Abstract
Evidence-based guidelines for long-term follow-up of early-stage breast cancer patients developed by oncology societies in the United States and Europe recommend that breast cancer survivors undergo regular evaluation with history and physical examination, as well as annual mammography. Routine blood tests, circulating tumor markers, and/or surveillance imaging studies beyond mammography are not recommended in the absence of concerning symptoms or physical examination findings because of lack of supportive clinical evidence. Despite these guidelines, studies have shown that 20% to 40% of oncologists assess serum tumor markers as part of routine monitoring of early-stage breast cancer patients. As part of efforts to both address the financial challenges confronting the health-care system and optimize patient outcomes, the American Society of Clinical Oncology’s Cost of Care Task Force identified adherence to breast cancer surveillance guidelines as an opportunity to improve care and reduce cost. However, these recommendations are based on trials done in an era of outdated technology and limited therapeutic options. It is possible that recent improvements in diagnostics and treatments could make earlier detection of recurrent disease important for improving both survival and quality of life outcomes. Research is necessary to further inform optimal breast cancer follow-up strategies, which could impact these recommendations. At this time, outside of well-conducted clinical trials, there is no role for ordering routine serial blood or imaging tests in monitoring for recurrence in early-stage breast cancer patients.
In a 2009 editorial in the New England Journal of Medicine addressing medicine’s ethical responsibility in health-care reform, medical specialties were challenged with creating a “Top Five” list of commonly ordered diagnostic tests or treatments that are expensive but lack evidence of meaningful benefit to patients (1). The American Society of Clinical Oncology (ASCO) Cost of Care Task Force subsequently developed a Top Five list of cancer practices that are frequently used but have not been proven to promote quality patient care or improve outcomes (2). The task force identified lack of adherence to breast cancer surveillance guidelines as one of these Top Five practices in oncology. In particular, serial assessment of blood-based and/or radiographic imaging tests as part of routine monitoring of early-stage breast cancer patients, performed by many oncologists, is not supported by clinical evidence and is specifically recommended against in ASCO clinical practice guidelines (3,4). This commentary focuses on the available clinical evidence underlying this recommendation, which is applicable to asymptomatic patients with breast cancer who have no evidence of persistent disease after primary therapy and who are undergoing monitoring for occult relapse (Figure 1).
Figure 1.
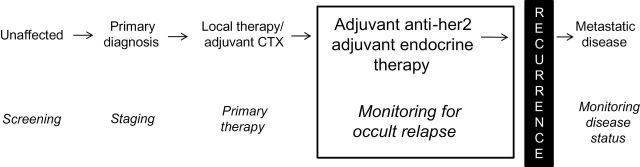
Schematic depicting the different phases of treatment and monitoring for patients before and after breast cancer diagnosis. Monitoring for occult relapse, the phase highlighted in the box, is the focus of this commentary. CTX = chemotherapy; Her2 = human epidermal growth factor receptor 2.
Current Recommendations: Routine Assessment of Breast Cancer Patients After Primary Therapy
The recently published ASCO guidelines update for follow-up and management of early-stage breast cancer recommend that all patients undergo regular evaluation with history and physical examination, as well as annual mammography (4). Routine blood tests and/or surveillance imaging studies beyond mammography are not recommended in the absence of concerning symptoms or physical examination findings. In 2007, ASCO published specific recommendations against routine assessment of tumor markers for detection of recurrence of disease after primary therapy in asymptomatic patients (3). Other guideline development groups, including the National Comprehensive Cancer Network, have also concluded that, based on lack of evidence of survival or quality-of-life benefit, the routine use of blood tests and nonbreast radiologic imaging in the asymptomatic early-stage breast cancer patient is not recommended (8–10).
The Evidence Supporting the Guidelines
Fundamentally, there are two reasons to conduct surveillance testing of women who are free of disease and asymptomatic after primary and adjuvant therapy for breast cancer: improved overall survival (OS) or quality of life (QOL). One might hope that early detection of occult metastases, with intervention before development of symptoms, might lead to longer survival. Even if OS is not improved, QOL might be improved by either accurately reassuring patients that they are free of disease or, alternatively, by detecting disease before development of symptoms and, by virtue of early intervention, delaying the time to development of symptoms.
Several studies have evaluated the role of intensive surveillance after primary breast cancer therapy (Table 1). None supports intensive surveillance to accomplish either of these two potential outcomes. Two large, prospective, randomized studies performed in Italy in the late 1980s investigated the impact of more intensive vs standard, symptom-based follow-up strategies on OS. In the Gruppo Interdisciplinare Valutazione Interventi in Oncologia (GIVIO) trial, 1320 patients were randomly assigned to intensive surveillance vs usual care (11). The intensive surveillance group underwent physical examination and liver function tests every 3 months, chest x-ray every 6 months, and bone scan, liver ultrasound, and mammography annually. The control group underwent only physical examination and mammography. After a median follow-up of 71 months, there was no difference in OS between the two groups. In the Italian National Research Council Project on Breast Cancer Follow-up trial, 1243 patients were randomized to clinical vs intensive follow-up (12,13). The clinical follow-up group underwent physical examination every 3 to 6 months and annual mammogram, whereas the intensive follow-up group also underwent chest x-ray and bone scan every 6 months. Five and 10-year OS rates were similar between the two groups. Although these trials were large, randomized, and had prolonged follow-up, they used imaging modalities with low sensitivity, set prolonged time periods between imaging assessments, and did not include assessments of tumor markers.
Table 1.
Randomized clinical trials of intensive surveillance after primary breast cancer therapy*
| Trial (reference) | No. of patients | Surveillance (randomized to the listed evaluation) | Overall survival | Quality of life |
|---|---|---|---|---|
| GIVIO (11) | 1320 | 1) Clinic visits, alkaline phosphatase, GGT every 3 months CXR every 6 months Bone scan, liver US every 1 year 2) Clinic visits every 3 months |
No difference at 71 months | No difference |
| Del Turco et al., Palli et al. (12,13) | 1243 | 1) Clinic visit every 3–6 months CXR, bone scan every 6 months 2) Clinic visit every 3–6 months |
No difference at 5 or 10 years | Not assessed |
| Kokko et al. (14) | 472 | 1) Clinic visits every 3 months 2) Clinic visits every 6 months 3) Clinic visits, lab tests (blood tests, ESR, liver tests), CA15-3 every 3 months CXR every 6 months Bone scan and liver US every 2 years 4) Clinic visits, lab tests (blood tests, ESR, liver tests), CA15-3, CXR every 6 months Bone scan and liver US every 2 years |
No difference at 4.2 years | Not assessed |
* CXR = chest x-ray; ESR = erythroid sedimentation rate; GGT = gamma-glutamyltransferase; GIVIO = Gruppo Interdisciplinare Valutazione Interventi in Oncologia; US = ultrasound.
A Finnish study of 472 early-stage breast cancer patients conducted in the early 1990s randomly assigned patients to follow-up every 3 vs every 6 months after primary treatment and to routine use of diagnostic examinations vs usual care (14). The routine diagnostic evaluations included laboratory studies (including the breast cancer tumor marker CA15-3 at each visit) and imaging tests (chest x-ray every 6 months and liver ultrasound and bone scan every other year). After a mean follow-up of 4.2 years, there was no difference in disease-free survival or OS based on frequency or intensity of follow-up (14). Limitations included a relatively small sample size and, more important, short follow-up and therefore very few events in any arm, as well as use of imaging tests with low sensitivity.
An additional retrospective, observational study examined more intensive vs less intensive follow-up for 129 patients with recurrent disease. The individuals had been enrolled in a prospective database before relapse and were categorized into two groups based on the method of detection of their recurrent disease: minimalist or intensive (15). The minimalist group (79% of subjects) included patients diagnosed with disease recurrence because of symptoms, physical examination, or mammography. The intensive group (21% of subjects) included those who were asymptomatic at the time of diagnosis but were found to have recurrent disease based on bone scan, computed tomography scan, chest x-ray, or elevation in liver function test or tumor marker. There was no difference in time to detection of disease or OS between groups.
Taken together, these randomized and observational studies indicate that intensive surveillance offers no substantial survival advantage after primary therapy for early-stage breast cancer. Indeed, a 2005 Cochrane Collaboration–sponsored meta-analysis of these trials also found no benefit in OS or disease-free survival from intensive surveillance (10).
Improved Diagnostic Technology in Early-Stage Breast Cancer
Although prospective randomized trials have failed to demonstrate improved OS or QOL through surveillance of asymptomatic patients, the majority of these studies were performed in the remote past before advances in diagnostics and therapies. Subsequently, several diagnostic technologies have been developed regarding both circulating protein biomarkers and cancer imaging modalities.
Circulating Biomarkers
The Italian studies did not include serial circulating tumor markers in their trials. Several uses of circulating tumor markers in breast cancer have been proposed, including detecting occult relapse after primary therapy (3,17). Circulating MUC-1 antigen can be detected in the peripheral blood using either the CA 15-3 or CA 27.29 assay. In addition, carcinoembryonic antigen (CEA) can be detected in the peripheral blood at the time of recurrence, although less frequently than the MUC-1 antigen. CA-125, although traditionally used for monitoring ovarian cancer, can also be detected in the serum of patients with breast cancer (18).
CA15-3 and CEA can detect breast cancer relapse before clinical and radiological evidence of disease, and the simultaneous use of both markers can increase the sensitivity for early diagnosis of metastases (3). CA 15-3 has a slightly higher sensitivity for detection of recurrence than CEA (63%–73% vs 46%–53%) (3). Both markers have been shown to be more useful in detection of visceral recurrence, in contrast with locoregional recurrence (19). One difficulty with using tumor markers for assessment is the inconsistent definition of “positive.” For example, a positive value has been defined as an increase above an absolute threshold, as a percentage increase compared with a prior value, and as a combination of the two methods. In addition, some studies require a repeat assessment a few weeks after the original measurement for confirmation of the increase.
A number of prospective studies have evaluated serum tumor marker concentrations for monitoring for occult recurrence of disease in patients with early-stage breast cancer. In one study of 924 patients, the sensitivity and specificity of CA15-3 ever being elevated above the study’s predefined cutoff in a patient who developed distant disease recurrence were 40.2% and 95.8%, respectively (20). In a separate study of 243 women, CA15-3 was elevated on at least one occasion in 36% of patients with relapsed disease (21). In yet another study of 1023 patients who were followed with serial serum assessment of CA15-3, 54% had an elevation in tumor marker before the diagnosis of distant recurrence, with a lead time of 4.2 months (19). Specificity for subsequent breast cancer metastasis over the ensuing 9 months was 99%. Similar findings were found for CA27.29, with a sensitivity of 57.7%, specificity of 97.9%, positive predictive value of 83.3%, negative predictive value of 92.6%, and mean lead time of 5.3 months (22).
Diagnostic Imaging
Although the existing data do not support surveillance for occult metastases, substantial evidence supports routine annual mammographic evaluation for detection of in-breast recurrence or new primary breast cancers in the follow-up of patients diagnosed with early-stage breast cancer. A recent meta-analysis of 13 studies evaluating routine follow-up strategies focusing on the early detection of curable recurrences demonstrated an impact on survival for early detection of a local recurrence of breast cancer as compared with late detection (hazard ratio (HR) = 1.68; 95% confidence interval (CI) = 1.48 to 1.91) (26). Survival was better when the recurrence was found by mammography instead of physical examination or in patients without symptoms as compared with those with symptoms (HR = 2.44, 95% CI = 1.78 to 3.35; HR = 1.56, 95% CI = 1.36 to 1.79, respectively). In contrast, regularly scheduled chest radiographs, bone scans, and abdominal ultrasounds did not change survival outcomes in the two randomized trials of intensive surveillance conducted in in Italy in the 1980s (11–13).
Impact of Treatment Initiated at the Time of Tumor Marker Elevation
A few studies have attempted to evaluate the impact of early initiation of treatment on long-term disease outcomes in asymptomatic breast cancer patients with suspicion of recurrence based on serum tumor markers and clinically and radiographically undetectable disease. In one study of 61 patients with increasing serum levels of mucin-like carcinoma-associated antigen but no evidence of metastatic disease, subjects were randomized to tamoxifen at the time of tumor marker evaluation vs delayed until evidence of disease relapse (27,28). Although 1-year evaluation showed a statistically significant difference in relapse rates favoring the tamoxifen arm (24.1% vs 0%; P = .01), 5-year follow-up data revealed a less substantial difference (25.8% vs. 17.4%; P = .46). Patterns of relapse were similar between the two arms. In a second trial, patients underwent mastectomy followed by intensive monitoring every 4 to 6 months, including assessment of tumor markers (29,30). Patients (n = 109) with two or more consecutive elevated tumor marker values and without radiographic evidence of recurrent disease were assigned to either early salvage treatment (n = 36) or delayed salvage treatment after imaging confirmation of relapse (n = 32). There was a statistically significant difference in average time from tumor marker increase to definitive image-detected relapse between the two groups (early: 17.3 months; delayed: 2.9 months; P < .001) and 7-year survival from time of mastectomy (early: 42%; delayed: 19%; P = .009). The authors concluded that “tumor marker guided” early salvage therapy resulted in substantial improvements in survival.
Frequency of Serum Tumor Marker Imaging Testing in Early-Stage Breast Cancer
In a substudy of CALGB trial 8541 in women with stage II breast cancer treated with adjuvant chemotherapy, 37% of patients reported having tumor markers assessed at least once in the previous year during routine follow-up, and 11% to 18% reported undergoing imaging studies (computed tomography and bone scans) (5). Similar findings were reported in a Surveillance, Epidemiology, and End Results (SEER)–Medicare data analysis of patients diagnosed in the 1990s, with even higher rates reported for patients who were followed by a medical oncologist (6). In addition, a SEER–Medicare study evaluating patients with stage I and stage II breast cancer diagnosed between 1998 and 2003 found that 40% of patients had at least one high technology radiologic imaging test between 1 and 3 years after diagnosis, compared with 25% of control patients (16). Finally, a survey in 2007 asked 900 US oncologists how they would monitor a patient with stage IIIA breast cancer after completion of chemotherapy (7). Twenty-two percent stated they would order CA15-3 at every follow-up visit, and 16% reported that they would check chest radiographs every 6 months. Interestingly, although all guidelines support annual mammography in this population, only 82% of oncologists responded that they would order them.
Potential Adverse Effects of Intensive Follow-up of Early-Stage Breast Cancer
Radiation Risks
The estimated attributable risk for cancer from computed tomography scans is 1.5% to 2% (31). Indeed, the Institute of Medicine has reported that exposure to ionizing radiation is one of the two environmental factors most strongly associated with development of breast cancer (32). Therefore, in a population of breast cancer survivors with a high likelihood of cure of their disease, there is concern about unnecessary exposure to radiation that could increase risk of malignancy.
False-Positive Results
False-positive results may precipitate extensive and expensive diagnostic workups and cause considerable anxiety. Unnecessary testing can lead to harm through unneeded invasive procedures, additional radiation exposure, potential overtreatment, and misdiagnosis. False-positive elevations of tumor markers have been reported in 3% of patients with nonmalignant conditions, including autoimmune and liver disease (19,21,33). Elevation of a tumor marker generally leads to additional noninvasive and invasive interventions, including multiple imaging studies and biopsies, to identify the reason for the increase in tumor marker concentration.
As described above, several studies have demonstrated a 4- to 6-month lag time between tumor marker elevation and subsequent development of clinically evident disease recurrence (19). Therefore, a false-positive test result can also result in possible inappropriate treatment that is initiated based on the result, without evidence of recurrent disease identified.
If early initiation of therapy does not improve OS, then treatment of asymptomatic recurrent disease is the equivalent of “upfront palliation.” This approach has the risk of causing treatment-related symptoms in an otherwise asymptomatic patient to delay cancer-related symptoms without increasing survival. If initiation of cytotoxic chemotherapy is required, this approach is questionable given the high likelihood of treatment-related toxicity, but it may be acceptable with newer less toxic endocrine or targeted therapies.
Quality of Life
Intensive follow-up may impact breast cancer patients’ QOL, including psychological distress and anxiety around the testing encounter itself as well as false-positive results. In contrast, some studies suggest that breast cancer survivors can obtain reassurance from the intensive testing. The GIVIO group evaluated the impact of intensive vs standard surveillance for breast cancer recurrence on health-related QOL (11). No difference in health-related QOL (overall health and QOL perception, emotional well-being, body image, social functioning, symptoms, and satisfaction with care) was detected between the two groups. The GIVIO trial did not find that intensive surveillance provided increased reassurance, nor did it find that frequent testing increased stress and anxiety.
Multiple studies have examined patient preferences about routine surveillance. A UK study of conventional follow-up visit intervals vs less frequent clinic visits with a back-up hotline support if symptoms arose found that twice as many patients expressed a preference for reducing rather than increasing follow-up visit frequency (34). A Dutch study of patients’ needs and preferences in routine follow-up of early breast cancer found, not surprisingly, that women tend to vary in their views of different aspects of follow-up (35). In general, patients preferred additional investigations (such as x-ray and blood tests) to be part of routine follow-up visits. Less satisfaction with interpersonal aspects, higher scores on a depression scale, and greater fear of recurrence were related to stronger preferences for a more intensive follow-up schedule. A 2005 German survey of 801 breast cancer patients regarding surveillance practices and views indicated that one-third to one-half of patients asked for more laboratory and imaging procedures during their follow-up exams (36). More than 80% of respondents stated that intensification of follow-up was associated with an increased sense of security, whereas approximately 10% felt it led to an increased sense of fear. Up to one-third of patients indicated that they would prefer not to constantly analyze the disease. More than 80% of responders stated that regularly scheduled visits during follow-up care were important to come to terms with the disease.
Cost of Screening for Occult Metastases
Regardless of whether the patient remains free of suggestive findings or has a false- or true-positive finding, intensive follow-up of breast cancer survivors adds to the burgeoning cost of medical care. The current financial impact of ongoing intensive follow-up of breast cancer survivors is unknown. In 1990, costs of a minimalist surveillance scheme vs an intensive surveillance scheme (similar to the arms of the GIVIO trial) were estimated for the United States. The minimalist approach was suggested to result in a $636 million cost savings in 1990 (37). Implementing routine intensive follow-up not only consumes more resources but also leads to additional testing because of equivocal results of tests performed by protocol. A follow-up that is not guideline compliant costs 2.2 to 3.6 times more than a guideline-compliant follow-up (38). In the previously described Finnish study, the mean cost of follow-up per patient differed by 2.2-fold between those with the most- vs those with the least-intense follow-up (14). Studies are underway in the United States to determine the current frequency and intensity of testing by oncologists and its financial impact.
Could Earlier Initiation of Modern Therapies for Metastatic Disease Impact Outcomes?
We have indicated that studies conducted to date fail to demonstrate that intensive surveillance after primary therapy for early-stage breast cancer positively impacts QOL or OS. However, there are important limitations to the prior studies that must be considered. First, very few prospective and/or randomized studies have been conducted; they are all small with relatively low power, and all were performed in the remote past. No study has evaluated whether there are certain cohorts of survivors who might benefit from more intensive surveillance—for example, based on intrinsic subtype (39). In this regard, those with estrogen receptor–, progesterone receptor–, and Her2–negative breast cancer (so-called “triple negative”) are at high risk of recurrence and may benefit from this strategy but were likely poorly represented in the large previously conducted trials given the low incidence of the disease subtype.
Only one of the three prospective, randomized, controlled trials incorporated serum tumor markers in the serial assessment. Importantly, the two large trials were performed in an era before the development of more sensitive imaging modalities and many of the currently available anticancer therapies. Many of the new treatment regimens are more effective and less toxic than the older medications; in addition, the available supportive care therapies are also substantially improved. As a result of these limitations, at least one group, the European Group on Tumor Markers, supports monitoring using serial CA15-3 and CEA every 2 to 4 months during the initial 5 years and then less frequently thereafter for early detection of recurrence, although the group acknowledges that the impact of these results on patient outcome is unclear (40).
The rationale for monitoring of asymptomatic women is that the efficacy of breast cancer treatment is better, at least in theory, when tumor burden is lowest (41,42). Adjuvant systemic therapy is given to eliminate undetectable micrometastatic disease, decreasing the risk of distant relapses and increasing OS (43). There are no statistically significant data that demonstrate that early treatment of relapsed breast cancer will improve clinical outcomes; however, it has been hypothesized that patients with early and limited metastatic recurrence may be curable (44,45).
Considerations for a Prospective, Randomized, Controlled Trial to Evaluate the Efficacy of Serum Tumor Marker Testing on Patient QOL and Survival
SWOG, in partnership with members of the National Cancer Institute’s Clinical Trials Network, is currently designing a prospective clinical trial evaluating the impact of serial testing of tumor markers in patients with stage II and III breast cancer to determine whether early initiation of therapy at the time of tumor marker–detected relapse can result in an improvement in OS. The current trial design calls for testing serum tumor markers at a central laboratory every 3 to 6 months for 5 years, with patients and physicians blinded to the assay results. In cases where a tumor marker became elevated during follow-up, the patient would be randomly assigned either to reporting the results to the treating physician (unblinded) or to no reporting (blinded). In the unblinded group, patients with confirmed marker elevation would undergo confirmation of metastatic disease, and physicians and patients would be strongly recommended to initiate or change therapy. Patients randomly assigned to the blinded group would continue usual clinical follow-up and undergo evaluation and treatment at the time of clinical evidence of disease recurrence.
There are a number of hurdles to face when designing and implementing such a trial. A very large sample size is required for a number of reasons: 1) a minority of patients will experience disease recurrence, and 2) only a portion of patients who experience disease recurrence will have a tumor marker elevation. A large sample size substantially increases the cost of conducting the trial, in addition to impacting the ability to meet accrual goals and comply with study procedures. Potential subjects may be reluctant to be randomized to not being told if their tumor marker is elevated. Many physicians still routinely assess tumor markers and/or obtain surveillance imaging studies in their patients with early-stage breast cancer and may be unwilling to forgo assessment in trial participants. Therefore, patient and physician education about the known risks and benefits of tumor marker assessment will be imperative for maximizing the feasibility of such a trial.
Despite these potential limitations, conduct of this trial could have far-reaching benefits beyond evaluating the impact of serial tumor marker assessment on breast cancer outcomes. Enrollment of a large number of patients with breast cancer could yield important information about late effects of cancer diagnosis and treatment, including long-term physical and emotional consequences and the impact on QOL (46). In addition, the associated biorepository of serial serum samples could be invaluable for evaluating and validating prospective new biomarkers that are more sensitive and specific for the diagnosis and monitoring of breast cancer.
Summary
It is increasingly recognized that cancer survivorship is an area for which further research is needed to develop evidence-based, comprehensive, and compassionate follow-up care (47). A primary goal of breast cancer follow-up is the early recognition and treatment of potentially curable disease recurrence. The growing number of breast cancer survivors provides a strong reason for investigating how to optimally monitor for and treat recurrence to maximize benefit to the patient and reduce potential harms. Detection of an isolated recurrence may lead to curative treatment, but the benefit of early detection of distant metastases has not been established. To date, there is no evidence from randomized trials that earlier detection of asymptomatic, metastatic breast cancer recurrence improves survival. Research is necessary to further inform optimal breast cancer follow-up strategies, which could impact these recommendations. At this time, outside of well-conducted clinical trials, there is no role for ordering routine serial blood or imaging tests in monitoring for recurrence in early-stage breast cancer patients.
Funding
This work was supported by the Breast Cancer Research Foundation to J.R.G. and D.F.H.; and by the Fashion Footwear Association of New York/Shoes on Sale to D.F.H.
References
- 1. Brody H. Medicine’s ethical responsibility for health care reform—the top five list. N Engl J Med. 2010;362(4):283–285. [DOI] [PubMed] [Google Scholar]
- 2. Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724. [DOI] [PubMed] [Google Scholar]
- 3. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. [DOI] [PubMed] [Google Scholar]
- 4. Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31(7):961–965. [DOI] [PubMed] [Google Scholar]
- 5. Hensley ML, Dowell J, Herndon JE. 2nd, et al. Economic outcomes of breast cancer survivorship: CALGB study 79804. Breast Cancer Res Treat. 2005;91(2):153–161. [DOI] [PubMed] [Google Scholar]
- 6. Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25(9):1074–1081. [DOI] [PubMed] [Google Scholar]
- 7. Foster JA, Abdolrasulnia M, Doroodchi H, McClure J, Casebeer L. Practice patterns and guideline adherence of medical oncologists in managing patients with early breast cancer. J Natl Compr Canc Netw. 2009;7(7):697–706. [DOI] [PubMed] [Google Scholar]
- 8. Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9(2):136–222. [DOI] [PubMed] [Google Scholar]
- 9. Grunfeld E, Dhesy-Thind S, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update). CMAJ. 2005;172(10):1319–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rojas MP, Telaro E, Russo A, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;2005(1):CD001768. [DOI] [PubMed] [Google Scholar]
- 11. GIVIO Investigators. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. JAMA. 1994;271(20):1587–1592. [DOI] [PubMed] [Google Scholar]
- 12. Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994;271(20):1593–1597. [DOI] [PubMed] [Google Scholar]
- 13. Palli D, Russo A, Saieva C, et al. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA. 1999;281(17):1586. [DOI] [PubMed] [Google Scholar]
- 14. Kokko R, Hakama M, Holli K. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat. 2005;93(3):255–260. [DOI] [PubMed] [Google Scholar]
- 15. Joseph E, Hyacinthe M, Lyman GH, et al. Evaluation of an intensive strategy for follow-up and surveillance of primary breast cancer. Ann Surg Oncol. 1998;5(6):522–528. [DOI] [PubMed] [Google Scholar]
- 16. Panageas KS, Sima CS, Liberman L, Schrag D. Use of high technology imaging for surveillance of early stage breast cancer. Breast Cancer Res Treat. 2012;131(2):663–670. [DOI] [PubMed] [Google Scholar]
- 17. McCarthy NJ, Swain SM. Tumor markers: should we or shouldn’t we? Cancer J. 2001;7(3):175–177. [PubMed] [Google Scholar]
- 18. Perey L, Hayes DF, Tondini C, et al. Elevated CA125 levels in patients with metastatic breast carcinoma. Br J Cancer. 1990;62(4):668–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molina R, Zanon G, Filella X, et al. Use of serial carcinoembryonic antigen and CA 15.3 assays in detecting relapses in breast cancer patients. Breast Cancer Res Treat. 1995;36(1):41–48. [DOI] [PubMed] [Google Scholar]
- 20. Mariani L, Miceli R, Michilin S, Gion M. Serial determination of CEA and CA 15.3 in breast cancer follow-up: an assessment of their diagnostic accuracy for the detection of tumour recurrences. Biomarkers. 2009;14(2):130–136. [DOI] [PubMed] [Google Scholar]
- 21. Kokko R, Holli K, Hakama M. Ca 15-3 in the follow-up of localised breast cancer: a prospective study. Eur J Cancer. 2002;38(9):1189–1193. [DOI] [PubMed] [Google Scholar]
- 22. Chan DW, Beveridge RA, Muss H, et al. Use of Truquant BR radioimmunoassay for early detection of breast cancer recurrence in patients with stage II and stage III disease. J Clin Oncol. 1997;15(6):2322–2328. [DOI] [PubMed] [Google Scholar]
- 23. Hayes DF, Smerage J. Is there a role for circulating tumor cells in the management of breast cancer? Clin Cancer Res. 2008;14(12):3646–3650. [DOI] [PubMed] [Google Scholar]
- 24. Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–695. [DOI] [PubMed] [Google Scholar]
- 25. Rack BK, Schindlbeck C, Andergassen U, et al. Use of circulating tumor cells (CTC) in peripheral blood of breast cancer patients before and after adjvuant chemotherapy to predict risk for relapse: The SUCCESS trial. J Clin Oncol. 2010;28(Suppl):abstract 1003. [Google Scholar]
- 26. Lu WL, Jansen L, Post WJ, et al. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009;114(3):403–412. [DOI] [PubMed] [Google Scholar]
- 27. Kovner F, Merimsky O, Hareuveni M, Wigler N, Chaitchik S. Treatment of disease-negative but mucin-like carcinoma-associated antigen-positive breast cancer patients with tamoxifen: preliminary results of a prospective controlled randomized trial. Cancer Chemother Pharmacol. 1994;35(1):80–83. [DOI] [PubMed] [Google Scholar]
- 28. Merimsky O, Kovner F, Inbar M, et al. Tamoxifen for disease-negative but MCA-positive breast cancer patients. Oncol Rep. 1997;4(4):843–847. [DOI] [PubMed] [Google Scholar]
- 29. Nicolini A, Carpi A, Michelassi C, et al. “Tumour marker guided” salvage treatment prolongs survival of breast cancer patients: final report of a 7-year study. Biomed Pharmacother. 2003;57(10):452–459. [DOI] [PubMed] [Google Scholar]
- 30. Nicolini A, Anselmi L, Michelassi C, Carpi A. Prolonged survival by “early” salvage treatment of breast cancer patients: a retrospective 6-year study. Br J Cancer. 1997;76(8):1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 32. Smith-Bindman R. Environmental causes of breast cancer and radiation from medical imaging: findings from the Institute of Medicine report. Arch Intern Med. 2012;172(13):1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colomer R, Ruibal A, Genolla J, et al. Circulating CA 15-3 levels in the postsurgical follow-up of breast cancer patients and in non-malignant diseases. Breast Cancer Res Treat. 1989;13(2):123–133. [DOI] [PubMed] [Google Scholar]
- 34. Gulliford T, Opomu M, Wilson E, Hanham I, Epstein R. Popularity of less frequent follow up for breast cancer in randomised study: initial findings from the hotline study. British Med J. 1997;314(7075):174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Bock GH, Bonnema J, Zwaan RE, et al. Patient’s needs and preferences in routine follow-up after treatment for breast cancer. Br J Cancer. 2004;90(6):1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hans-Joachim S, Dorit L, Petra S, et al. The reality in the surveillance of breast cancer survivors-results of a patient survey. Breast Cancer (Auckl). 2008;1:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schapira DV. Breast cancer surveillance—a cost-effective strategy. Breast Cancer Res Treat. 1993;25(2):107–111. [DOI] [PubMed] [Google Scholar]
- 38. Mille D, Roy T, Carrere MO, et al. Economic impact of harmonizing medical practices: compliance with clinical practice guidelines in the follow-up of breast cancer in a French Comprehensive Cancer Center. J Clin Oncol. 2000;18(8):1718–1724. [DOI] [PubMed] [Google Scholar]
- 39. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. [DOI] [PubMed] [Google Scholar]
- 40. Molina R, Barak V, van Dalen A, et al. Tumor markers in breast cancer—European Group on Tumor Markers recommendations. Tumour Biol. 2005;26(6):281–293. [DOI] [PubMed] [Google Scholar]
- 41. De Vita VT. The relationship between tumor mass and resistance to chemotherapy. Implications for surgical adjuvant therapy of cancer. Cancer. 1983;51(7):1209–1220. [DOI] [PubMed] [Google Scholar]
- 42. Skipper HE. Kinetics of mammary tumor cell growth and implications for therapy. Cancer. 1971;28(6):1479–1499. [DOI] [PubMed] [Google Scholar]
- 43. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 44. Hortobagyi G. Can we cure limited metastatic breast cancer? J Clin Oncol. 2002;20(3):620–623. [DOI] [PubMed] [Google Scholar]
- 45. Buzdar AU, Blumenschein GR, Montague ED, et al. Combined modality approach in breast cancer with isolated or multiple metastases. Am J Clin Oncol. 1984;7(1):45–50. [DOI] [PubMed] [Google Scholar]
- 46. Ganz PA, Land SR, Antonio C, et al. Cancer survivorship research: the challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J Cancer Surviv. 2009;3(3):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: a new challenge in delivering quality cancer care. J Clin Oncol. 2006;24(32):5101–5104. [DOI] [PubMed] [Google Scholar]


