Microdissection of maize leaf primordia reveals that genes utilized during initiation of leaves and branches are redeployed at the ligule blade-sheath boundary.
Abstract
Development of multicellular organisms proceeds via the correct interpretation of positional information to establish boundaries that separate developmental fields with distinct identities. The maize (Zea mays) leaf is an ideal system to study plant morphogenesis as it is subdivided into a proximal sheath and a distal blade, each with distinct developmental patterning. Specialized ligule and auricle structures form at the blade-sheath boundary. The auricles act as a hinge, allowing the leaf blade to project at an angle from the stem, while the ligule comprises an epidermally derived fringe. Recessive liguleless1 mutants lack ligules and auricles and have upright leaves. We used laser microdissection and RNA sequencing to identify genes that are differentially expressed in discrete cell/tissue-specific domains along the proximal-distal axis of wild-type leaf primordia undergoing ligule initiation and compared transcript accumulation in wild-type and liguleless1-R mutant leaf primordia. We identified transcripts that are specifically upregulated at the blade-sheath boundary. A surprising number of these “ligule genes” have also been shown to function during leaf initiation or lateral branching and intersect multiple hormonal signaling pathways. We propose that genetic modules utilized in leaf and/or branch initiation are redeployed to regulate ligule outgrowth from leaf primordia.
INTRODUCTION
Morphogenesis proceeds from positional determinants that establish the body axis. Plant morphogenesis relies on positional information in part because plant cells do not move, but instead respond in place to diverse morphogenetic signals. Shoot apical meristems (SAMs), which contain populations of plant stem cells that produce lateral organs such as leaves, are the primary starting points for establishing positional information in the plant shoot. Once initiated from the SAM, leaf primordia acquire distinct polarity relative to the meristem: Cells in the leaf initial are either proximal or distal to the SAM and are either adjacent to (adaxial) or away from (abaxial) the meristem. Cell and tissue differentiation proceeds along these proximal-distal and adaxial-abaxial axes to generate anatomically and functionally discrete regions of the leaf. The positional cues required to initiate and maintain these axial domains and their boundaries during plant development are largely unknown.
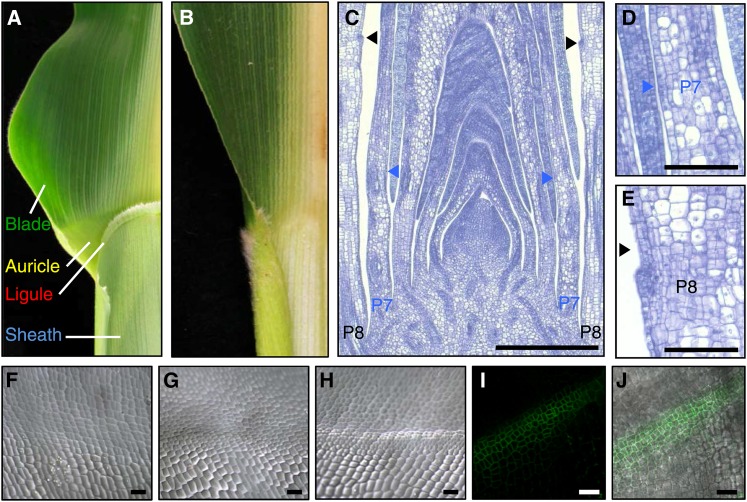
The maize (Zea mays) leaf is particularly useful to study positional information because it is subdivided into unique regions along its relatively linear axis. Maize leaves have a proximal sheath and distal blade, separated by a specialized fringe-like ligule adjacent to an auricle (Figure 1A). Together, the ligule and auricle act as a hinge, extending the leaf blade at an angle at the blade-sheath boundary. The first morphological sign of ligule and auricle development is the formation of the preligule band (PLB), a uniquely linear band of smaller cells that runs perpendicular to the proximal-distal axis of the developing leaf (Figures 1C to 1H). The PLB forms by localized anticlinal divisions (new cell walls perpendicular to the existing cell walls) in the adaxial epidermis. PLB cells accumulate the ZmPIN1a auxin efflux transporter at high levels, suggesting a role for auxin in ligule positioning (Moon et al., 2013) (Figures 1I and 1J). Cell divisions associated with the PLB are likely to occur in response to signals that occur earlier in development. Ligule outgrowth then proceeds via periclinal divisions, which are parallel to the leaf surface (Sharman, 1941; Sylvester et al., 1990).
Figure 1.
Morphology and Development of the Maize Ligule.
(A) Mature wild-type maize leaf showing auricle and ligule structures.
(B) Mature lg1-R leaf. Leaves in (A) and (B) have been cut in half along the midrib.
(C) to (E) Lateral longitudinal section through maize shoot apex showing early stages of ligule development. In this example, the PLB is apparent as a band of smaller cells in the adaxial epidermis of the P7 primordium (D). The initiating ligule is apparent as a bump projecting from the plane of the leaf at P8 (E). Arrowheads indicate position of PLB. P indicates plastochron number.
(F) to (H) Scanning electron micrographs of leaf primordia adaxial surfaces showing early stages of ligule development. (F) P5, (G) P6, and (H) P7.
(I) and (J) Confocal images of ZmPIN1a-YFP localization in PLB. (I) ZmPIN1a-YFP fluorescence; (J) bright-field overlay.
Bars = 500 μm in (C), 100 μm in (D) and (E), and 25 μm in (F) to (J).
Genes that play a role in leaf proximal-distal patterning have been identified by analyses of mutations that alter the ligule and leaf angle. One such gene, liguleless1 (lg1) encodes SQUAMOSA PROMOTER BINDING PROTEIN (SBP), which is required for developmental patterning of the blade-sheath boundary (Moreno et al., 1997). Recessive lg1 mutations delete the ligule and auricle; lg1-R leaves are narrower and more upright than wild-type siblings, and the blade-sheath boundary is less distinct (Figure 1B) (Emerson, 1912; Becraft et al., 1990; Sylvester et al., 1990; Foster et al., 2004). knotted1-like homeobox (knox) genes encode transcription factors that function in meristem maintenance and leaf initiation. knotted1 (kn1) transcripts accumulate at high levels in the maize SAM but are restricted from incipient and emerging leaf primordia where auxin levels are high (Jackson et al., 1994; Bolduc et al., 2012a; O’Connor et al., 2014). Dominant maize mutants that ectopically express knox genes displace proximal sheath tissue into the distal blade, whereas mutants that are defective in auxin transport exhibit ectopic accumulation of KNOX proteins and similar proximal-distal leaf patterning defects (Freeling and Hake, 1985; Sinha and Hake, 1990; Fowler and Freeling, 1996; Foster et al., 1999; Tsiantis et al., 1999; Scanlon et al., 2002; Ramirez et al., 2009). These observations support a model whereby KNOX accumulation specifies the proximal sheath compartment of very young primordia, and auxin restricts KNOX protein accumulation from distal leaf domains (Bolduc et al., 2012a). Such antagonism between auxin and KNOX is a module that acts in multiple contexts during plant development (Scanlon, 2003; Hay et al., 2006; Gallavotti et al., 2008; Hay and Tsiantis, 2010).
In this study, we analyzed the transcriptome associated with ligule formation using laser microdissection RNA-sequencing (LM-RNAseq). We quantified transcript accumulation in the PLB and adjacent preblade and presheath regions of wild-type leaf primordia in order to identify candidate genes involved in proximal-distal patterning at the blade-sheath boundary. We also compared transcript accumulation in lg1-R mutants and wild-type siblings to identity genes acting downstream of LG1. We discovered that a suite of genes specifically upregulated in the preligule region is also expressed at developmental boundaries of leaves and branches. These results suggest that the genetic network used to initiate lateral organs is redeployed to make the ligule.
RESULTS AND DISCUSSION
LM-RNAseq of the Primordial Blade-Sheath Boundary
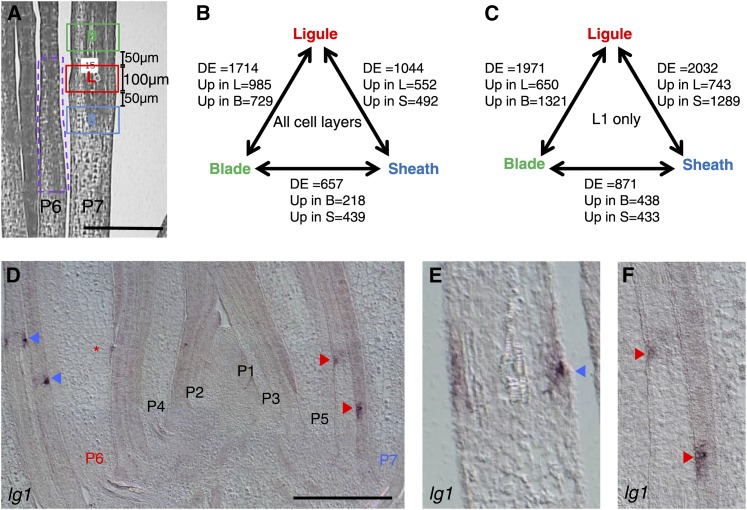
To identify transcripts that are differentially expressed (DE) along the leaf proximal-distal axis, we employed LM-RNAseq of the PLB and adjacent preblade and presheath regions of Plastochron 7 (P7; which describes the primordium that is seven leaves from the SAM) stage leaf primordia (Figure 2A; Supplemental Figure 1, red, green, and blue boxes, respectively). Given that the ligule arises from periclinal divisions within the L1-derived epidermis, we also performed LM-RNAseq of just the adaxial epidermal cell layer in the initiating ligule, preblade, and presheath regions.
Figure 2.
Laser Microdissection of Leaf Primordial Domains.
(A) Scheme for laser microdissection of leaf primordial domains. Preligule tissue (red box) was microdissected from 100-μm-high rectangles, centered on the PLB. Preblade (green) and presheath (blue) tissue was microdissected from 100-μm rectangles 50 μm above and below the preligule selection, respectively. For the comparison of wild-type and lg1-R transcriptomes, tissue between 400 and 900 μm from the base of P6 leaf primordia (purple dashed line) was microdissected from lateral sections.
(B) and (C) Number of DE genes in pairwise comparisons between leaf regions.
(B) Microdissection of all cell layers.
(C) Microdissection of adaxial L1 only.
(D) to (F) lg1 in situ hybridization. Arrowheads indicate PLB at margins. Asterisk indicates preligule region at midrib. P indicates plastochron number.
Bars 300 μm in (A) and 500 μm in (D). B, blade; L, ligule; S, sheath.
An analysis of transcript accumulation within all the microdissected tissue layers found a total of 2359 DE genes, with a false discovery rate of <0.05 (Supplemental Data Set 1). Specifically, 1714 genes were DE between ligule and blade, 1044 genes were DE between ligule and sheath, and 657 genes were DE between blade and sheath (Figure 2B; Supplemental Data Set 2). lg1, which is expressed specifically at the ligule (Moon et al., 2013), served as a useful control to verify tissue specificity of the microdissections. In both epidermal LM as well as microdissections of all cell layers, lg1 transcript accumulation was significantly higher in ligule tissue than in either blade or sheath (all-cell-layers analysis; log fold-change ligule versus sheath = 5.925, log fold-change ligule versus blade = 6.615), indicating that our tissue sampling was accurate (Supplemental Figure 2A). In situ hybridizations confirmed that accumulation of lg1 transcript is restricted to the PLB and the emerging ligule regions (Figures 2D to 2F, arrowheads).
To verify the specificity of our epidermal microdissections, we compared transcript accumulation for several layer-specific genes in epidermis-only microdissections to the data from all cell layers (Supplemental Data Set 3). The genes brown midrib1, Ran BINDING PROTEIN2 (specific to vascular tissues), and Rubisco small subunit are predicted to be expressed only in subepidermal cell layers (Humphreys and Chapple, 2002; Takacs et al., 2012). In our data sets, these transcripts were not represented in epidermis-only captures or were present at low levels relative to the all-cell-layers captures, thus supporting the accuracy of the captures. Epidermis-specific genes are expected to be higher in the epidermis-only microdissections but also represented in the all-cell-layers data, since these microdissections included the epidermis as well as internal cell layers. As predicted, accumulation of epidermal gene transcripts such as Outer Cell Layer 1 (Ingram et al., 2000), Protodermal Factor2-like (Abe et al., 2003), and AT Meristem L1-like (Lu et al., 1996) was higher in the epidermis-only microdissections than in the all-cell-layers microdissections.
An analysis of transcript accumulation in the adaxial epidermis found a total of 3128 DE genes; 1971 genes were DE between ligule and blade, 2032 genes were DE between ligule and sheath, and 871 genes were DE between blade and sheath (Figure 2C; Supplemental Data Set 4). Thus, in both data sets there were more genes DE between ligule and blade and ligule and sheath than between blade and sheath. This might be due to the fact that the ligule is a specialized group of cells that is undergoing more rapid cell division and growth than surrounding cells. Based on these combined data, we constructed lists of genes that are specifically enriched in each leaf region; preblade, preligule, and presheath (Table 1; Supplemental Data Sets 5 and 6).
Table 1. Number of Genes Significantly Upregulated or Downregulated in Specific Leaf Primordial Domains.
| Up in Blade | Up in Ligule | Down in Ligule | Up in Sheath | |
|---|---|---|---|---|
| All cell layers | 218 | 373 | 246 | 439 |
| Adaxial epidermis | 438 | 287 | 778 | 433 |
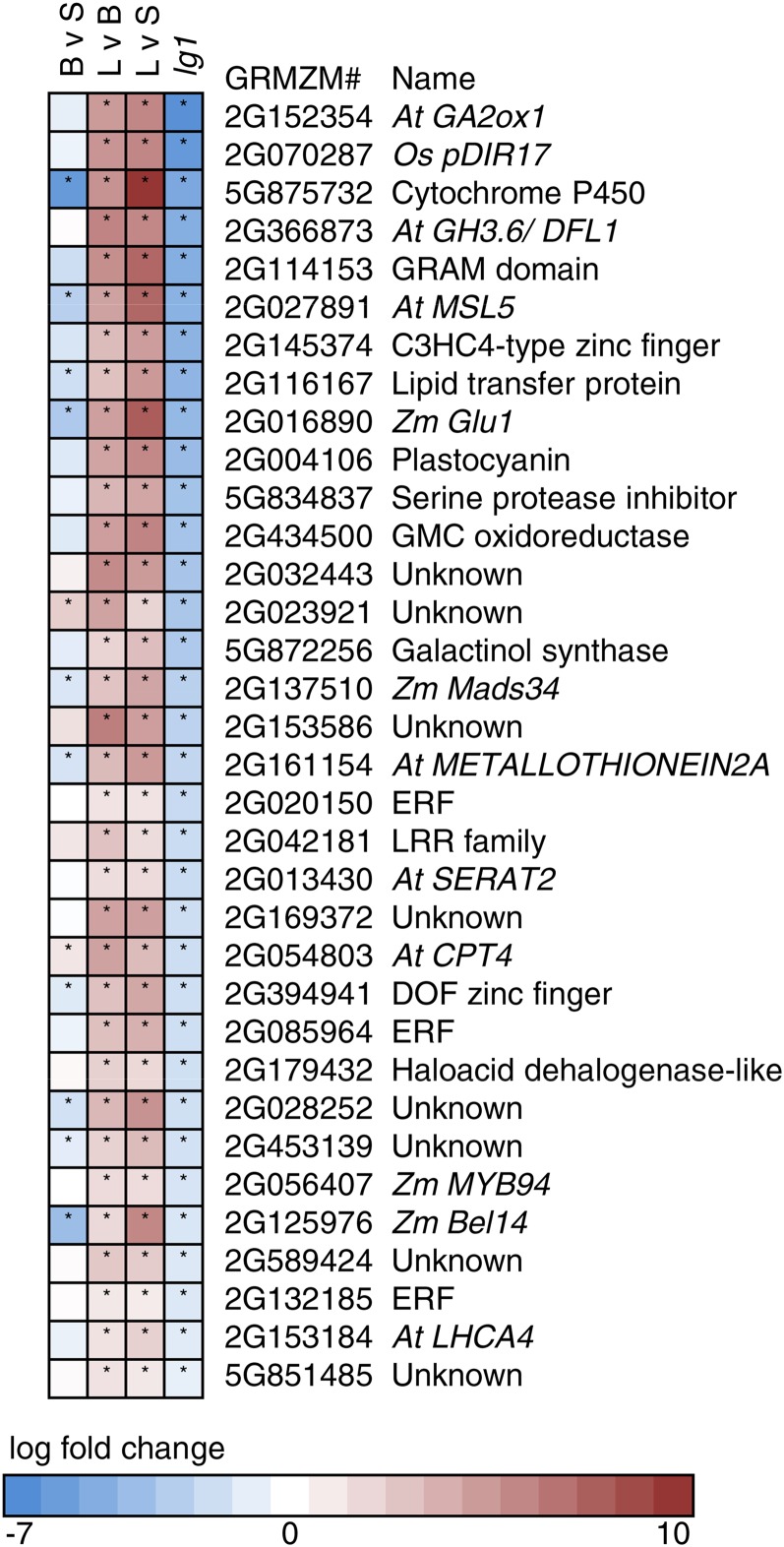
To identify genes that are DE in lg1-R mutant leaf primordia, we conducted LM-RNAseq and compared transcript accumulation in wild-type and lg1-R P6 leaf primordia (dashed box, Figure 2A). Ninety-six genes were DE with a false discovery rate of <0.05, 59 were downregulated in lg1-R mutants, and 37 were upregulated (Supplemental Data Sets 7 and 8). Of these 96 genes DE in lg1-R, 34 were also significantly upregulated in preligule tissue in our analysis of all cell layers and thereby comprise an especially interesting subset of ligule-enriched transcripts (Figure 3). All 34 genes that were upregulated in preligule tissue and DE in lg1-R were downregulated in lg1-R mutants.
Figure 3.
Genes Upregulated in the Preligule Region and DE in lg1-R Mutants.
BvS, blade relative to sheath; LvB, ligule relative to blade; LvS, ligule relative to sheath; lg1=lg1-R, mutant relative to wild-type sibling. Asterisk indicates P < 0.05. “Zm” indicates maize gene name, “At” indicates name of closest Arabidopsis gene, and “Os” indicates name of closest rice gene.
Functional Category Enrichment
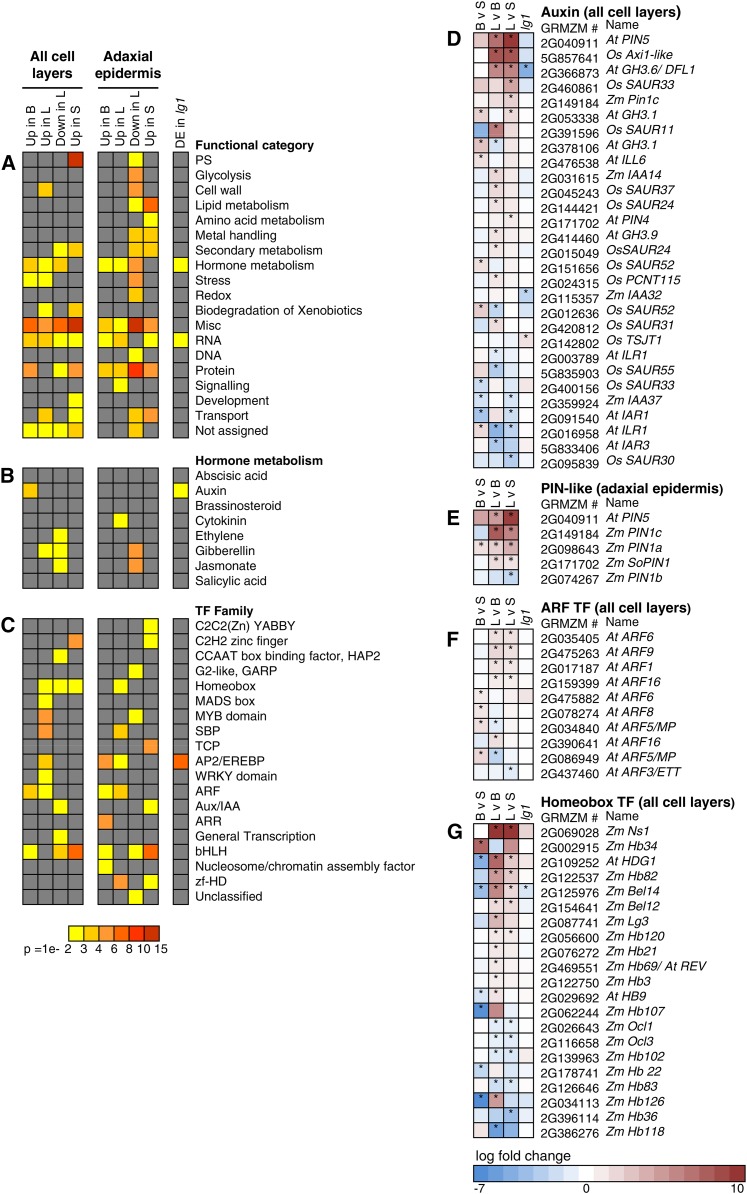
All expressed genes were assigned to MapMan functional categories (Thimm et al., 2004); tests for enrichment of functional categories in proximal-distal leaf regions and in lg1-R mutant primordia were performed (Figures 4A to 4C). Genes that are DE in lg1-R mutants are enriched for hormone metabolism and RNA MapMan categories (Figure 4A). Blade and ligule genes are also enriched for hormone metabolism, and lists for blade, ligule, and sheath were all enriched for RNA (Figure 4A). The ligule gene list was also enriched for cell wall genes, possibly reflecting cell wall modifications required for ligule outgrowth (Figure 4A).
Figure 4.
Enrichment of Functional Categories and Expression Profiles for DE Genes.
(A) Enrichment of functional categories for DE gene lists.
(B) Enrichment of hormone metabolism subcategories.
(C) Enrichment of transcription factor subcategories. Gray, not significantly enriched. Up in B, genes significantly upregulated in blade; Up in L, genes significantly upregulated in ligule; Down in L, genes significantly downregulated in ligule; Up in S, genes significantly upregulated in sheath; genes DE in lg1, genes DE in lg1-R versus the wild type.
(D) Expression profiles for DE auxin-related genes (all-cell-layers LM).
(E) Expression profiles for DE pin genes (epidermis-only LM).
(F) Expression profiles for DE ARF transcription factor genes (all-cell-layers LM).
(G) Expression profiles for DE homeobox transcription factor genes (all-cell-layers LM). BvS, blade relative to sheath; LvB, ligule relative to blade; LvS, ligule relative to sheath; lg1, lg1-R mutant relative to wild-type sibling. Asterisk indicates P < 0.05. “Zm” indicates maize gene name, “At” indicates name of closest Arabidopsis gene, and “Os” indicates name of closest rice gene.
A breakdown of transcription factor families revealed that the ligule gene list is enriched for seven transcription factor families, including MADS box, MYB, and SBP (Figure 4C). LG1 is itself a SBP expressed specifically in the PLB and emerging ligule (Figures 2D to 2F), and our LM-RNAseq data reveal that several SBP gene family paralogs exhibit domain-specific transcript accumulation during patterning of the blade-sheath boundary (Figure 4A; Supplemental Figures 2A and 2F). Both the ligule and sheath gene lists are enriched for homeobox transcription factors (Figure 4C). Intriguingly, whereas the blade and ligule DE gene lists are enriched for AUXIN RESPONSE FACTOR (ARF) transcription factor family members (Figure 4F), the ligule and epidermal sheath show significant enrichment for Aux/IAA genes that encode repressors of ARF activity (reviewed in Hagen and Guilfoyle, 2002; Figure 4C).
Transcripts Implicated in Maize Leaf Initiation Are Redeployed during Patterning of the Primordial Blade-Sheath Boundary
A surprising number of transcription factor transcripts and hormonal genes differentially accumulating at the blade-sheath boundary of maize leaf primordia were first described as key developmental regulators of early stages in leaf initiation from the SAM. The most highly upregulated homeobox gene in the ligule region of P7-staged leaf primordia is narrow sheath1 (ns1) (GRMZM2G069028) (Figure 4G). ns1 encodes a WUSCHEL-LIKE HOMEOBOX3 protein that is required for recruitment of lateral leaf founder cells during early stages of maize leaf development (Scanlon et al., 1996; Nardmann et al., 2004). At least two Class III HOMEODOMAIN LEUCINE ZIPPER (HD-ZIPIII) genes with homology to the Arabidopsis thaliana leaf polarity genes REVOLUTA (GRMZM2G469551) and PHABULOSA (GRMZM2G469551) also display specific expression patterns in both emerging leaf primordia and in developing ligules (Supplemental Figures 2B and 3)(Juarez et al., 2004). Two additional transcription factor genes implicated in abaxial patterning of initiating maize leaf primordia (the ARF3 paralogs GRMZM2G030710 and GRMZM2G441325) are likewise upregulated in the preligule region epidermis of maize leaf primordia (Supplemental Figures 2B and 2C). According to a model proposed by Waites and Hudson (1995), the juxtaposition of adaxial (upper leaf) and abaxial (lower leaf) developmental fields generates a new leaf axis that promotes laminar outgrowth (Waites and Hudson, 1995; Timmermans et al., 1998; Nogueira et al., 2007; Candela et al., 2008; Douglas et al., 2010). This mechanism may have been co-opted to generate new growth axes in leaves with nonplanar morphologies, such as the maize prophyll (Johnston et al., 2010). We hypothesize that ARF3 and HD-ZIPIII paralogs also function during ligule outgrowth from later-staged leaf primordia in a manner analogous to laminar outgrowth at the earliest stages of maize leaf initiation.
In support of this hypothesis, a number of transcripts implicated in phytohormone metabolism, transport, and signaling during maize leaf initiation are likewise redeployed during establishment of the blade-sheath boundary. These include four PIN-like genes, previously described to function during the earliest known events in lateral organ initiation (Benková et al., 2003; Reinhardt et al., 2003; Carraro et al., 2006; Gallavotti et al., 2008; Lee et al., 2009), that are upregulated in the preligule L1 (Figures 4D and 4E). These data are consistent with previous reports that ZmPIN1a-YFP accumulates in the PLB (Moon et al., 2013) (Figures 1I and 1J). Three paralogous GRETCHEN HAGEN3 (GH3)-like genes, which are predicted to encode IAA-amide-synthetase proteins that function to convert the auxin IAA into biologically inactive conjugates (Zhao et al., 2013), are also highly upregulated in the ligule and downregulated in lg1-R mutants (Figure 4D). Intriguingly, misexpression of a rice (Oryza sativa) homolog of GH3 conditions mutant phenotypes at the blade-sheath boundary that alter leaf inclination (Zhao et al., 2013).
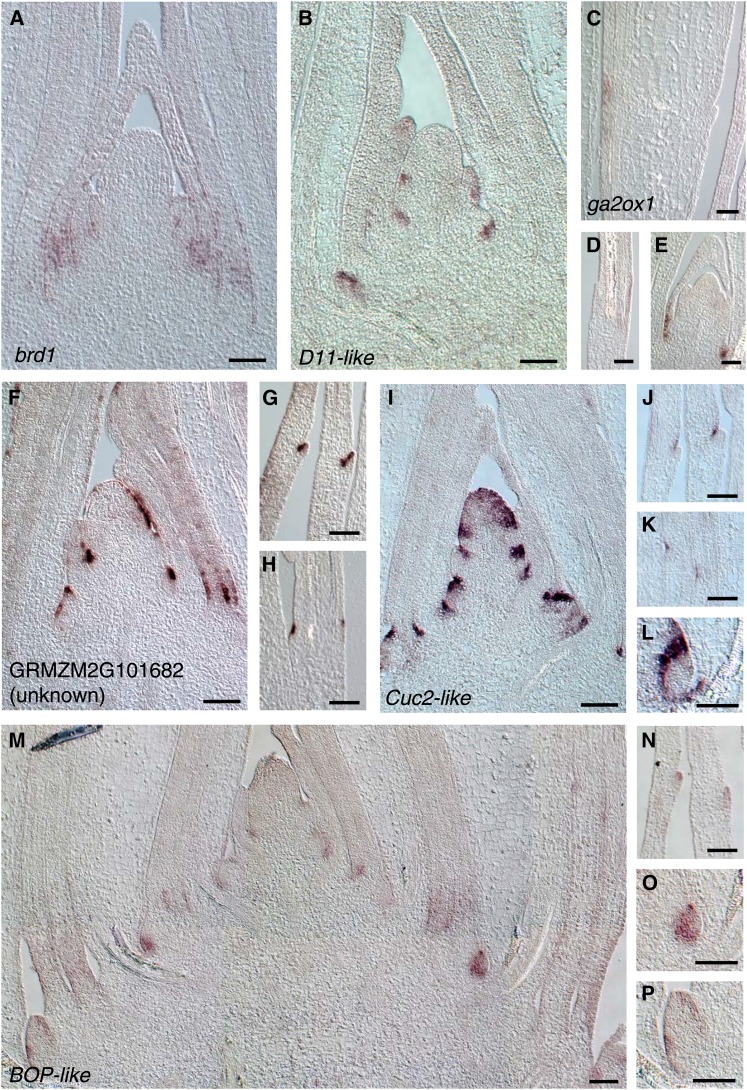
Two genes implicated in brassinosteroid biosynthesis, brassinosteroid-deficient dwarf1 (brd1; GRMZM2G103773) and a gene with high similarity to rice Dwarf11 (ZmD11; GRMZM2G107199), were significantly upregulated in our LM-RNAseq analyses of preligule tissue (Supplemental Data Set 5). Likely due to their low transcript abundance, neither brd1 nor ZmD11 transcripts are detected by in situ hybridization in emerging ligules, although transcripts are observed near the primordial leaf base and in initiating lateral organs, respectively (Figures 5A and 5B). Maize brd1-m1 mutants exhibit enlarged auricles and an indistinct blade-sheath boundary, whereas rice d11 mutants have upright leaves (Tanabe et al., 2005; Makarevitch et al., 2012). Our data reinforce previous findings implicating brassinosteroid biosynthesis during patterning of the blade-sheath boundary in maize.
Figure 5.
Hormonal Signaling Pathways and Organ Boundary Genes in Leaf Proximal-Distal Patterning.
In situ hybridization of maize wild-type B73 seedlings using the indicated probes. Bars = 100 μm.
(A) brd1, lateral longitudinal section through shoot apex.
(B) D11-like, longitudinal section through shoot apex.
(C) to (E) ga2ox1.
(C) Longitudinal section through leaf primordium at midrib.
(D) Longitudinal section through leaf primordium near margin.
(E) Axillary bud.
(F) to (H) GRMZM2G101682 (unknown gene).
(F) Longitudinal section through shoot apex.
(G) Longitudinal section through developing ligule near leaf margins.
(H) Longitudinal section through PLB.
(I) to (L) CUC2-like.
(I) Longitudinal section through shoot apex.
(J) Longitudinal section through developing ligule near leaf margins.
(K) Longitudinal section through PLB.
(L) Axillary meristem.
(M) to (P) BOP-like.
(M) Longitudinal section through shoot apex.
(N) Longitudinal section through developing ligule near leaf margins.
(O) Axillary meristem.
(P) Axillary bud.
Transcripts of two genes with predicted functions in gibberellin metabolism are also DE during ligule initiation. A maize homolog of the Arabidopsis GIBBERELLIN2-OXIDASE1 gene (ga2ox1), which functions in gibberellin catabolism, was significantly upregulated in the preligule region of wild-type leaves and downregulated in lg1-R mutants (Figure 3; Supplemental Figure 2D). In situ hybridization revealed that ga2ox1 transcripts accumulate on the abaxial domains of the developing ligular/auricle region and at the base of lateral organ primordia in axillary buds (Figures 5C to 5E).
Two cytokinin oxidase genes were upregulated in the preligule epidermis (Supplemental Figure 2E). Cytokinin oxidases degrade cytokinin (CK), suggesting that epidermal-localized CK degradation occurs in preligule cells (Houba-Hérin et al., 1999; Schmülling et al., 2003). A gene with similarity to ARABIDOPSIS THALIANA RESPONSE REGULATOR9, a type-A response regulator and negative regulator of CK signaling, was also upregulated at the blade-sheath boundary (Supplemental Figure 2E; Kiba et al., 2003; To et al., 2004). Lateral organ founder cells exhibit low ratios of CK/auxin (Jasinski et al., 2005; Yanai et al., 2005). Our data suggest that reduced CK levels in preligule cells may promote cell expansion and ligule outgrowth in a manner similar to leaf initiation.
Ligule Genes Are Expressed at Multiple Organ Boundaries
In situ hybridization of genes that are DE along the leaf proximal-distal axis revealed that many are also expressed at organ boundaries in the SAM. Several of these genes are putative orthologs of genes that function in boundary specification in other species. One grass-specific gene of unknown function, GRMZM2G101682, was highly upregulated in the preligule region (Supplemental Data Set 5; Figures 5F to 5H). In situ hybridizations reveal that GRMZM2G101682 is expressed in one or a few cells immediately distal to the PLB (Figure 5H). Transcripts accumulate at the emerging ligule cleft and on the adaxial side of the ligule at later stages of development (Figure 5G). Strikingly, accumulation of this unknown transcript is also detected at boundaries between initiating organs and the SAM and at the base of young lateral organs (Figure 5F). Transcript is largely confined to L1-derived cells in all these tissues and developmental stages.
Another gene that is highly upregulated in the ligule region is GRMZM2G393433, similar to Arabidopsis CUP-SHAPED COTYLEDON2 (CUC2) (Supplemental Data Set 5). In Arabidopsis embryos, CUC activity represses growth between the cotyledons (Aida et al., 2002). These organ boundaries are associated with low auxin levels, in keeping with models wherein CUC2 expression is negatively regulated by auxin (Furutani et al., 2004; Bilsborough et al., 2011; Q. Wang et al., 2014; Y. Wang et al., 2014). In situ hybridization revealed that transcripts of the maize CUC2-like gene accumulate in the PLB region, prior to ligule outgrowth, and in the cleft above developing ligules (Figures 5J and 5K). Also expressed in the SAM and in the boundaries between initiating organs and the shoot apex, CUC2-like transcripts are not detected in initiating leaves per se (Figure 5I). A band of CUC2-like accumulation is also seen between initiating leaf margins and axillary meristems, presumably demarcating the boundary between the primary shoot and lateral branches (Figure 5L). These results suggest that common mechanisms specify the blade-sheath boundary and other developmental boundaries in the maize shoot.
A homolog of Arabidopsis BLADE-ON-PETIOLE1/2 (BOP1/BOP2) (GRMZM2G039867) is upregulated in sheath relative to blade (Supplemental Data Set 5). Arabidopsis BOP genes function in leaf proximal-distal patterning (Ha et al., 2003, 2004, 2007), and our results suggest that this role is conserved in maize. In situ hybridization of the maize BOP-like gene reveals transcript accumulation at organ boundaries, the base of leaf primordia (presheath domain), in axillary meristems, and in developing ligules (Figures 5M to 5P). A second BOP-like gene (GRMZM2G060723) was also upregulated in sheath and ligule relative to blade, but transcripts of this paralog accumulate at much lower levels (Supplemental Data Set 5). These results suggest that BOPs may specify the proximal (sheath) portion of the leaf primordium, contributing to specification of the blade-sheath boundary and later ligule outgrowth.
The correct establishment of developmental boundaries is required for leaf initiation and lateral branching. Mutants that are defective in boundary specification, such as Arabidopsis knox and bell mutants, exhibit aberrant branching patterns (Byrne et al., 2003; Smith and Hake, 2003; Ragni et al., 2008). In maize, the male inflorescence (tassel) has long branches that develop from indeterminate meristems at the base of the inflorescence (Tanaka et al., 2013). In contrast, axillary meristems initiated by female inflorescence meristems are determinate, resulting in ears that lack long branches. Maize ramosa1 (ra1) mutants are characterized by abnormal branching in the ear, a phenotypic anomaly that mimics the branching patterns normally observed in the developing tassel (Gallavotti et al., 2010). Recent data show that LG1 accumulates in the axils of tassel branches (Eveland et al., 2014; Lewis et al., 2014). lg1 mutants exhibit reduced tassel angle and three SBP genes, including lg1, are implicated in tassel branching, suggesting that LG1 functions in boundary specification in the tassel (Brown et al., 2011; Eveland et al., 2014; Lewis et al., 2014). Our finding that genes such as CUC2-like have specific expression patterns at the blade-sheath boundary and other developmental boundaries prompted us to ask if other genes that are DE at the blade-sheath boundary are also implicated in lateral branching. Thus, we looked for overlap between our transcriptomic data sets and genes implicated in lateral branching (Eveland et al., 2014). Genes that are common in multiple data sets are likely to play evolutionarily conserved roles during regulation of developmental processes.
We compared transcripts that are DE specifically in preligule tissue and lg1-R mutants to genes that are either DE between branched tassel and unbranched ear primordia or DE in ra1 mutant ears compared with the wild type (Supplemental Figure 4) (Eveland et al., 2014). Of 619 genes that are DE in preligule tissue, 227 were also DE in ra1 ear primordia relative to wild-type ears and 151 were DE in tassel primordia relative to ear primordia (Supplemental Figures 4A and 4B). Of 96 genes that are DE in lg1-R mutant primordia, 45 were also DE in ra1 ears and 26 were DE in tassel versus ear primordia (P < 1e-2 for all comparisons) (Supplemental Figures 4C and 4D). Given that lg1 is ectopically expressed in the highly branched ra1 mutant ear primordia, but not in wild-type ears (Eveland et al., 2014), it is likely that DE genes common to both lg1-R and ra1 mutants act downstream of LG1 and are involved in branching.
Interactive Networks: KN1-Bound and Modulated
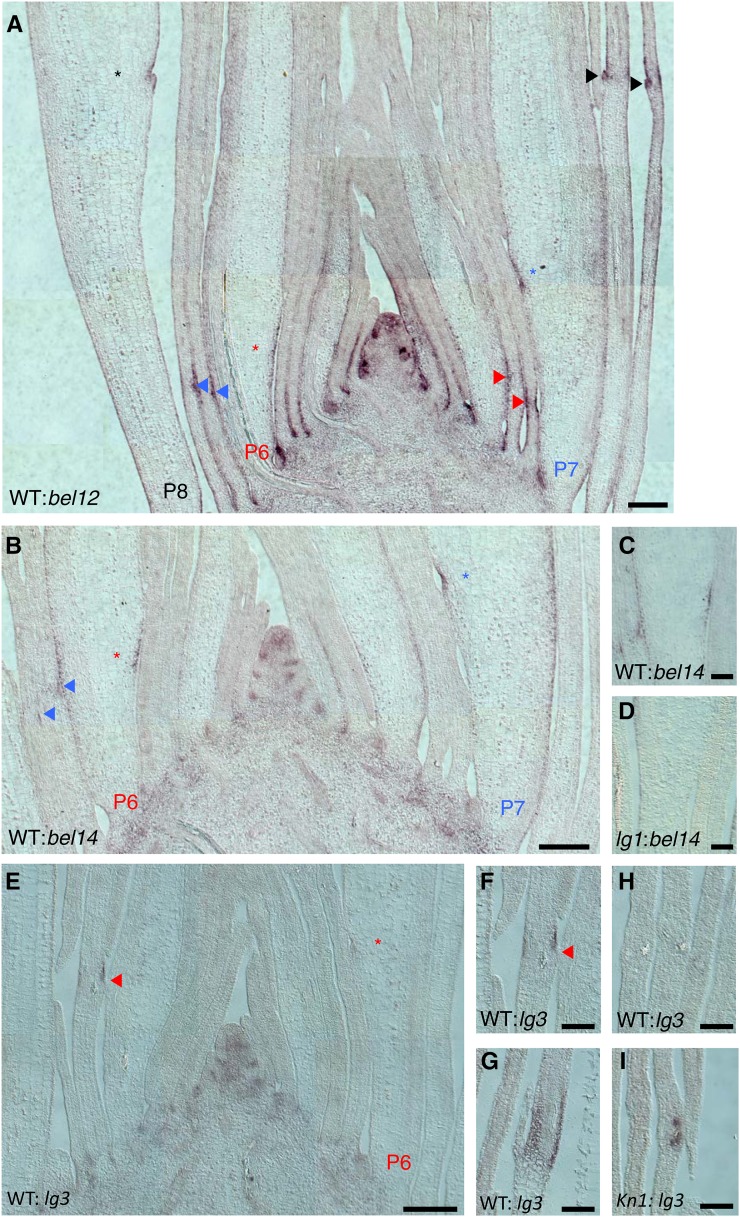
KNOX accumulates at the base of wild-type leaf primordia and knox misexpression in the leaf blade causes cells to differentiate as proximal tissues, such as sheath. These observations form the basis of a model in which KNOX specifies the presheath domain during early leaf development (Bolduc et al., 2012a). Given the proposed role of KNOX in leaf proximal-distal patterning, we investigated genes that are DE in our data and are also bound and modulated by KN1 protein (Bolduc et al., 2012b). In Arabidopsis, BEL1-like homeodomain (BELL) proteins form heterodimers with KNOX proteins in combinations that determine target selection and subcellular localization (Bellaoui et al., 2001; Smith et al., 2002; Bhatt et al., 2004; Hackbusch et al., 2005; Cole et al., 2006). Our data show that maize bel12 (GRMZM2G154641) is significantly upregulated in the preligule region, whereas bel14 (GRMZM2G125976) is expressed at low levels in the blade, moderate levels in the sheath, and most highly in the ligule region (Figure 4G; Supplemental Table 4). Both bel12 and bel14 are bound by KN1 and are upregulated in Kn1-N/Kn1-N leaves compared with the wild type, although the difference is not statistically significant in the case of bel14 (Bolduc et al., 2012b).
In situ hybridization analyses of bel12 reveal transcript accumulation at lateral organ boundaries in the SAM and where older leaf primordia insert at the stem (Figure 6A). The in situ analyses confirm increased transcript accumulation in the preligule region of P6 and P7 primordia, as identified in our LM-RNAseq, and also reveal transcript accumulation in the proximal domain of younger leaf primordia. Similar to the pattern observed for bel12, bel14 transcripts accumulate within lateral organ boundaries in the SAM and stem and in the preligule region of P6 and P7 leaf primordia (Figures 6B and 6C). Intriguingly, both our lg1-R RNAseq data and in situ hybridizations of lg1-R mutant leaf primordia revealed decreased accumulation of bel14 in the preligule region of P6 mutant leaves (Figures 3A, 6C, and 6D). The finding that bel14 transcript is significantly lower in lg1-R mutants suggests that LG1 function may be required to maintain bel14 expression specifically in the ligule region. The dynamic expression of bel12 and bel14, and the finding that bel14 is DE in lg1-R mutants, suggest that they may be important factors in leaf proximal-distal patterning.
Figure 6.
In Situ Hybridization of KN1-Bound and Modulated Genes.
(A) bel12, longitudinal section through shoot apex.
(B) to (D) bel14.
(B) Longitudinal section through shoot apex.
(C) Longitudinal section through wild-type midrib showing transcript accumulation in preligule region.
(D) Longitudinal section through lg1-R midrib at equivalent stage and position to “C” showing absence of transcript accumulation.
(E) to (I) lg3.
(E) Longitudinal section through shoot apex.
(F) Longitudinal section through PLB.
(G) Longitudinal section through ligule.
(H) Longitudinal section through wild-type preblade showing absence of transcript accumulation.
(I) Longitudinal section through Kn1-N preblade showing transcript accumulation around vascular tissue.
P indicates plastochron number. Asterisks indicate height of PLB at the midrib. Arrowheads indicate height of PLB at the leaf margins. Asterisks and arrowheads are color-coded by plastochron number: red, P6; blue, P7; black, P8. Bars = 200 μm in (A), (B), and (E) and 100 μm in (C), (D), and (F) to (I).
KN1 accumulation overlaps with bel12 and bel14 transcripts in the SAM and proximal portion of early leaf primordia; thus, BEL12 and BEL14 may interact with KN1 in these domains. However, kn1 transcript is very low in the preligule region of P7 leaf primordia where bel12 and bel14 transcripts accumulate, suggesting another KNOX protein may interact with BEL proteins in this domain. One KNOX protein that potentially interacts with BEL12 and BEL14 in the ligule is LG3. Dominant Lg3 mutants are characterized by leaf patterning defects that include displacement of the ligule over the midrib or deletion of the ligule (Fowler and Freeling, 1996; Muehlbauer et al., 1997, 1999). lg3 is bound by KN1 and RNAseq data show that lg3 is highly upregulated in Kn1-N leaves (Bolduc et al., 2012b). We found that lg3 transcript is significantly higher in preligule tissue than in blade tissue and is also higher in preligule than in sheath tissue, although this latter difference was not statistically significant (Figure 4G). In situ hybridization shows lg3 transcript accumulation in the preligule region and distal to the ligule after ligule outgrowth (Figures 6E to 6G). Kn1-N mutant blades were examined to test whether accumulation of lg3 is also ectopically upregulated in this gain-of-function mutant. In wild-type leaf blades, lg3 transcript is not detected (Figure 6H); however, ectopic lg3 transcript accumulates around the veins of Kn1-N mutant leaf blades (Figure 6I). These observations further support the upregulation of lg3 by KN1.
lg3 is most similar to KNAT6 in Arabidopsis. KNAT6 is expressed at organ boundaries and contributes to boundary establishment via interactions with the KNOX protein SHOOT-MERISTEMLESS1 and with CUC (Belles-Boix et al., 2006). We speculate that LG3 may have a similar function during boundary establishment in maize. The role of KNOX proteins in establishing the blade-sheath boundary may be analogous to the formation of dissected or compound leaves, where reactivation of knox gene expression juxtaposed to PIN-induced auxin maxima creates new boundaries that lead to leaflet formation (Hay et al., 2006; Hay and Tsiantis, 2006; Barkoulas et al., 2008; Shani et al., 2009). Interactions between CUC2, whose expression is antagonized by auxin (Bilsborough et al., 2011), and its posttranscriptional regulator miR164 are important in the control of leaf serration and lobing (Nikovics et al., 2006). Given that KNOX-BEL-auxin modules act in multiple contexts during plant development, it is possible that different SBP family members are expressed depending on context, thus contributing to the elaboration of different developmental boundaries.
Intriguingly, two auxin transport genes, Zm-PIN1a and So-PIN1, are upregulated in preligule epidermal cells and are also bound by KN1 and upregulated in Kn1-N mutant leaves (Bolduc et al., 2012b). This finding suggests that KN1 directly activates Zm-PIN1a and SoPIN1 transcription at ectopic boundaries and suggests a model in which auxin transport by PIN proteins is activated at the KNOX presence/absence boundary at the base of wild-type leaf primordia.
A Model for Ligule Development
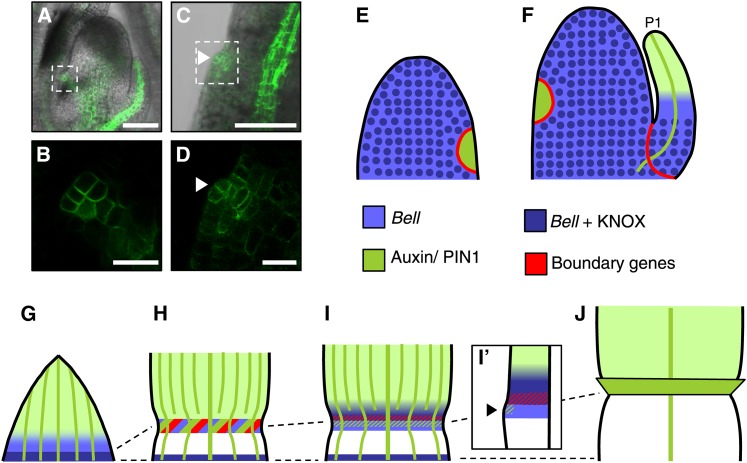
We employed LM-RNAseq to identify genes expressed at early stages of ligule formation and compared these with adjacent blade and sheath. Many of the genes we identified are also expressed at lateral organ boundaries or in initiating lateral organs. We hypothesize that patterning mechanisms that operate in the SAM during organogenesis are reiterated at the blade-sheath boundary during ligule initiation. This model is supported by differential coexpression, in both the primordial ligule and initiating lateral organs, of multiple transcription factors involved in lateral organ patterning, including ns1, arf3a, HD-ZIPIII family members, and CUC2-like (Figure 4G; Supplemental Figures 2B and 2C). Hormonal signaling networks typically associated with organogenesis and branching are also recapitulated during ligule biogenesis in the young primordium. PIN1 accumulation within the PLB strongly resembles PIN1 maxima at the site of leaf initiation (Figures 7A to 7D) (Carraro et al., 2006; Gallavotti et al., 2008; Lee et al., 2009).
Figure 7.
Ligule Initiation Reiterates Leaf Initiation at the SAM.
(A) ZmPIN1a-YFP accumulation in longitudinal section through the SAM.
(B) Enlargement of boxed region in (A) showing ZmPIN1a-YFP accumulation in leaf buttress.
(C) ZmPIN1a-YFP accumulation in longitudinal section through initiating ligule.
(D) Enlargement of boxed region in (C) showing ZmPIN1a-YFP accumulation in initiating ligule. Arrowheads indicate PLB. Bars = 100 μm in (A) and (C) and 25 μm in (B) and (D).
(E) to (J) Model for proximal-distal patterning of the maize leaf.
(E) KNOX proteins accumulate throughout the SAM except for the P0 primordium (green), where PIN-mediated auxin transport is correlated with KNOX downregulation. Boundary genes, such as CUC2-like, are expressed between P0 and the SAM.
(F) and (G) KNOX accumulates at the base of P1 and older primordia and overlaps with bel12 transcript, which is expressed more broadly. KNOX and BEL proteins interact to activate target genes. KNOX accumulation defines the presheath domain. Auxin accumulation in the distal portion of the primordium restricts KNOX to the base of the primordium and defines the preblade region.
(H) Initially, ligule genes are expressed at the presumptive blade-sheath boundary in a broad band. LG1 activates PIN1a expression. White portion represents growth of the presheath domain.
(I) lg3 expression is reactivated at the blade-sheath boundary (indicated by dark purple). Antagonism between auxin and boundary genes restricts boundary genes to cells that will form the ligule cleft.
(I’) Illustrates longitudinal section through primordium depicted in (I).
(J) Leaf after ligule outgrowth.
Our data support a model in which KNOX accumulation at the base of the leaf primordium and auxin accumulation and signaling in the distal portion of the primordium provide positional cues that demarcate the blade-sheath boundary. KNOX proteins accumulate throughout the SAM and are excluded from the P0 cells of the incipient leaf primordium (Smith et al., 1992; Jackson et al., 1994). Localization of PIN-mediated auxin transport at the P0 correlates with subsequent knox gene downregulation (Figure 7E) (Hay et al., 2006). At P1 and later, KNOX accumulates at the base of the primordium (Figures 7F and 7G), while the distal portion of the primordium becomes an auxin source (Jackson, 2002; Bolduc et al., 2012a). Our data show that the preblade region is enriched for auxin-related transcripts, whereas the preligule and presheath regions are enriched for homeobox transcription factors. We propose that KNOX proteins interact with BEL12 to define the basal presheath domain prior to formation of the PLB. Auxin in the distal portion of the leaf restricts KNOX accumulation to this basal domain, and the resultant boundary between auxin and KNOX signaling specifies the position of the blade-sheath boundary. A presumed reduced concentration of auxin in this basal, presheath domain is supported by the downregulation of arf gene expression in the presheath (Figures 4C and 4F). This model is supported by evidence that reduced auxin transport can alter the position and elaboration of the blade-sheath boundary (Tsiantis et al., 1999; Scanlon et al., 2002).
lg1, lg3, and other ligule genes are expressed at the boundary between preblade and presheath, thereby activating bel14 expression and reactivating transcription of bel12 (Figure 7H). Initially, lg1 is expressed in a broad domain and activates PIN1a expression at the PLB (Figure 7H). We propose that subsequent antagonism between auxin and boundary genes, such as CUC2-like and lg3, restricts boundary gene expression to cells that will form the ligule cleft (Figure 7I). LG3 interacts with BEL14 and/or BEL12 during boundary specification. PIN-mediated auxin accumulation promotes ligule outgrowth, while boundary genes restrict cell division/growth in the ligule cleft (Figure 7I’). In mutants with ectopic knox expression, BEL proteins are ectopically expressed and misplaced ligules are formed. In lg1-R mutants, bel14 expression at the PLB is deleted (Figures 6C and 6D) and no ligule is formed.
In summary, our model proposes that elements of meristematic function observed at the SAM and branch meristem are repeated at developmental boundaries throughout the plant shoot, in this particular case at the blade-sheath boundary. Consistent with this model, we found similar patterns of transcript accumulation in the developing ligule and at the boundaries of lateral organs, such as leaves and tassel branches.
METHODS
Plant Material and Growth Conditions
B73 plants were used for the LM-RNAseq analysis of wild-type leaf domains. Homozygous lg1-R plants introgressed into the “Freeling B73” background and wild-type siblings were used for LM-RNAseq analysis of lg1-R mutants and in situ hybridization in lg1-R. B73 was used for wild-type in situ hybridization. Kn1-N and Lg3-O introgressed into B73 were used for in situ hybridization in these mutant backgrounds. ZmPIN1a-YFP transgenic seed were provided by D. Jackson (Cold Spring Harbor Laboratory).
Plants for LM were grown in growth chambers with a cycle of 15 h light at 25°C and 9 h dark at 20°C. Plants for in situ hybridization were grown under standard greenhouse conditions. All analyses were performed on 14-d-old seedlings.
Laser Microdissection and Sequencing
For analysis of wild-type leaf domains, plants were sectioned along the lateral axis, perpendicular to the midrib-margin axis (Figure 2A; Supplemental Figures 1A and 1B). Sites targeted for microdissection were identified by the appearance of the late PLB as it emerged from the plane of the leaf at about P7. For the preligule region, a 100-μm-high rectangle centered on the PLB was selected (Figure 2A). Preblade and presheath domains were microdissected from similar sized rectangles 50 μm above and below the ligule region, respectively (Figure 2A; Supplemental Figure 1, red, green, and blue boxes, respectively).
To identify genes that are DE in lg1-R mutant leaf primordia, we conducted LM-RNAseq and compared transcript accumulation in wild-type and lg1-R P6 stage leaf primordia (Figure 2D). LM was used to isolate tissue from a region between 400 and 900 μm from the base of each P6 primordium, which encompasses the domain and developmental stage where lg1 is first expressed (Figure 2A, dashed box). Three replicates of five to six plants each were used for each tissue type.
Laser microdissection was performed using the Positioning and Ablation with Laser Microbeams system (P.A.L.M. Microlaser Technologies). RNA extraction and amplification, cDNA library preparation, and Illumina sequencing were performed as described previously (Takacs et al., 2012).
Bioinformatics
The sequencing reads were aligned to the maize (Zea mays) B73 RefgenV2 using TopHat (v2.0.11) (Trapnell et al., 2009). Reads that were mapped to multiple positions were filtered out by Bamtools (Barnett et al., 2011). The Cuffdiff software (2.1.0) (Trapnell et al., 2010) was used to quantify the raw read count in each gene, using the version 5a gene annotation (working set genes) provided by the maize genome sequencing project . The gene read counts were based on the Cuffdiff output file “genes.read_group_tracking.” The Bioconductor edgeR package (Robinson et al., 2010) was used to identify differentially expressed genes. This analysis used the generalized linear model approach. The trimmed means of M-values were used as scaling factors for data normalization, and the Benjamini and Hochberg’s algorithm was used to control the false discovery rate (tested on a subset of genes with cpm > 0.1 in at least three samples). MapMan gene annotation (Thimm et al., 2004; Usadel et al., 2009) was used for function enrichment analyses. The binomial test was used to identify enrichment of differentially expressed gene sets compared with all maize gene sets in each of the MapMan function bins, using a P value cutoff of 0.01.
Construction of Domain-Enriched Gene Lists
Lists of genes that are specifically enriched in each leaf domain were constructed. Genes that were DE between blade and sheath and upregulated in the sheath were considered to be sheath-enriched, whereas genes that were upregulated in the blade compared with the sheath were considered to be blade-enriched. Genes that were DE in both ligule versus blade and ligule versus sheath and that were upregulated in ligule tissue in both comparisons were considered to be ligule-enriched (Table 1; Supplemental Data Sets 5 and 6).
In Situ Hybridization and Confocal Imaging
In situ hybridization was conducted as described previously (Jackson et al., 1994). Primer sequences used for probe synthesis are provided in Supplemental Table 1. Confocal imaging was performed as described (Shimizu et al., 2009).
Data Access
All the Illumina RNA-seq raw data (FASTQ) and processed data (read counts per gene) used in this study have been deposited at NCBI Gene Expression Omnibus under accession number GSE61333.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases; all accession numbers for genes mentioned in this study are given in Supplemental Data Set 2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Scheme for Laser Microdissection of Leaf Primordial Domains.
Supplemental Figure 2. Expression Profiles for DE Genes.
Supplemental Figure 3. In Situ Hybridization of the ZmPHB Gene.
Supplemental Figure 4. Ligule Genes Overlap with Genes Implicated in Regulation of Branching.
Supplemental Table 1. Primer Sequences Used for Synthesis of in Situ Hybridization Probes.
Supplemental Data Set 1. Alignment Statistics.
Supplemental Data Set 2. RNAseq Data All-Cell-Layers Analysis, All Expressed Genes.
Supplemental Data Set 3. Transcript Accumulation of Selected Layer-Specific Genes in Epidermis-Only and All-Cell-Layers Microdissections.
Supplemental Data Set 4. RNAseq Data Adaxial Epidermis Only, All Expressed Genes.
Supplemental Data Set 5. Genes Upregulated in Primordial Blade, Ligule, or Sheath (All-Cell-Layers Analysis).
Supplemental Data Set 6. Genes Upregulated in Primordial Blade, Ligule, or Sheath (Adaxial Epidermis-Only Analysis).
Supplemental Data Set 7. RNAseq Data lg1-R Mutants versus Wild-Type Siblings.
Supplemental Data Set 8. Genes DE in lg1-R Mutants.
Supplementary Material
Acknowledgments
We thank the reviewers for helpful comments that improved the article. We thank J. Moon for images used in Figure 1. We are grateful to S. Leiboff for helpful reviews of the article. We thank X. Rebocho, A. Richardson, and E. Coen for stimulating discussions of maize ligule development. We thank N.R.R. Todt for assistance in performing in situ hybridizations. We thank Mamta Srivastava at the Plant Cell Imaging Center, Boyce Thompson Institute for Plant Research for assistance with confocal microscopy. This study was supported by National Science Foundation Grant MCB 1052051.
AUTHOR CONTRIBUTIONS
R.J., S.H., A.W.S., and M.J.S. designed the research. R.J. performed the research. R.J., M.W., Q.S., S.H., A.W.S., and M.J.S. analyzed the data. R.J. wrote the article, and S.H., A.W.S., Q.S., and M.J.S. edited the article.
Glossary
- SAM
shoot apical meristem
- PLB
preligule band
- LM-RNAseq
laser microdissection RNA-sequencing
- DE
differentially expressed
- CK
cytokinin
Footnotes
Online version contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abe M., Katsumata H., Komeda Y., Takahashi T. (2003). Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643. [DOI] [PubMed] [Google Scholar]
- Aida M., Vernoux T., Furutani M., Traas J., Tasaka M. (2002). Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129: 3965–3974. [DOI] [PubMed] [Google Scholar]
- Barkoulas M., Hay A., Kougioumoutzi E., Tsiantis M. (2008). A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40: 1136–1141. [DOI] [PubMed] [Google Scholar]
- Barnett D.W., Garrison E.K., Quinlan A.R., Strömberg M.P., Marth G.T. (2011). BamTools: a C++ API and toolkit for analyzing and managing BAM files. Bioinformatics 27: 1691–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft P.W., Bongard-Pierce D.K., Sylvester A.W., Poethig R.S., Freeling M. (1990). The liguleless-1 gene acts tissue specifically in maize leaf development. Dev. Biol. 141: 220–232. [DOI] [PubMed] [Google Scholar]
- Bellaoui M., Pidkowich M.S., Samach A., Kushalappa K., Kohalmi S.E., Modrusan Z., Crosby W.L., Haughn G.W. (2001). The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell 13: 2455–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles-Boix E., Hamant O., Witiak S.M., Morin H., Traas J., Pautot V. (2006). KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell 18: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- Bhatt A.M., Etchells J.P., Canales C., Lagodienko A., Dickinson H. (2004). VAAMANA—a BEL1-like homeodomain protein, interacts with KNOX proteins BP and STM and regulates inflorescence stem growth in Arabidopsis. Gene 328: 103–111. [DOI] [PubMed] [Google Scholar]
- Bilsborough G.D., Runions A., Barkoulas M., Jenkins H.W., Hasson A., Galinha C., Laufs P., Hay A., Prusinkiewicz P., Tsiantis M. (2011). Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA 108: 3424–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc N., O’Connor D., Moon J., Lewis M., Hake S. (2012a). How to pattern a leaf. Cold Spring Harb. Symp. Quant. Biol. 77: 47–51. [DOI] [PubMed] [Google Scholar]
- Bolduc N., Yilmaz A., Mejia-Guerra M.K., Morohashi K., O’Connor D., Grotewold E., Hake S. (2012b). Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 26: 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P.J., Upadyayula N., Mahone G.S., Tian F., Bradbury P.J., Myles S., Holland J.B., Flint-Garcia S., McMullen M.D., Buckler E.S., Rocheford T.R. (2011). Distinct genetic architectures for male and female inflorescence traits of maize. PLoS Genet. 7: e1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M.E., Groover A.T., Fontana J.R., Martienssen R.A. (2003). Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130: 3941–3950. [DOI] [PubMed] [Google Scholar]
- Candela H., Johnston R., Gerhold A., Foster T., Hake S. (2008). The milkweed pod1 gene encodes a KANADI protein that is required for abaxial/adaxial patterning in maize leaves. Plant Cell 20: 2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro N., Forestan C., Canova S., Traas J., Varotto S. (2006). ZmPIN1a and ZmPIN1b encode two novel putative candidates for polar auxin transport and plant architecture determination of maize. Plant Physiol. 142: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M., Nolte C., Werr W. (2006). Nuclear import of the transcription factor SHOOT MERISTEMLESS depends on heterodimerization with BLH proteins expressed in discrete sub-domains of the shoot apical meristem of Arabidopsis thaliana. Nucleic Acids Res. 34: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R.N., Wiley D., Sarkar A., Springer N., Timmermans M.C., Scanlon M.J. (2010). ragged seedling2 Encodes an ARGONAUTE7-like protein required for mediolateral expansion, but not dorsiventrality, of maize leaves. Plant Cell 22: 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R.A. (1912). The inheritance of the ligule and auricle of corn leaves. Neb. Agric. Exp. Sta. An. Rep. 25: 81–85. [Google Scholar]
- Eveland A.L., et al. (2014). Regulatory modules controlling maize inflorescence architecture. Genome Res. 24: 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T., Hay A., Johnston R., Hake S. (2004). The establishment of axial patterning in the maize leaf. Development 131: 3921–3929. [DOI] [PubMed] [Google Scholar]
- Foster T., Yamaguchi J., Wong B.C., Veit B., Hake S. (1999). Gnarley1 is a dominant mutation in the knox4 homeobox gene affecting cell shape and identity. Plant Cell 11: 1239–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J.E., Freeling M. (1996). Genetic analysis of mutations that alter cell fates in maize leaves: dominant Liguleless mutations. Dev. Genet. 18: 198–222. [DOI] [PubMed] [Google Scholar]
- Freeling M., Hake S. (1985). Developmental genetics of mutants that specify knotted leaves in maize. Genetics 111: 617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani M., Vernoux T., Traas J., Kato T., Tasaka M., Aida M. (2004). PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131: 5021–5030. [DOI] [PubMed] [Google Scholar]
- Gallavotti A., Long J.A., Stanfield S., Yang X., Jackson D., Vollbrecht E., Schmidt R.J. (2010). The control of axillary meristem fate in the maize ramosa pathway. Development 137: 2849–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallavotti A., Yang Y., Schmidt R.J., Jackson D. (2008). The relationship between auxin transport and maize branching. Plant Physiol. 147: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C.M., Jun J.H., Nam H.G., Fletcher J.C. (2004). BLADE-ON-PETIOLE1 encodes a BTB/POZ domain protein required for leaf morphogenesis in Arabidopsis thaliana. Plant Cell Physiol. 45: 1361–1370. [DOI] [PubMed] [Google Scholar]
- Ha C.M., Jun J.H., Nam H.G., Fletcher J.C. (2007). BLADE-ON-PETIOLE 1 and 2 control Arabidopsis lateral organ fate through regulation of LOB domain and adaxial-abaxial polarity genes. Plant Cell 19: 1809–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C.M., Kim G.T., Kim B.C., Jun J.H., Soh M.S., Ueno Y., Machida Y., Tsukaya H., Nam H.G. (2003). The BLADE-ON-PETIOLE 1 gene controls leaf pattern formation through the modulation of meristematic activity in Arabidopsis. Development 130: 161–172. [DOI] [PubMed] [Google Scholar]
- Hackbusch J., Richter K., Müller J., Salamini F., Uhrig J.F. (2005). A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc. Natl. Acad. Sci. USA 102: 4908–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. (2002). Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49: 373–385. [PubMed] [Google Scholar]
- Hay A., Barkoulas M., Tsiantis M. (2006). ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133: 3955–3961. [DOI] [PubMed] [Google Scholar]
- Hay A., Tsiantis M. (2006). The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat. Genet. 38: 942–947. [DOI] [PubMed] [Google Scholar]
- Hay A., Tsiantis M. (2010). KNOX genes: versatile regulators of plant development and diversity. Development 137: 3153–3165. [DOI] [PubMed] [Google Scholar]
- Houba-Hérin N., Pethe C., d’Alayer J., Laloue M. (1999). Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. Plant J. 17: 615–626. [DOI] [PubMed] [Google Scholar]
- Humphreys J.M., Chapple C. (2002). Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 5: 224–229. [DOI] [PubMed] [Google Scholar]
- Ingram G.C., Boisnard-Lorig C., Dumas C., Rogowsky P.M. (2000). Expression patterns of genes encoding HD-ZipIV homeo domain proteins define specific domains in maize embryos and meristems. Plant J. 22: 401–414. [DOI] [PubMed] [Google Scholar]
- Jackson D. (2002). Double labeling of KNOTTED1 mRNA and protein reveals multiple potential sites of protein trafficking in the shoot apex. Plant Physiol. 129: 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D., Veit B., Hake S. (1994). Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413. [Google Scholar]
- Jasinski S., Piazza P., Craft J., Hay A., Woolley L., Rieu I., Phillips A., Hedden P., Tsiantis M. (2005). KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 15: 1560–1565. [DOI] [PubMed] [Google Scholar]
- Johnston R., Candela H., Hake S., Foster T. (2010). The maize milkweed pod1 mutant reveals a mechanism to modify organ morphology. Genesis 48: 416–423. [DOI] [PubMed] [Google Scholar]
- Juarez M.T., Twigg R.W., Timmermans M.C. (2004). Specification of adaxial cell fate during maize leaf development. Development 131: 4533–4544. [DOI] [PubMed] [Google Scholar]
- Kiba T., Yamada H., Sato S., Kato T., Tabata S., Yamashino T., Mizuno T. (2003). The type-A response regulator, ARR15, acts as a negative regulator in the cytokinin-mediated signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 44: 868–874. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Johnston R., Yang Y., Gallavotti A., Kojima M., Travençolo B.A., Costa Lda.F., Sakakibara H., Jackson D. (2009). Studies of aberrant phyllotaxy1 mutants of maize indicate complex interactions between auxin and cytokinin signaling in the shoot apical meristem. Plant Physiol. 150: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.W., Bolduc N., Hake K., Htike Y., Hay A., Candela H., Hake S. (2014). Gene regulatory interactions at lateral organ boundaries in maize. Development 141: 4590–4597. [DOI] [PubMed] [Google Scholar]
- Lu P., Porat R., Nadeau J.A., O’Neill S.D. (1996). Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell 8: 2155–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevitch I., Thompson A., Muehlbauer G.J., Springer N.M. (2012). Brd1 gene in maize encodes a brassinosteroid C-6 oxidase. PLoS ONE 7: e30798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Candela H., Hake S. (2013). The Liguleless narrow mutation affects proximal-distal signaling and leaf growth. Development 140: 405–412. [DOI] [PubMed] [Google Scholar]
- Moreno M.A., Harper L.C., Krueger R.W., Dellaporta S.L., Freeling M. (1997). liguleless1 encodes a nuclear-localized protein required for induction of ligules and auricles during maize leaf organogenesis. Genes Dev. 11: 616–628. [DOI] [PubMed] [Google Scholar]
- Muehlbauer G.J., Fowler J.E., Freeling M. (1997). Sectors expressing the homeobox gene liguleless3 implicate a time-dependent mechanism for cell fate acquisition along the proximal-distal axis of the maize leaf. Development 124: 5097–5106. [DOI] [PubMed] [Google Scholar]
- Muehlbauer G.J., Fowler J.E., Girard L., Tyers R., Harper L., Freeling M. (1999). Ectopic expression of the maize homeobox gene liguleless3 alters cell fates in the leaf. Plant Physiol. 119: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J., Ji J., Werr W., Scanlon M.J. (2004). The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 131: 2827–2839. [DOI] [PubMed] [Google Scholar]
- Nikovics K., Blein T., Peaucelle A., Ishida T., Morin H., Aida M., Laufs P. (2006). The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18: 2929–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira F.T., Madi S., Chitwood D.H., Juarez M.T., Timmermans M.C. (2007). Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev. 21: 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D.L., Runions A., Sluis A., Bragg J., Vogel J.P., Prusinkiewicz P., Hake S. (2014). A division in PIN-mediated auxin patterning during organ initiation in grasses. PLOS Comput. Biol. 10: e1003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni L., Belles-Boix E., Günl M., Pautot V. (2008). Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell 20: 888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J., Bolduc N., Lisch D., Hake S. (2009). Distal expression of knotted1 in maize leaves leads to reestablishment of proximal/distal patterning and leaf dissection. Plant Physiol. 151: 1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D., Pesce E.R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon M.J. (2003). The polar auxin transport inhibitor N-1-naphthylphthalamic acid disrupts leaf initiation, KNOX protein regulation, and formation of leaf margins in maize. Plant Physiol. 133: 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon M.J., Henderson D.C., Bernstein B. (2002). SEMAPHORE1 functions during the regulation of ancestrally duplicated knox genes and polar auxin transport in maize. Development 129: 2663–2673. [DOI] [PubMed] [Google Scholar]
- Scanlon M.J., Schneeberger R.G., Freeling M. (1996). The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122: 1683–1691. [DOI] [PubMed] [Google Scholar]
- Schmülling T., Werner T., Riefler M., Krupková E., Bartrina y Manns I. (2003). Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J. Plant Res. 116: 241–252. [DOI] [PubMed] [Google Scholar]
- Shani E., Burko Y., Ben-Yaakov L., Berger Y., Amsellem Z., Goldshmidt A., Sharon E., Ori N. (2009). Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell 21: 3078–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman B.C. (1941). Development of the ligule in Zea mays L. Nature 147: 641. [Google Scholar]
- Shimizu R., Ji J., Kelsey E., Ohtsu K., Schnable P.S., Scanlon M.J. (2009). Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol. 149: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N., Hake S. (1990). Mutant characters of knotted maize leaves are determined in the innermost tissue layers. Dev. Biol. 141: 203–210. [DOI] [PubMed] [Google Scholar]
- Smith H.M., Boschke I., Hake S. (2002). Selective interaction of plant homeodomain proteins mediates high DNA-binding affinity. Proc. Natl. Acad. Sci. USA 99: 9579–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.M., Hake S. (2003). The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15: 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.G., Greene B., Veit B., Hake S. (1992). A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116: 21–30. [DOI] [PubMed] [Google Scholar]
- Sylvester A.W., Cande W.Z., Freeling M. (1990). Division and differentiation during normal and liguleless-1 maize leaf development. Development 110: 985–1000. [DOI] [PubMed] [Google Scholar]
- Takacs E.M., Li J., Du C., Ponnala L., Janick-Buckner D., Yu J., Muehlbauer G.J., Schnable P.S., Timmermans M.C., Sun Q., Nettleton D., Scanlon M.J. (2012). Ontogeny of the maize shoot apical meristem. Plant Cell 24: 3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Ashikari M., Fujioka S., Takatsuto S., Yoshida S., Yano M., Yoshimura A., Kitano H., Matsuoka M., Fujisawa Y., Kato H., Iwasaki Y. (2005). A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17: 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka W., Pautler M., Jackson D., Hirano H.Y. (2013). Grass meristems II: inflorescence architecture, flower development and meristem fate. Plant Cell Physiol. 54: 313–324. [DOI] [PubMed] [Google Scholar]
- Thimm O., Bläsing O., Gibon Y., Nagel A., Meyer S., Krüger P., Selbig J., Müller L.A., Rhee S.Y., Stitt M. (2004). MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 37: 914–939. [DOI] [PubMed] [Google Scholar]
- Timmermans M.C., Schultes N.P., Jankovsky J.P., Nelson T. (1998). Leafbladeless1 is required for dorsoventrality of lateral organs in maize. Development 125: 2813–2823. [DOI] [PubMed] [Google Scholar]
- To J.P., Haberer G., Ferreira F.J., Deruère J., Mason M.G., Schaller G.E., Alonso J.M., Ecker J.R., Kieber J.J. (2004). Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantis M., Brown M.I., Skibinski G., Langdale J.A. (1999). Disruption of auxin transport is associated with aberrant leaf development in maize. Plant Physiol. 121: 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B., Poree F., Nagel A., Lohse M., Czedik-Eysenberg A., Stitt M. (2009). A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, maize. Plant Cell Environ. 32: 1211–1229. [DOI] [PubMed] [Google Scholar]
- Waites R., Hudson A. (1995). PHANTASTICA: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121: 2143–2154. [Google Scholar]
- Wang Q., Kohlen W., Rossmann S., Vernoux T., Theres K. (2014). Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell 26: 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang J., Shi B., Yu T., Qi J., Meyerowitz E.M., Jiao Y. (2014). The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 26: 2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O., Shani E., Dolezal K., Tarkowski P., Sablowski R., Sandberg G., Samach A., Ori N. (2005). Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr. Biol. 15: 1566–1571. [DOI] [PubMed] [Google Scholar]
- Zhao S.Q., Xiang J.J., Xue H.W. (2013). Studies on the rice LEAF INCLINATION1 (LC1), an IAA-amido synthetase, reveal the effects of auxin in leaf inclination control. Mol. Plant 6: 174–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.