-points of gait speed used in the original definition of frailty phenotype (5) were applied to the results obtained from a 4-m walk test.
- Poor muscle strength. Handgrip strength was measured using a Jamar handheld dynamometer (Bolingbrook, IL). The original gender- and body mass index-specific cut-points (5) were used for defining this criterion.
The presence of ≥3 criteria identifies frailty, with 1–2 representing prefrailty, and the absence of criteria indicating absence of frailty (5). Observations were included in the analytical sample when, even in the presence of missing frailty criteria items, a definitive classification of presence of frailty could be obtained.
Other variables
Main sociodemographic and behavioral characteristics were recorded at the baseline assessment. Prevalence of chronic conditions was determined using physician-diagnosed disease information self-reported by participants.
Statistical analysis
Chi-square and t test were used to compare the participants’ characteristics from the two randomization groups. Estimates of frailty prevalence and mean number of frailty criteria between randomization groups over time were calculated using repeated measures generalized linear models (ie, mixed effects models for continuous outcomes and generalized estimating equations with a logit link for binary frailty outcomes). Unstructured covariance matrices were used within both types of models. The baseline value of each frailty outcome (presence/absence or number of criteria), gender (stratifying variable for randomization), intervention assignment, assessment visit, and the interaction term of randomization group × assessment visit were included in the models. Models also included the baseline indicator of diabetes variable due to the highly significant difference it showed between the two groups at baseline. Although it is generally advised against wide-scale testing of the hypothesis of equality between baseline covariates in randomized trials, the low p value (p = .005) of diabetes may meet the higher standard justifying its inclusion (13). Evaluation of the effect of the intervention within baseline subgroups was performed for comparisons of the mean number of frailty criteria by inclusion of the subgroup covariate and relevant interaction terms in the above models. Because we report post-hoc analyses of a clinical trial, we view all significance tests as hypothesis generating and have reported nominal p values. Analyses were performed using IBM SPSS version 20.0 for Mac (Armonk, NY) and SAS 9.3 (Cary, NC).
Results
The main characteristics of the LIFE-P study sample (n = 424, mean age 76.8 [SD = 4.2] years, women = 68.9%) according to randomization group are presented in Table 1.
Table 1.
Sample Population (n = 424) According to Randomization Group
| Successful Aging (n = 211) | Physical Activity (n = 213) | p | |
|---|---|---|---|
| Age (years) | 77.0±4.3 | 76.5±4.2 | .24 |
| Gender (women) | 69.2 | 68.5 | .89 |
| Race | |||
| White | 73.5 | 75.1 | |
| Black | 19.0 | 17.4 | .91 |
| Other | 7.6 | 7.5 | |
| Education | |||
| 8th grade or below | 3.8 | 2.8 | |
| High school | 27.4 | 27.5 | .86 |
| College or above | 69.8 | 68.7 | |
| Body mass index (kg/m2) | 29.7±5.8 | 30.7±6.2 | .10 |
| Current smoking | 3.3 | 3.3 | .99 |
| Cancer | 17.1 | 17.8 | .83 |
| Congestive heart failure | 6.2 | 5.2 | .66 |
| Depression | 16.6 | 17.4 | .83 |
| Diabetes | 16.1 | 27.2 | .005 |
| Hypertension | 68.7 | 69.5 | .87 |
| Lung disease | 13.7 | 13.6 | .97 |
| Myocardial infarction | 7.1 | 11.3 | .14 |
| Osteoarthritis | 20.4 | 23.5 | .44 |
| Stroke | 5.7 | 3.8 | .35 |
| Number chronic conditions ≥3 | 22.3 | 27.7 | .20 |
Note: Results are shown as percentages, or mean ± SD.
Figure 1 reports results from the generalized linear models showing adjusted prevalence of frailty (panel A) and adjusted mean number of frailty criteria (panel B) at the different study assessment visits according to randomization group. After 12 months of follow-up, the estimates of prevalence were 10.0% (95% confidence interval [CI] = 6.5%, 15.1%) for the PA group and 19.1% (95% CI = 13.9%, 25.6%) for the SA group (p = .01); the adjusted odds ratio at the 12-month visit was 2.12 (95% CI = 1.17, 3.84). The mean number of frailty criteria decreased in the PA group (6 months: −0.43, 95% CI = −0.57, −0.29; 12 months: −0.48, 95% CI = −0.62, −0.33) as well as in the SA group (6 months: −0.20, 95% CI = −0.33, −0.06; 12 months: −0.21, 95% CI = −0.35, −0.06). Statistically significant differences in the number of frailty criteria were observed between the PA and SA groups at the 6-month (∆ = −0.23, 95% CI = −0.42, −0.04; p = .02) and 12-month (∆ = −0.27, 95% CI = −0.47, −0.06; p = 0.01) assessments.
Figure 1.
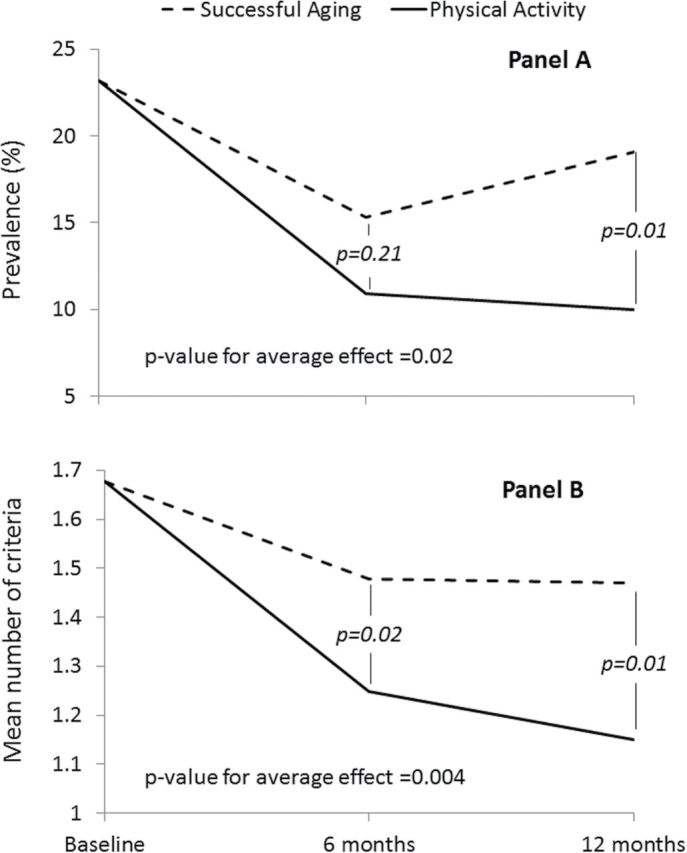
Results from general linear models showing the prevalence of frailty at the different study visits according to randomization group. Results are reported as means (95% CI). Gender (stratifying variable for randomization), number of frailty criteria at the baseline (A) or prevalence of frailty at the baseline (B), and diabetes are included as covariates of the model. CI = confidence interval.
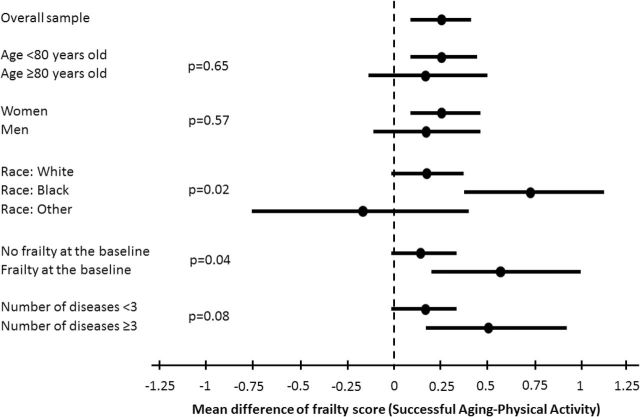
Figure 2 shows the results of stratified analyses estimating the effects of PA on the number of frailty criteria according to different subgroups. Results are presented as the mean number of frailty criteria in the SA group minus the mean number of frailty criteria in the PA group, averaged over the 6- and 12-month follow-up visits. Significant differences existed for the effect of the intervention within subgroups defined by race (p = .02) and baseline frailty (p = .04). Noteworthy reductions in the number of frailty criteria associated with the PA versus the SA intervention were observed for younger subjects (0.27, 95% CI = 0.07, 0.46), blacks (0.74, 95% CI = 0.35, 1.14), participants with frailty (0.60, 95% CI = 0.21, 0.98), and those with multimorbidity (0.52, 95% CI = 0.17, 0.87).
Figure 2.
Estimated effects of the intervention on the mean number of frailty criteria according to specific subgroups. Results are reported as means (95% CI). Estimates represent the average of the differences of mean levels between SA and PA groups over 6- and 12-mo visits obtained from a contrast estimated within a mixed effects model for the repeated frailty outcomes. Each model contained a prerandomization term representing the number of frailty conditions, an intervention effect, a visit effect, a term representing subgroup factor, and interactions between these last three terms. For the frailty subgroup analysis, the continuous baseline frailty outcome was dropped from the model. The reported p values for equality of difference between subgroups are obtained as a contrast from mixed effects model. CI = confidence interval; PA = physical activity; SA = successful aging.
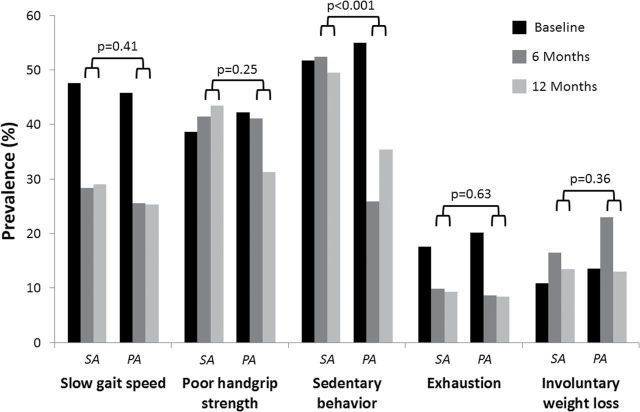
To understand which of the five frailty criteria was more positively affected by the study intervention, we conducted additional exploratory analyses looking at the variation of each single criterion according to randomization group (Figure 3). After adjustment for baseline levels of each frailty criterion, sedentary behavior was the only frailty criterion showing a significant difference between randomized groups in prevalence over the follow-up (adjusted odds ratio for the average effect = 2.37, 95% CI = 1.64, 3.43; p < .001). No other significant difference between the two groups was found. When the sedentary behavior criterion was omitted from the definition of the frailty phenotype, there was no significant difference in the modifications of frailty prevalence (adjusted odds ratio for average effect = 1.02, 95% CI = 0.49, 2.12; p = .97) or the number of frailty criteria (−0.04, 95% CI = −0.18, 0.09; p = .18) between the two groups.
Figure 3.
Estimated effects of the intervention on the prevalence of each of the frailty criterion according to the randomization group. Results are reported as percentages. The reported p values represent a test of the average prevalence at the month 6 and 12 visits in the PA vs the SA groups. Tests are adjusted for baseline levels for each frailty measure. PA = physical activity; SA = successful aging.
Discussion
To our knowledge, this is the first randomized clinical trial that has evaluated the effects of PA on frailty status in older persons. Our findings support the hypothesis that a PA program significantly reduced the presence and severity of frailty in a sample of sedentary elders, thus potentially modifying their risk profile. Secondary analyses suggest that participants benefiting most from the intervention included those with frailty and multimorbidity at baseline.
The present results are of major interest for the development of preventive interventions against disability. First, these findings support the concept that frailty is a reversible condition and, as such, might indeed represent an important target to consider for preventive programs aimed at the disability cascade in elders. Second, although the improvement of frailty status from the PA intervention was primarily related to the reduction of sedentary behavior, as opposed to the other components of frailty, this does not change the beneficial effect on the individual’s condition of risk. In fact, if the amelioration of the risk profile comes from the isolated action of the intervention on a single component of frailty (sedentary behavior in this case) or from a more general effect on multiple criteria, the final practical result (ie, reduction of frailty) still remains the same. Third, the lack of significant improvements reported for the frailty criteria other than sedentary behavior may provide important insights about the nature of this syndrome. In our study, PA seems to not uniformly affect the criteria designated to capture the frailty syndrome. Although the LIFE-P intervention was not designed to prevent/reduce frailty, our results suggest the existence of differences in the (sub)clinical mechanisms underlying each of the frailty criteria. From a research viewpoint, this indicates the need for exploring the single components of frailty in separate analyses in order to understand the differential responsiveness of criteria to specific interventions or their underlying pathophysiological mechanisms. On the other hand, using a more clinical perspective, the hypothesis that multidomain interventions are necessary in order to achieve the most successful results in the treatment of frailty might find support (14). The addition of preventive programs targeting different aspects of frailty (eg, malnutrition) may enhance the benefits that the frail older person may experience relative to a PA protocol alone.
Another important finding was obtained in subjects with frailty or higher comorbidity at the baseline. Our results, consistent with previous evidence (7,15), suggest that elders with higher risk profiles may still benefit from preventive strategies and should not be excluded a priori from interventions to prevent disability.
It might be argued that the improvement of frailty status per se may not necessarily translate into a reduction of frailty-related outcomes (eg, mortality, disability). Previous evidence from the LIFE-P study demonstrates that the adopted intervention is able to significantly improve physical performance measures and produce nonsignificant (but suggestive) results on “harder” endpoints such as mobility disability (7). Unfortunately, the pilot nature of the study does not allow to adequately clarifying this issue.
It might appear surprising that the PA intervention had nonsignificant effects on the slowness and weakness criteria of frailty. The lack of significant results is likely due to the categorization of the variables of interest. In our analyses, we explored the capacity of the intervention to improve gait speed and muscle strength above the thresholds of risk proposed by the original definition of frailty phenotype (5). Dichotomizing these features of frailty likely reduced statistical power, partly explaining the lack of significant differences. Moreover, by virtue of its inclusion criteria (7,8), the LIFE-P population was frailer than that recruited in the Cardiovascular Health Study (from which the defining criteria of frailty were derived). Consequently, it is possible that the relative improvements due to PA in the LIFE-P sample might have been of an insufficient magnitude for overcoming the predetermined thresholds of risk. Thus, it can be argued that the frailty phenotype may not be suitable for measuring health status modifications in the most vulnerable elders (16). Finally, it is possible that the frailty improvement follows a nonlinear exponential trend according to the severity of the condition, and/or some features (eg, sedentary behavior) are more quickly modified by the PA intervention compared with others.
Several limitations of our study need to be mentioned. The generalizability of our results might be limited due to the LIFE-P eligibility criteria, which were mainly focused at (i) defining the “at risk” group that might most benefit from the intervention, and (ii) ensuring that the PA program would be safe for participants. These findings can be applied to sedentary elders with some degree of functional limitation. While each of the frailty criteria was evaluated, two (sedentary behavior and weight loss) were slightly modified to accommodate the available data. For example, we do not have information on whether any observed weight loss was intentional or unintentional. Moreover, given the lack of information about weight loss at the baseline assessment, the loss of appetite was used as surrogate for defining the specific frailty criterion at this timepoint. Although weight loss and appetite loss are associated (17), their correlation might be modest (r ≅ .35) (18). Nevertheless, since we were comparing two treatment groups in the context of a randomized clinical trial, these modifications should not have had a substantial effect on our results. Unfortunately, the detail about the presence of macrovascular complications in diabetes is not present in the LIFE-P database. Such information might have supported specific analyses aimed at confirming recent findings that indicate complicated diabetes as a significant predictor of frailty progression (19). Finally, the PA program consisted of a suite of endurance, strengthening, and flexibility and balance exercises. This makes it difficult to formally tease apart which components of the intervention might be particularly important in reducing frailty. Future studies that systematically dismantle these different components may help to shed further light on this issue.
In conclusion, our results demonstrate a novel effect that regular PA may exert on the health status of older persons, that is the improvement of frailty syndrome. Given that such benefits seem to be particularly evident in frailer individuals, the exclusion of such persons from engaging in such types of PA is not justified when clinical contraindications are not present. If our findings will be confirmed, the promotion of PA programs adapted to older persons might have to be strengthened by public health authorities in order to reduce the incidence of frailty and disability and limit related healthcare expenditures. Future studies should be aimed at testing the possible enhanced benefits produced by multidomain interventions on geriatric syndromes.
Funding
The LIFE-P Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1-AG22376 and sponsored in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health.
Acknowledgments
Authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Research Investigators for Pilot Phase of LIFE: Cooper Institute, Dallas, TX. Steven N. Blair, PED (Field Center Principal Investigator [PI]); Timothy Church, MD, PhD, MPH (Field Center Co-PI); Jamile Ashmore, PhD; Judy Dubreuil, MS; Georita Frierson, PhD; Alexander N. Jordan, MS; Gina Morss, MA; RubenRodarte, MS; Jason M. Wallace, MPH. National Institute on Aging. Jack Guralnik, MD, PhD (Co-PI of the Study); Evan Hadley, MD; Sergei Romashkan, MD, PhD. Stanford University, Palo Alto, CA. Abby C. King, PhD (Field Center PI); William L. Haskell, PhD (Field Center Co-PI); Leslie Pruitt, PhD; Kari Abbott-Pilolla, MS; Karen Bolen, MS; Stephen Fortmann, MD; Ami Laws, MD; Carolyn Prosak, RD; Kristin Wallace, MPH. Tufts University, Boston, MA. Roger Fielding, PhD; Miriam Nelson, PhD. Dr. Fielding’s contribution is partially supported by the US Department of Agriculture, under agreement No. 58-1950-4-401. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. University of California, Los Angeles, CA. Robert M. Kaplan, PhD, MA. VA San Diego Healthcare System and University of California, San Diego, CA. Erik J. Groessl, PhD. University of Florida, Gainesville, FL. Marco Pahor, MD (PI of the Study); Michael Perri, PhD; Connie Caudle; Lauren Crump, MPH; Sarah Hayden; Latonia Holmes; Cinzia Maraldi, MD; Crystal Quirin. University of Pittsburgh, Pittsburgh, PA. Anne B. Newman, MD, MPH (Field Center PI); Stephanie Studenski, MD, MPH (Field Center Co-PI); Bret Goodpaster, PhD, MS; Nancy W. Glynn, PhD; Erin Aiken, BS; Steve Anthony, MS; Sarah Beck (for recruitment papers only); Judith Kadosh, BSN, RN; Piera Kost, BA; Mark Newman, MS; Jennifer Rush, MPH (for recruitment papers only); Roberta Spanos (for recruitment papers only); Christopher Taylor, BS; Pam Vincent, CMA; Diane Ives, MPH. The Pittsburgh Field Center was partially supported by the Pittsburgh Claude D. Pepper Center P30-AG024827. Wake Forest University, Winston-Salem, NC. Stephen Kritchevsky, PhD (Field Center PI); Peter Brubaker, PhD; Jamehl Demons, MD; Curt Furberg, MD, PhD; Jeffrey A. Katula, PhD, MA; Anthony Marsh, PhD; Barbara Nicklas, PhD; Jeff D. Williamson, MD, MPH; Rose Fries, LPM; Kimberly Kennedy; Karin Murphy, BS, MT (ASCP); Shruti Nagaria, MS; Katie Wickley-Krupel, MS. Data Management, Analysis and Quality Control Center (DMAQC) - Michael E. Miller, PhD (DMAQC PI); Mark Espeland, PhD (DMAQC Co-PI); Fang-Chi Hsu, PhD; Walter J. Rejeski, PhD; Don Babcock, Jr., PE; Lorraine Costanza; Lea Harvin; Lisa Kaltenbach, MS; Wei Lang, PhD; Wesley Roberson; Julia Rushing, MS; Scott Rushing; Michael Walkup, MS. The Wake Forest University Field Center is, in part, supported by the Claude D. Pepper Center #1-P30-AG21332. Yale University, New Haven, CT. Thomas M. Gill, MD. Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
References
- 1. Groessl EJ, Kaplan RM, Rejeski WJ, et al. Health-related quality of life in older adults at risk for disability. Am J Prev Med. 2007;33:214–218. 10.1016/j.amepre.2007.04.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried TR, Bradley EH, Williams CS, Tinetti ME. Functional disability and health care expenditures for older persons. Arch Intern Med. 2001;161:2602–2607. 10.1001/archinte.161.21.2602 [DOI] [PubMed] [Google Scholar]
- 3. Rodríguez-Mañas L, Féart C, Mann G, et al. ; FOD-CC group (Appendix 1). Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 6. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446–1461. 10.1016/j.mayocp.2013.08.020 [DOI] [PubMed] [Google Scholar]
- 7. Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci 2006;61:1157–1165. [DOI] [PubMed] [Google Scholar]
- 8. Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26:141–154. 10.1016/j.cct.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 9. Physical activity and health - A report of the Surgeon General. 1996:1–300.
- 10. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 11. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 12. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. [DOI] [PubMed] [Google Scholar]
- 13. Roberts C, Torgerson DJ. Understanding controlled trials: baseline imbalance in randomised controlled trials. BMJ. 1999;319:185. 10.1136/bmj.319.7203.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cesari M, Vellas B, Gambassi G. The stress of aging. Exp Gerontol. 2013;48:451–456. 10.1016/j.exger.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 15. Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. 10.1056/NEJM199406233302501 [DOI] [PubMed] [Google Scholar]
- 16. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- 17. Wilson MM, Thomas DR, Rubenstein LZ, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82:1074–1081. [DOI] [PubMed] [Google Scholar]
- 18. Solheim TS, Blum D, Fayers PM, et al. Weight loss, appetite loss and food intake in cancer patients with cancer cachexia: three peas in a pod? - analysis from a multicenter cross sectional study. Acta Oncol. 2014;53:539–546. 10.3109/0284186X.2013.823239 [DOI] [PubMed] [Google Scholar]
- 19. Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. 2012;60:652–660. 10.1111/j.1532-5415.2010.03153.x [DOI] [PMC free article] [PubMed] [Google Scholar]