Abstract
Background.
The Foundation for the National Institutes of Health Sarcopenia Project developed data-driven cut-points for clinically meaningful weakness and low lean body mass. This analysis describes strength and function response to interventions based on these classifications.
Methods.
In data from four intervention studies, 378 postmenopausal women with baseline and 6-month data were evaluated for change in grip strength, appendicular lean mass corrected for body mass index, leg strength and power, and short physical performance battery (SPPB). Clinical interventions included hormones, exercise, and nutritional supplementation. Differences in outcomes were evaluated between (i) those with and without weakness and (ii) those with weakness and low lean mass or with one but not the other. We stratified analyses by slowness (walking speed ≤ 0.8 m/s) and by treatment assignment.
Results.
The women (72±7 years; body mass index of 26±5kg/m2) were weak (33%), had low lean mass (14%), or both (6%). Those with weakness increased grip strength, lost less leg power, and gained SPPB score (p < .05) compared with nonweak participants. Stratified analyses were similar for grip strength and SPPB. With lean mass in the analysis, individuals with weakness had larger gains in grip strength and SPPB scores regardless of low lean mass (p < .01).
Conclusions.
Older women with clinically meaningful muscle weakness increased grip strength and SPPB, regardless of the presence of low lean mass following treatment with interventions for frailty. Thus, results suggest that muscle weakness, as defined by the Foundation for the National Institutes of Health Sarcopenia Project, appears to be a treatable symptom.
Key Words: Strength, Function, Sarcopenia, Gait speed, Clinical trials.
The age-related loss of muscle mass and strength, termed sarcopenia, represents a significant healthcare burden in the United States, reflecting $18.5 billion in costs in 2000 (1). Based on these statistics, reducing the incidence of sarcopenia by 10% nationwide would result in a potential saving of $1.1 billion (1), thus highlighting the importance of establishing proven interventions. To advance research, consensus on the clinical definition of sarcopenia is required. Currently, estimates of sarcopenia prevalence vary widely due to use of multiple parameters to define the condition (2).
In 1989, Rosenberg and colleagues (3) first coined the term sarcopenia as “a reduction in muscle mass with aging.” This definition stimulated others to characterize this condition (4,5). In 1998, Baumgartner and colleagues (4) first defined sarcopenia using appendicular skeletal lean mass, measured via dual-energy x-ray absorptiometry, as less than two standard deviations below the mean of a reference group of young persons (4). Yet muscle function and lean mass are not clearly associated, thus recent studies have suggested broader parameters to define sarcopenia (5,6). Weakness is associated with disability and mortality, but the role of muscle mass is less clear (7–9). Several working groups have made recommendations for defining sarcopenia based on review of the literature and expert opinion (5,10). Most recently, the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project used data pooled from numerous large epidemiologic studies and clinical trials to develop data-driven criteria for clinically meaningful weakness and low lean mass based on cut-points for grip strength and dual-energy x-ray absorptiometry-based appendicular lean mass (ALM), respectively (6). In the FNIH Sarcopenia Project, individuals classified as weak had a higher risk for incident mobility disability after 3 years of follow-up (11), yet it is unclear whether these proposed cut-points identify individuals who will benefit from interventions to improve mobility. The FNIH Sarcopenia Project specifically included studies of clinical trials to address this question of whether response to interventions was altered by sarcopenia, as defined by the data-driven cut-points.
The current study seeks to determine whether individuals, when categorized by the FNIH parameters for clinically meaningful weakness and low lean mass, respond to interventions for frailty. We used pooled data from four randomized trials of several different frailty interventions to compare the changes in muscle strength and function among groups of older adults defined as weak and having low lean mass according to the FNIH proposed criteria. We hypothesized that participants with weakness, with or without low lean mass, would respond equally well to interventions as those without weakness.
Methods
Participants
This study included data from four different clinical trials in women conducted at the University of Connecticut, CT. Recruitment and enrollment criteria varied and included (i) 6-month trial with 99 women (mean 77 ± 6 years) with frailty and low dehydroepiandrosterone sulfate (DHEA-S) levels who received 50 mg/d DHEA or placebo and participated in an exercise program (chair aerobics or chair yoga) (12); (ii) 6-month trial with 126 postmenopausal women (mean age 75 ± 7 years) with frailty treated with fish oil or placebo (13); (iii) a 2-year trial with 189 women aged 59–78 years on hormone therapy for a minimum of 2 years testing moderate resistance training (two to three sets of 10–14 repetitions) effects on femoral bone quality (14); (iv) a 3-year trial with 167 postmenopausal women (75±6 years), community-dwelling but frail, who received 0.25 mg 17-beta estradiol (15). All participants regardless of intervention or control group assignment were provided with calcium citrate and vitamin D supplements; the calcium dose ranged from 630 to 1300mg of calcium to meet a combined nutritional/supplement goal of 1500mg/d and the vitamin D dose ranged from 400 to 1000 IU/d. Study durations ranged from 6 to 36 months; however, data presented are on interventions from baseline to 6 months. Data on body composition and/or physical performance from the randomization visits were available in 378 women.
The Institutional Review Board at the University of Connecticut Health Center approved all studies and all women provided written informed consent. Eligibility information has been previously reported.
Measures
Short physical performance battery.—
The short physical performance battery (SPPB) includes three elements (gait, transfers, and balance), all components important in maintaining independence; it predicts mortality, nursing home admission (16), subsequent disability, and loss of independent ambulation. The SPPB which includes ability to rise from a chair, static balance, and the 8 foot walk (16) was assessed using the published protocol. Gait speed was defined as the length of the walking course divided by the time it took participants to walk the course at their usual pace. If more than one walking test was administered, the average gait speed (m/s) was used. Participants were asked to rise once from a standard chair without using their arms. If able to rise once successfully, participants were then asked to complete five chair stands and the time to complete the chair stands was recorded in seconds.
Lower extremity strength.—
Maximum lower extremity strength (Newtons) and power (Watts) was measured on a Keiser seated leg press (14).
Upper extremity strength.—
Grip strength was measured by a handheld Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL), and the maximum value from either hand was analyzed.
Body composition.—
Total body fat mass and total bone-free lean mass (kg) were acquired from total body scans using a fan-beam dual energy x-ray absorptiometer (Lunar, Madison, WI). Standard image analysis protocols were followed. The ALM was defined as the sum of lean mass from both arms and legs. Participants with invalid lean mass measurements for an arm or leg were excluded; this was usually due to metallic joint replacement. The validity and reproducibility of dual-energy x-ray absorptiometry have been reported previously (4).
Definitions of clinically meaningful weakness and low lean mass.—
The FNIH cut-points were used to determine weakness and low lean mass (grip strength <16kg, ALM/BMI < 0.512, respectively); these cut-points found to be predictive of future mobility impairment (11). We compared those with weakness to those without weakness. In addition, we compared those with weakness and low lean mass, those with weakness without low lean mass, and those without weakness.
Outcome measures.—
Grip strength, low lean mass (ALMBMI), leg strength (LS), leg power (LP), and SPPB were evaluated at baseline and follow-up (6 months). Scores for absolute change were calculated as follows: [6 month measure − baseline measure].
Covariates.—
Height (cm) was measured on Harpenden stadiometers and weight (kg) was measured on standard balance beam using standard protocols. Body mass index was calculated as weight divided by height2 (kg/m2). Chronic diseases including diabetes, congestive heart failure, chronic obstructive pulmonary disease, cancer were collected where possible, definitions were study specific and dichotomized as present or absent.
Statistical Analysis
The baseline characteristics were compared between treatment groups using chi-square tests for categorical data or one-way analysis of variance for continuous data. One-way analysis of variance was used to compare the absolute changes in grip strength, LS, LP, walking speed, SPPB, and ALM between those classified as weak to those who were not weak, and among groups defined as weak with low lean mass, weak with normal lean mass, and not weak. The studies were combined to provide enough participants to address the question of differential response to treatment in those with FINH-defined sarcopenia. We felt this appropriate as all interventions (even the control of calcium and vitamin D) have been used in studies for the treatment of frailty, sarcopenia, or falls. In addition to analyzing all participants combined, analyses were conducted stratified by treatment assignment (active vs. control) and mobility status (gait speed < 0.8 m/s, yes/no). Expert opinion has estimated gait speed less than 0.8 m/s as a cut-point predictive of shorter life expectancy, poor health, and decreased function (17). We did further subanalyses base on exercise (any exercise vs no exercise and resistance exercise vs no resistance exercise). Statistical analyses were performed using SPSS version 19.0 (Chicago, IL).
Results
Data were collected from 378 postmenopausal women ages 71.9 ± 7 years with a body mass index of 26.0 ± 4.8 at baseline and 6-months follow-up. Table 1 outlines baseline characteristics for study participants. The majority of participants had walking speeds > 0.8 m/s (n = 355); 33% of participants had weakness, 14% had low lean mass, and 6% of participants had both.
Table 1.
Baseline Age, Physical Performance, and Body Composition Characteristics of Study Populations
| Total (n = 377) | Control (n = 168) | Active (n = 209) | p Value | |
|---|---|---|---|---|
| Age (y) | 71.7±6.8 | 71.9±6.8 | 71.6±6.8 | .734 |
| BMI (kg/m2) | 25.9±4.9 | 25.9±5.0 | 25.9±4.8 | .954 |
| SPPB score | 10.5±1.7 | 10.6±1.6 | 10.5±1.7 | .629 |
| Baseline GS (kg) | 19.9±6.4 | 20.1±6.8 | 19.7±6.1 | .570 |
| Baseline GS/BMI (kg/kg/m2) | 0.8±0.3 | 0.8±0.3 | 0.8±0.3 | .623 |
| Baseline ALM (kg) | 15.6±2.3 | 15.5±2.4 | 15.7±2.2 | .452 |
| Baseline ALM/BMI (kg/kg/m2) | 0.6±0.1 | 0.6±0.1 | 0.6±0.1 | .315 |
| Baseline leg power (Watts) | 217±76 | 209±72 | 225±79 | .110 |
| Baseline gait speed (m/s) | 1.1±0.2 | 1.1±0.2 | 1.1±0.2 | .163 |
| Absolute change in | ||||
| SPPB | 0.3±1.7 | 0.2±1.9 | 0.4±1.5 | .187 |
| Grip strength (kg) | −0.2±4.2 | −0.2±4.1 | −0.3±4.3 | .849 |
| GS/BMI (kg/kg/m2) | −0.1±0.2 | −0.1±0.2 | −0.1±0.2 | .885 |
| ALM (kg) | −0.0±0.6 | −0.1±0.6 | 0.0±0.7 | .075 |
| ALM/BMI (kg/kg/m2) | 0.0±0.2 | 0.0±0.3 | −0.0±0.1 | .187 |
| Leg power (Watts) | −20.1±49.5 | −17.1±47.6 | −22.9±51.3 | .364 |
| Gait speed (m/s) | −0.0±0.1 | −0.0±0.1 | −0.0±0.2 | .149 |
Notes: Mean ± SD; ALM = appendicular lean mass; BMI = body mass index; GS = grip strength; SPPB score = short physical performance battery score. Categories are distinguished by weakness at baseline (defined by GS < 16kg) and low ALM < 15.02 kg, and low lean mass by ALMBMI < 0.512. p value indicates differences between active treatment and placebo groups. Active group included the following interventions: 50mg dehydroepiandrosterone (DHEA) + chair aerobics/chair yoga; 1.2g fish oil; lower extremity progressive resistance training; 0.25mg 17-beta estradiol. Control groups received the following interventions: placebo for DHEA + chair aerobics/chair yoga, 1.2g olive oil, upper extremity nonprogressive resistance exercise, and placebo for estradiol. All participants (active and control) received calcium and vitamin D supplementation.
In analysis of the entire group, those with weakness increased SPPB scores compared with those without weakness. Similar results were seen when stratified by slowness and treatment assignment though not always reaching statistical significance due to sample size (Table 2, Figure 1). Those without weakness at baseline demonstrated loss of grip strength, whereas those with weakness improved. The results were similar in all subgroup analyses with the exception of those with slow walk speed, most likely due to the small sample size (Table 2, Supplementary Figure 3a). The LP declined regardless of weakness classification, although less in those with weakness. Results were similar when stratified by slowness or treatment assignment. Table 2, Supplementary Figure 3b). Subgroup analyses were also completed by comparing exercise (upper/lower extremity resistance, yoga, aerobics vs. nonexercise (estrogen, fish oil) and resistance training (upper/lower extremity resistance) against other (yoga, aerobic, estrogen, fish oil) in the same classifications as other analyses to address impact of exercise against other interventions. The results are of similar magnitude but may lose significance due to smaller sample sizes (data not shown).
Table 2.
Absolute Change in Muscle Quality Variables for Normal and Low Grip Strength Groups
| Variables | All Participants | Slowness Present? | Treatment Assignment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Active | Control | |||||||
| Not Weak (N = 252) | Weak (N = 124) | Not Weak (N = 246) | Weak (N = 106) | Not Weak (N = 6) | Weak (N = 17) | Not Weak (N = 133) | Weak (N = 65) | Not Weak (N = 113) | Weak (N = 41) | |
| Grip strength | −0.97* | 1.21* | −0.97* | 1.01* | −1.00 | 2.53 | −0.78* | 0.68* | −1.19* | 1.51* |
| Leg strength (GS) | −9.84 | −31.30 | −9.00 | −21.38 | −64.33 | −112.70 | −0.46 | 2.94 | −17.90* | −55.71* |
| Leg power | −23.65* | −5.50* | −24.33* | −5.92* | 20.33 | −2.10 | −30.36* | 6.67* | −18.05 | −23.68 |
| Walking speed | −0.02 | −0.03 | −0.02 | −0.04 | 0.05 | 0.03 | −0.02 | −0.02 | −0.03 | −0.07 |
| SPPB | 0.02* | 0.84* | 0.00* | 0.68* | 0.83 | 1.53 | 0.16 | 0.52 | −0.18* | 0.93* |
| ALMBMI | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | −0.00 | −0.00 | −0.02 | 0.03 | 0.00 |
Notes: ALMBMI = appendicular lean mass standardized to body mass index; GS = grip strength; SPPB = short physical performance battery. Categories are distinguished by weakness at baseline (defined by GS < 16kg).
*Significance between those with normal and low GS within the grouping (ie, whole group).
Figure 1.
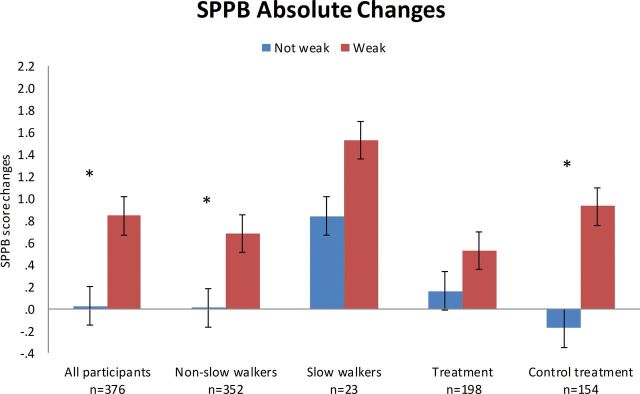
Measured change in short physical performance battery after 6 months in all participants, nonslow (>0.8 m/s) and slow walkers (≤0.8 m/s), active or placebo treatment (various interventions). Categories are distinguished by weakness at baseline (defined by grip strength < 16kg). *Significant at p < .05.
Participants were further classified into three groups (not weak [n = 251], weak with low lean mass [n = 24], weak without low lean mass [n = 100]; Table 3, Figure 2). SPPB, grip strength, and LP had significant absolute changes, whereas LS, walking speed and ALMBMI did not (Table 3) for each of the three groups. The SPPB increased in both weak groups compared with not weak grip strength (Figure 2). Similar results were seen in those stratified by with slowness and treatment (control and active) (Figure 2). Grip strength increased in those with weakness, regardless of the presence of low lean mass at baseline, and this change was significant only in the weak without low lean mass group. (Table 3, Supplementary Figure 4a). Similar results were seen when stratified for slowness and treatment group (control) (Supplementary Figure 4a). Overall, those with normal grip strength lost more LP than the weak groups, although the differences did not always reach significance (Supplementary Figure 4b).
Table 3.
Absolute Change in Muscle Function Variables for Not Weak; Weak, Low Lean; and Weak, Not Low Lean Groups
| All Participants | Slowness Present | Treatment Assignment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Active | Control | ||||||||||||
| Not Weak (N = 251) | Weak Low Lean (N = 24) | Weak Not Low Lean (N = 100) | Not Weak (N = 245) | Weak Low Lean (n = 21) | Weak Not Low Lean (N = 85) | Not Weak (N = 6) | Weak Low Lean (N = 3) | Weak Not Low Lean (N = 14) | Not Weak (N = 133) | Weak Low Lean (N = 11) | Weak Not Low Lean (N = 54) | Not Weak (N=112) | Weak Low Lean (N = 10) | Weak Not Low Lean (N = 31) | |
| Grip strength (GS) | −0.98* | 0.94* | 1.28*,† | −0.98* | 0.88* | 1.05*,† | −1.00 | 1.33 | 2.79 | −0.78 | 1.75 | 0.48 | −1.22* | 0.09* | 2.00*,† |
| Leg strength | −9.84 | −43.00 | −27.18 | −9.00 | −43.00 | −12.43 | −64.33 | NA | −112.70 | −0.46 | −28.08 | 13.28 | −17.90 | −57.92 | −54.50 |
| Leg power | −23.65 | −4.08 | −6.00 | −24.33 | −4.08 | −6.67 | 20.33 | NA | −2.10 | −30.36* | −2.17* | 9.61*,† | −18.05 | −6.00 | −33.32 |
| Walking speed | −0.02 | −0.06 | −0.03 | −0.02 | −0.08 | −0.03 | 0.05 | 0.06 | 0.02 | −0.02 | −0.08 | −0.01 | −0.03 | −0.07 | −0.07 |
| SPPB | 0.02* | 1.21* | 0.75*,‡ | 0.00* | 1.29* | 0.53*,‡ | 0.83 | 0.67 | 1.71 | 0.16* | 1.82* | 0.26* | −0.18* | 0.70* | 1.00*,† |
| ALMBMI | 0.01 | 0.01 | −0.01 | 0.01 | 0.01 | −0.02 | 0.01 | 0.00 | −0.00 | −0.00 | 0.02 | −0.02 | 0.03 | 0.00 | −0.00 |
Notes: ALMBMI = appendicular lean mass standardized to body mass index; SPPB = short physical performance battery. Categories are distinguished by weakness at baseline (defined by GS < 16kg) and low lean mass (defined by ALMBMI < 0.512).
*Significance within grouping.
†Post hoc analysis: Not weak is significantly different from weak, not low lean p < .001.
‡Post hoc analysis: Not weak is significantly different from both weak low lean and weak, not low lean p < .01.
Figure 2.
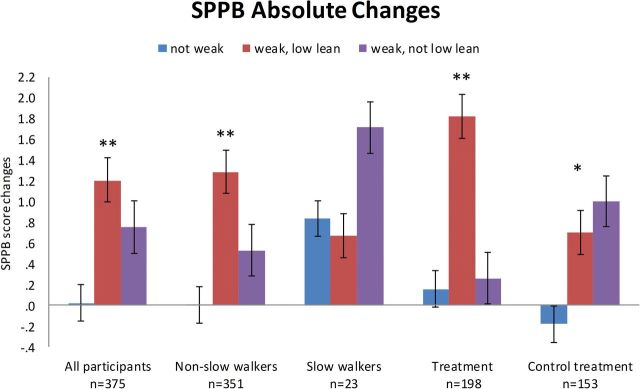
Measured change in short physical performance battery after 6 months in all participants, nonslow (>0.8 m/s) and slow walkers (≤0.8 m/s), active or placebo treatment (various interventions). Categories are distinguished by weakness at baseline (defined by grip strength < 16kg and low lean mass by ALMBMI < 0.512). ALMBMI = appendicular lean mass corrected for body mass index. Post hoc statistical analysis: *not weak is significantly different from weak, not low lean p < .001; **not weak is significantly different than both weak low lean and weak, not low lean p < .01.
Discussion
Compared with those without weakness at baseline, those with weakness demonstrated better response to interventions aimed at improving strength and physical function. This work is consistent with prior work demonstrating the ability of weak individuals to improve strength and function (18–20). Whether observed increases from frailty interventions are clinically meaningful are yet to be determined. However, recent work from the FNIH Sarcopenia Project revealed that muscle weakness, defined as grip strength < 16kg in women, increased the likelihood of mobility impairment, regardless of lean mass (11). Thus, if achieved increases in strength bring weak individuals above the previously established thresholds for clinically meaningful weakness (21), strength improvements may be considered clinically meaningful.
Although all weak individuals improved their grip strength more than those who were not weak, those without low lean mass had the greatest response. These results suggest that the preservation of lean mass was beneficial in the response to interventions to improve frailty. We are uncertain if this has been demonstrated before. Fiatarone demonstrated improved muscle strength in very frail, old nursing home participants with resistance training (18), whereas others have failed to demonstrate gains in frail adults (20,22). In our analyses of SPPB score, those with weakness had similar improvements regardless of the low lean mass category; those with weakness also had greater improvements compared with individuals without weakness. The differences between gains in strength against gain in function may be due to adiposity, although our definition of lean mass corrects for body mass and should mitigate some of this effect. Functional gains may be more difficult in those with increase adiposity (23) and those with increased adiposity are more likely to have normal lean mass (24).
In the present study those without weakness declined in strength or function even with interventions. The biological process of aging is associated with declines in muscle mass, strength, and function (3). A 12-year study found a significant decrease in skeletal muscle strength with an annual decline ranging from 1.4% to 2.5% (25), reductions in LS (2.5 N-m/y [in men] and 1.8 N-m/y [in women]) and quality of ~10% per decade (26) and decline in walking speed (27). Mechanisms to explain the decline include inflammation, oxidative stress, mitochondrial dysfunction, and alterations in fiber types with age (28,29). The reason for the lack of response is not clear, although may be explained by regression to the mean (30). Previous studies have demonstrated changes in muscle strength in healthy adults (not limited to frail adults) with progressive resistance training (25,31,32) and in those with frailty (18,19). None of the interventions were progressive resistance training, so that the effects may have been unsuccessful in those with preserved function as they did not have a muscular challenge sufficient to change strength or function. According to the overload principle, exercise should be performed at intensity higher than the usual load to increase the metabolic demand and facilitate a training response (33).
In contrast to changes in grip strength, LS and power declined regardless of weakness, slowness, or treatment classification. It is unclear why lower extremity strength and power changes differed from observed changes in grip strength. However, muscles in the upper body may be more adaptable to interventions in older adults, as Sousa and colleagues (34) similarly found greater changes in upper body than lower body measures in older adults in response to an intervention. The reason for this anatomical difference is unknown, but may be related to the greater age-related longitudinal declines in lower body than upper body strength in women (7,35). The clinical relevance of changes in lower extremity measures are more difficult to interpret as unlike grip strength, no consensus thresholds based on large pooled analyses have been established for lower extremity measures. As earlier studies have reported stronger associations between strength and function when strength is assessed on the corresponding muscle group (36–39), further research is warranted on the clinical relevance of lower extremity strength and power measures.
The individuals in these randomized studies were selected for some level of frailty and if selected for a component not related to strength/sarcopenia, physiologic factors other than sarcopenia could have had more impact in strength and function. Fried and colleagues (39) established cut points to identify malfunctioning physiologic systems associated with frailty and demonstrated that three or more abnormal systems are 26 times more likely to result in a frailty diagnosis. The physiologic systems include hematologic, inflammatory, endocrine, adiposity, neuromuscular, and micronutrient, so that the affected system may or may not result in a significant impact on muscle (39). Furthermore, both frailty and sarcopenia are complex systems so that adjusting for one system may not be enough to correct frailty or sarcopenia (28,39). The interventions evaluated in the current analysis included anabolic (exercise and DHEA), hormonal (estrogen), and micronutrient (calcium and vitamin D) interventions; however, in frail and sarcopenic individuals, inflammation or hematologic systems are also frequently involved (40,41). Neglecting to include interventions for anemia and inflammation may have prevented improvements in some sarcopenic and frail patients.
Anabolic agents may be most beneficial to those with low strength. DHEA supplementation demonstrated no benefit in muscle strength or body composition in a trial of healthy, nonfrail older men and women (42). Studies that found improvement in physical strength or function with DHEA combined supplementation with exercise in older, frailer adults (43,44). The control group most likely benefited from calcium and vitamin D supplementation. Numerous studies demonstrate corrective effects of vitamin D on muscle strength and function in older adults (45–48), although not all (49). A 6-month study of calcium and vitamin D supplementation in 46 elderly institutionalized participants demonstrated improved hip flexor and knee extensor strength by 16.4% and 24.6% in those receiving calcium plus vitamin D group and no improvements in the calcium plus placebo group (48). Community dwelling elderly women in the lowest tertile of strength and mobility improved after 1 year of vitamin D supplementation (47). Vitamin D increased gait speed and decreased body sway whereas resistance training improved strength in a study of elderly participants with vitamin D levels ≤ 16 ng/mL (45).
The study has several limitations. The sample size is small and would benefit from a larger population; we may have missed differences on some measures. The data is pooled from diverse studies with different interventions. The different inclusion criteria specific to each study may have altered the ability to combine the studies. Furthermore, the interventions were varied [exercise, hormones, and nutrition] which also may limit the ability to combine the data. Although the individuals in the population were selected for frailty, those with the most severe frailty were not well represented and studies in a more frail population will need to be done to confirm results. Finally, the role of the FINH cut-points in men will also need further exploration.
In summary, the definitions of clinically meaningful muscle weakness proposed by FINH were able to differentiate response to interventions targeting frailty in older, frail postmenopausal women. The response was consistent when we evaluated all participants or when stratified by slowness or treatment assignment. Thus, muscle weakness appears to be a treatable symptom in sarcopenia diagnosis. The differentiation of whether lean mass preservation allows for more improvement in strength and whether those with low lean mass have more dramatic improvements in function with interventions will require further study and larger sample sizes.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
Funding support for the conference and the work of the consortium was provided by the National Institute on Aging (1U13AG041583 and P30 AG024827), the Food and Drug Administration, and through grants from the Foundation for the NIH, made possible by funding from Abbott Nutrition, Amgen, Eli Lilly, Merck, Novartis, and The Dairy Research Institute.
Supplementary Material
Acknowledgments
This work was funded by the following grants: MO1-RR06192, R01-AG18887, NNG04GK63G; 5P60AG13631; Patrick and Catherine Donaghue Medical Research Foundation; American Federation on Aging Paul Beeson Faculty Scholar Program.
References
- 1. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. [DOI] [PubMed] [Google Scholar]
- 2. Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13:708–712. [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995;123:727–728. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 5. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. :10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 8. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 9. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 10. Abellan van Kan G, André E, Bischoff Ferrari HA, et al. Carla Task Force on Sarcopenia: propositions for clinical trials. J Nutr Health Aging. 2009;13:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. :10.1093/gerona/glu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA, Kenny AM. Effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics. Age Ageing. 2010;39:451–458. :10.1093/ageing/afq043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutchins-Wiese HL, Kleppinger A, Annis K, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17:76–80. :10.1007/s12603-012-0415-3 [DOI] [PubMed] [Google Scholar]
- 14. Judge JO, Kleppinger A, Kenny A, Smith JA, Biskup B, Marcella G. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporos Int. 2005;16:1096–1108. [DOI] [PubMed] [Google Scholar]
- 15. Kenny AM, Kleppinger A, Wang Y, Prestwood KM. Effects of ultra-low-dose estrogen therapy on muscle and physical function in older women. J Am Geriatr Soc. 2005;53:1973–1977. [DOI] [PubMed] [Google Scholar]
- 16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 17. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. :10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 19. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. [DOI] [PubMed] [Google Scholar]
- 20. Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. [DOI] [PubMed] [Google Scholar]
- 21. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69: 559–566. :10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faber MJ, Bosscher RJ, Chin A Paw MJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87:885–896. [DOI] [PubMed] [Google Scholar]
- 23. Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Estrada M, Kleppinger A, Judge JO, Walsh SJ, Kuchel GA. Functional impact of relative versus absolute sarcopenia in healthy older women. J Am Geriatr Soc. 2007;55:1712–1719. [DOI] [PubMed] [Google Scholar]
- 25. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985). 2000;88:1321–1326. [DOI] [PubMed] [Google Scholar]
- 26. Newman AB, Haggerty CL, Goodpaster B, et al. ; Health Aging And Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. [DOI] [PubMed] [Google Scholar]
- 27. Ishizaki T, Furuna T, Yoshida Y, et al. ; TMIG-LISA Research Group. Declines in physical performance by sex and age among nondisabled community-dwelling older Japanese during a 6-year period. J Epidemiol. 2011;21:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans WJ, Paolisso G, Abbatecola AM, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. :10.1007/s10522-010-9297-0 [DOI] [PubMed] [Google Scholar]
- 29. Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. [DOI] [PubMed] [Google Scholar]
- 30. Cummings SR, Palermo L, Browner W, et al. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA. 2000;283:1318–1321. [DOI] [PubMed] [Google Scholar]
- 31. Fielding RA. The role of progressive resistance training and nutrition in the preservation of lean body mass in the elderly. J Am Coll Nutr. 1995;14:587–594. [DOI] [PubMed] [Google Scholar]
- 32. Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA. 1994;272:1909–1914. [DOI] [PubMed] [Google Scholar]
- 33. Hellebrandt FA, Houtz SJ. Mechanisms of muscle training in man: experimental demonstration of the overload principle. Phys Ther Rev. 1956;36:371–383. [DOI] [PubMed] [Google Scholar]
- 34. Sousa N, Mendes R, Abrantes C, Sampaio J. Differences in maximum upper and lower limb strength in older adults after a 12 week intense resistance training program. J Hum Kinet. 2011;30:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985). 1999;86:188–194. [DOI] [PubMed] [Google Scholar]
- 36. Bohannon RW. Is it legitimate to characterize muscle strength using a limited number of measures? J Strength Cond Res. 2008;22:166–173. [DOI] [PubMed] [Google Scholar]
- 37. Bohannon RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther. 1998;11:258–260. [DOI] [PubMed] [Google Scholar]
- 38. Bohannon RW. Dynamometer measurements of grip and knee extension strength: are they indicative of overall limb and trunk muscle strength? Percept Mot Skills. 2009;108:339–342. [DOI] [PubMed] [Google Scholar]
- 39. Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. [DOI] [PubMed] [Google Scholar]
- 41. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. [DOI] [PubMed] [Google Scholar]
- 42. Percheron G, Hogrel JY, Denot-Ledunois S, et al. ; Double-blind placebo-controlled trial. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163:720–727. [DOI] [PubMed] [Google Scholar]
- 43. Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58:1707–1714. :10.1111/j. 1532-5415.2010.03019.x [DOI] [PubMed] [Google Scholar]
- 44. Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008. [DOI] [PubMed] [Google Scholar]
- 45. Bunout D, Barrera G, Leiva L, et al. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41:746–752. [DOI] [PubMed] [Google Scholar]
- 46. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20:315–322. [DOI] [PubMed] [Google Scholar]
- 47. Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 2010;58:2063–2068. :10.1111/j.1532-5415.2010.03142.x [DOI] [PubMed] [Google Scholar]
- 48. Moreira-Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann Nutr Metab. 2009;54:291–300. [DOI] [PubMed] [Google Scholar]
- 49. Close GL, Leckey J, Patterson M, et al. The effects of vitamin D(3) supplementation on serum total 25[OH]D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47:692–696. :10.1136/bjsports-2012-091735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


