Proteasome-mediated protein homeostasis plays a role in the fine-tuning of plant flowering time in response to cold treatment via a series of genetic and epigenetic modifications.
Abstract
Winter-annual accessions of Arabidopsis thaliana require either exposure to cold stress or vernalization to initiate flowering via FRIGIDA (FRI). FRI acts as a scaffold protein to recruit several chromatin modifiers that epigenetically modify flowering genes. Here, we report that proteasome-mediated FRI degradation regulates flowering during vernalization in Arabidopsis. Our genetic and biochemical experiments demonstrate that FRI directly interacts with the BTB (Bric-a-Brac/Tramtrack/Broad Complex) proteins LIGHT-RESPONSE BTB1 (LRB1) and LRB2 as well as the CULLIN3A (CUL3A) ubiquitin-E3 ligase in vitro and in vivo, leading to proteasomal degradation of FRI during vernalization. The degradation of FRI is accompanied by an increase in the levels of the long noncoding RNA ColdAIR, which reduces the level of histone H3Lys4 trimethylation (H3K4me3) in FLOWERING LOCUS C chromatin to promote flowering. Furthermore, we found that the cold-induced WRKY34 transcription factor binds to the W-box in the promoter region of CUL3A to modulate CUL3A expression. Deficiency of WRKY34 suppressed CUL3A transcription to enhance FRI protein stability and led to late flowering after vernalization. Conversely, overexpression of WRK34 promoted FRI degradation and early flowering through inducing CUL3A accumulation. Together, these data suggest that WRKY34-induced and CUL3A-dependent proteolysis of FRI modulate flowering in response to vernalization.
INTRODUCTION
Flowering at the appropriate time is fundamental to the reproductive success of plants; thus, plants have evolved complex mechanisms to control the initiation of flowering in response to environmental cues or endogenous signals (Johanson et al., 2000; Kim et al., 2009). Many plant species require a long period of cold before flowering. In Arabidopsis thaliana, a major factor in determining flowering time in various accessions is the FRIGIDA (FRI) locus. FRI encodes a coiled-coil protein that activates the expression of FLOWERING LOCUS C (FLC), a MADS box transcription factor. In turn, FLC represses the expression of the so-called flowering pathway integrators FT, SUPPRESSOR OF OVEREXPRESSION OF CO1, and LEAFY. Thus, a high level of FLC expression results in late flowering in the winter-annual accessions of Arabidopsis, which begin vegetative growth during the fall and flower during the following spring (Kim et al., 2009). By contrast, the summer-annual forms of Arabidopsis have low FLC expression due to the absence of FRI and flower rapidly and thus complete their life cycle in a single growing season. Furthermore, allelic variation of FRI has been reported to be associated with variations in the flowering time of natural Arabidopsis accessions (Choi et al., 2011). For example, the Columbia and Landsberg erecta accessions of Arabidopsis both have early flowering times and possess deletion alleles at the FRI locus.
Screens for early-flowering mutants in a functional FRI-containing, winter-annual background led to the identification of genes, including FRIGIDA-LIKE1 (FRL1) and FRIGIDA ESSENTIAL1 (FES1), that are indispensable for the upregulation of FLC by FRI (Michaels et al., 2004; Schmitz et al., 2005; Choi et al., 2011). Additional components that affect the chromatin state of FLC have been reported to be involved in the FRI-mediated transcriptional activation of FLC (Geraldo et al., 2009; Choi et al., 2011). VERNALIZATION INDEPENDENCE/EARLY FLOWERING genes are components of the Paf complex, which is required for chromatin remodeling and transcriptional elongation (Dennis and Peacock, 2007). In addition, both the ATP-dependent chromatin remodeling protein PHOTOPERIOD-INDEPENDENT/EARLY FLOWERING1 and a putative component of a chromatin-remodeling complex, ACTIN-RELATED PROTEIN6, are required for the upregulation of FLC by FRI (Choi et al., 2011). Deficiency in mRNA metabolism, such as that caused by a mutation in the small (CBP20) or large (CBP80) subunit of cap binding protein, also suppresses FRI-mediated upregulation of FLC transcription (Geraldo et al., 2009). To date, most studies have focused on how FRI recruits its partner proteins to modulate FLC at the transcriptional or chromatin level; however, the effect of the stability of FRI itself on FLC transcription during vernalization is unclear.
Ubiquitin-mediated regulation of protein stability is a key regulatory mechanism in plant growth and development. Protein ubiquitination is largely regulated by E3 ubiquitin ligases, which direct the substrates to the 26S proteasome (Hua and Vierstra, 2011). To date, several hundred E3 ligases have been identified; among them, the most intensively studied subclass is the CULLIN-RING-type E3 ubiquitin ligase (CRL) superfamily. In this subclass, the CULLIN protein recruits a RING-finger protein at its C-terminal region, while its N terminus is used to bind substrate-adapter proteins. Four major E3 ligase families, which contain either a CULLIN protein (CUL1, CUL2, and CUL3) or the CULLIN-like protein APC2, have been reported in plants (Hua and Vierstra, 2011; Guo et al., 2013). The Arabidopsis genome encodes two CUL3 proteins, CUL3A and CUL3B. The disruption of both the encoding genes is embryo lethal, indicating the importance of these genes in plant development (Figueroa et al., 2005). The C-terminal region of CUL3 acts as a scaffold to bind the RING-finger protein RING-BOX PROTEIN1 (RBX1), while the N-terminal region recognizes proteins containing a BTB (Bric-a-Brac/Tramtrack/Broad Complex)/POZ (Pox virus and Zinc finger) fold. Arabidopsis contains over 80 BTB/POZ proteins, which can be divided into 12 subgroups based on their secondary domains. During the ubiquitination process, the BTB/POZ protein is required for CULLIN recruitment and also functions as an adaptor to allow binding to the substrate. Recently, roles of BTB/POZ proteins in plant responses to light, ethylene, abscisic acid, and fatty acid biosynthesis have been reported (Weber and Hellmann, 2009; Christians et al., 2012; Chen et al., 2013). LIGHT-RESPONSE BTB1 (LRB1) and BTB2 (LRB2) strongly influence Arabidopsis photomorphogenesis; the lrb1 lrb2 double mutant is hypersensitive to red light and altered in multiple developmental processes, including seed germination, cotyledon opening and expansion, chlorophyll accumulation, shade avoidance, and flowering time (Christians et al., 2012). LRB1/2 modulate the protein stability of phyB and phyD and are thus thought to play a role in proteasome-mediated protein degradation (Christians et al., 2012; Ni et al., 2014). In addition, CUL3-mediated ubiquitin ligases are involved in numerous physiological processes, including responses to attempted pathogen infection, root development, and blue light responses (Spoel et al., 2009; Thomann et al., 2009; Roberts et al., 2011). Arabidopsis HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1 (HOS1) encodes a RING-finger ubiquitin E3 ligase that modulates cold-responsive gene expression through the proteasome-mediated turnover of the transcription factor INDUCER OF CBF EXPRESSION1 (ICE1) (Chinnusamy et al., 2003). The hos1 mutant exhibits early flowering caused by the downregulation of FLC expression (Lee et al., 2001). Collectively, these findings suggest that CUL3-RING ligases might be required for flowering or vernalization in Arabidopsis.
To explore whether FRI homeostasis is involved in the Arabidopsis response to vernalization and to test for a possible role of the CUL3-mediated proteasome system in this process, we monitored the stability of FRI using transgenic Arabidopsis plants in which the expression of FRI fused to the green fluorescent protein (GFP) reporter was driven by the FRI promoter (pFRI:FRI-GFP). Our results showed that long-term cold stress promoted the degradation of FRI and promoted flowering by suppressing FLC transcription, a process dependent on CUL3A and associated with long noncoding RNA and chromatin modification. Furthermore, cold-induced WRKY34 transcription factors consistently accelerated CUL3A accumulation, which enhanced FRI proteolysis during vernalization. Our results establish mechanistic links between transcription factors, the ubiquitin proteasome pathway, and FRI homeostasis and highlight the sophisticated mechanisms that evolved to fine-tune plant flowering time in response to vernalization.
RESULTS
Expression of FRI-GFP Fusion Protein Delays Flowering Time in a Rapid-Flowering Arabidopsis Accession
FRI is required for FLC upregulation and thus its activity delays flowering time (Johanson et al., 2000). To investigate the mechanism underlying FRI function, we generated a FRI-GFP fusion protein driven by the native FRI promoter. The construct was transformed into the rapid-cycling accession Columbia-0 (Col-0), which carries recessive FRI alleles containing a 16-bp deletion that results in early flowering. As shown in Figure 1A, immunoblotting revealed that several transgenic ProFRI:FRI-GFP lines (hereafter termed FRI-GFP), represented by lines 3 and 7, accumulated FRI-GFP protein. Strong GFP fluorescence was also observed in the root tips and leaf epidermal cells of transgenic FRI-GFP lines (Supplemental Figure 1). The transgenic lines showed later flowering times without vernalization, but displayed similar flowering times as the wild-type Col-0 lines after 21 d of vernalization (Figures 1B and 1C), demonstrating that functional FRI can efficiently reverse the early-flowering phenotype in a rapid-cycle Arabidopsis accession by complementing the recessive loss-of-function FRI allele.
Figure 1.
Late-Flowering Phenotype of Transgenic FRI-GFP Lines.
(A) Immunoblot assay of FRI-GFP protein accumulation in Col-0 and FRI-GFP lines. Total protein was extracted from the 2-week-old wild-type Col-0 and transgenic FRI-GFP lines, and an anti-GFP antibody was used to measure the accumulation of FRI-GFP. Anti-ACTIN was used as a loading control. The experiment was repeated three times with similar results.
(B) Flowering phenotypes of Col-0 and FRI-GFP lines grown under long-day conditions (16 h light/8 h darkness). NV, without vernalization; 21DV, with 21 d of vernalization.
(C) Flowering time, as indicated by the rosette leaf number under long-day conditions (16 h light/8 h darkness) with or without vernalization. Data are the means ± sd for three biological replicates; for each line, 20 plants were scored. Bars with different letters are significantly different at P < 0.05 (Tukey’s test).
[See online article for color version of this figure.]
Expression and Subcellular Localization of FRI
We examined the subcellular localization of FRI in FRI-GFP lines. Using nucleus-specific 4′,6-diamidino-2-phenylindole (DAPI) staining, GFP fluorescence was observed only inside the nuclei of transgenic Arabidopsis leaf (upper panel, Figure 2A) and root (lower panel, Figure 2A) cells. Specifically, FRI-GFP fluorescence formed a compact body around the nucleus. This localization resembled that of the Cajal body, a well-studied nuclear compartment that is involved in the processing and maturation of several types of ribonucleoprotein complexes and also functions in epigenetic modulation. Arabidopsis Cajal bodies require a distant homolog of vertebrate coilin for Cajal body formation (Collier et al., 2006). To test the possibility that FRI colocalizes with the Cajal body, we generated a transgenic line expressing a fusion of Arabidopsis coilin with the red fluorescent protein (RFP) and termed this line Atcoilin-RFP. We then crossed FRI-GFP with the Atcoilin-RFP line and observed the localization of Cajal and FRI in the F1 generation of seedlings of the FRI-GFP Atcoilin-RFP lines. We observed colocalization between FRI-GFP fluorescence and Atcoilin-RFP fluorescence in the leaf epidermal and root cells (Figures 2C and 2D), and both FRI-GFP and Atcoilin-RFP fluorescence were localized inside the nucleus, as revealed by nucleus-specific DAPI staining (Figure 2B).
Figure 2.
FRI Localizes to the Cajal Body in the Nucleus.
(A) Subcellular localization of FRI in the leaf epidermal cells (upper panel) and root tip cells (lower panel) of 2-week-old FRI-GFP lines not subjected to vernalization treatment. From left to right: GFP fluorescence, DAPI staining showing the nuclei, bright-field (BF), merged image of GFP and DAPI, and merged image of GFP/DAPI/bright field. Bar = 20 μm.
(B) Colocalization of FRI-GFP and the Cajal body in the leaf epidermal (upper panels) and root tip (lower panels) cells of 2-week-old FRI-GFP Atcoilin-RFP lines not subjected to vernalization treatment. From left to right: RFP fluorescence, DAPI staining showing the nuclei, GFP fluorescence; bright field, merged image of RFP/GFP/DAP, and merged image of RFP/GFP/DAP/bright field. Bar = 20 μm.
Cold Stress Induces the Degradation of FRI
Vernalization treatment suppresses FLC transcription, whereas FRI efficiently upregulates FLC transcription (Kim et al., 2009). We hypothesized that FRI stabilization would influence FLC transcription under cold stress, in addition to the epigenetic regulation of FLC by FRI. To test this hypothesis, we first determined FRI protein stability in the transgenic FRI-GFP line after cold treatment. As shown in Figure 3A, we monitored GFP accumulation using a GFP-specific antibody and found that short-term cold stress, i.e., a 1- or 2-week treatment, did not cause a dramatic reduction of FRI-GFP protein in the FRI-GFP lines; however, a long-term cold treatment of over 2 weeks markedly reduced FRI-GFP accumulation (Figure 3A). Conversely, GFP accumulation was stable in the transgenic FRI-GFP line in the absence of cold stress (Figure 3A). We also monitored the levels of FRI-GFP transcript and found that cold stress did not significantly alter the levels of FRI-GFP transcript (Supplemental Figure 2), suggesting that the cold-induced reduction in FRI-GFP accumulation does not result from a change at the transcriptional level. To validate the dynamic changes in nuclear-localized FRI-GFP during cold stress, we also monitored the GFP fluorescence intensity in the leaf epidermal cells and root tip cells of FRI-GFP lines. We found that 3 or 4 weeks of cold treatment markedly suppressed GFP fluorescence in the leaf epidermal cells and root tip cells of FRI-GFP lines (Figures 3B and 3C).
Figure 3.
Vernalization Induces FRI Protein Degradation.
(A) Immunoblot analysis of FRI-GFP stability in the transgenic FRI-GFP lines over time either with (upper panel) or without (bottom panel) cold treatment. Two-week-old Arabidopsis seedlings were treated with cold stress at 4°C (upper panel) or without cold stress (bottom panel) for the indicated time periods, and total protein was extracted from the whole seedlings. Changes in the level of FRI-GFP protein were detected using an anti-GFP antibody; anti-ACTIN was used as a loading control. The experiment was repeated three times with similar results.
(B) and (C) Degradation of FRI-GFP in the leaf epidermal (upper panel) and root tip (lower panel) cells of transgenic FRI-GFP lines during vernalization stress. The transgenic 2-week-old FRI-GFP lines were subjected to cold stress for the indicated periods, and GFP fluorescence was monitored over time. From left to right: GFP, DAPI staining showing the nuclei, bright field (BF), merged image of GFP/DAP, and merged image of GFP/DAPI/bright field. Bars = 20 μm.
FRI Can Assemble CUL3 and LRB1/2 in Vivo and in Vitro
To explore the mechanism by which FRI regulates flowering time, we used FRI as the bait to screen an Arabidopsis cDNA library by the yeast two-hybrid (Y2H) assay. Among the several FRI-interacting proteins we identified was a member of the BTB protein family, LRB1. Y2H assays showed the strong interaction between LRB1 and FRI (Figures 4A and 4B). Since LRB2 is highly similar to LRB1 (Christians et al., 2012), we also tested for interactions between FRI and LRB2 in Y2H assays and found strong interactions between LRB2 and FRI (Figures 4A and 4B). After using SMART software to predict the location of the BTB domain in LRB1 and LRB2 (Supplemental Figure 3), we expressed truncated versions of the proteins in yeast. The N-terminal portion of LRB1, either including the BTB domain (1 to 250 amino acids, LRB1-T1) or lacking the BTB domain (1 to 136 amino acids, LRB1-T2), interacted with FRI in a Y2H assay, but the C-terminal portion lacking the BTB domain (250 to 561 amino acids, LRB1-T3) did not (Figure 4B). We generated the analogous LRB2 variants (Figure 4A) and similarly found that LRB2-T1 and LRB2-T2, but not LRB2-T3, interacted with FRI (Supplemental Figure 4). These findings indicate that the BTB domain in LRB1/2 is not necessary for their interaction with FRI in yeast. According to our SMART analysis, FRI contains a coiled-coil domain (Supplemental Figure 3). We generated truncated FRI proteins containing the N-terminal portion of FRI including the coiled-coil domain (1 to 300 amino acids, N-FRI) or the C-terminal portion of FRI (301 to 609 amino acids, C-FRI). N-FRI, but not C-FRI, interacted with LRB1 and LRB2 (Figure 4C), suggesting that the N-terminal region of FRI is important for its interaction with LRB1 and LRB2.
Figure 4.
Interaction of FRI with LRB1/2 and FRI in Yeast Cells.
(A) Schematic diagrams of full-length or truncated FRI, LRB1/2, and CUL3A proteins. The regions of site-directed mutagenesis in CUL3A are highlighted, and the coiled-coil, CH, and BTB domains are shown in black boxes. CH domain, cullin homology domain.
(B) and (C) FRI interacts with the N-terminal portion of LRB1 (LRB1-T1 and LRB2-T2; [B]), but only the N-terminal region of FRI interacts with the N-terminal region of LRB1/2 (C), as indicated by the ability of yeast cells to grow on synthetic dropout medium lacking Leu, Trp, His, and Ade in the presence of 3 mM 3-amino-1,2,4-triazole.
(D) and (E) FRI interacts with the N-terminal region of CUL3A (D), but only the C-terminal region of FRI is necessary for such an interaction (E), as indicated by the ability of yeast cells to grow on synthetic dropout medium lacking Leu, Trp, His, and Ade in the presence of 3 mM 3-amino-1,2,4-triazole.
[See online article for color version of this figure.]
Our Y2H screen also demonstrated a strong interaction between CUL3A and FRI. There are highly conserved N-terminal elements corresponding to the H2 helix in CUL3A, and mutation of the H2 helix domain (S48A/F49A) affects its interaction with BTB proteins (Figueroa et al., 2005). In addition, a K676R mutation in CUL3A abolishes the RUB (related to ubiquitin) modification of CUL3A (Figueroa et al., 2005). Here, we found that the CUL3A (S48A/F49A) and CUL3A (K676R) mutant proteins were still able to interact with FRI in yeast (Figures 4D and 4E), whereas the control, CUL4, did not. Furthermore, we confirmed that an N-terminal portion of CUL3A (1 to 400 amino acids, N-CUL3A), but not an C-terminal fragment (401 to 732 amino acids, C-CUL3A), interacted with FRI, suggesting that the C-terminal region of FRI and the N-terminal region of CUL3A are necessary for their interaction (Figure 4D).
In agreement with our Y2H assays, in vitro pull-down experiments using a glutathione S-transferase-fused FRI protein (GST-FRI) demonstrated that FRI coprecipitated with maltose binding protein-tagged CUL3A (MBP-CUL3A) or N-terminal CUL3A (MBP-N-CUL3A), but not C-terminal CUL3A (MBP-C-CUL3A). The control, GST alone, could not coprecipitate MBP-CUL3A, MBP-N-CUL3A, or MBP-C-CUL3A (Figures 5A and 5B). Furthermore, the GST-tagged C-terminal truncation of FRI (GST-C-FRI), but not the N-terminal truncation of FRI, coprecipitated with MBP-CUL3A or MBP-N-CUL3A, but not MBP-C-CUL3A (Figures 5A and 5B). Like MBP-CUL3A, the mutated variants MBP-CUL3A (S48A/F49A) and MBP-CUL3A (K676R) coprecipitated with GST-FRI and GST-N-FRI, but not with GST-N-FRI (Figures 5A and 5C). Similarly, we found that GST-FRI and GST-N-FRI, but not GST-C-FRI, coprecipitated with MBP-fused LRB1 or LRB2 (MBP-LRB1 and MBP-LRB2), or N-terminal truncations of LRB1 and LRB2 (MBP-N-LRB1 and MBP-N-LRB2) (Figure 5D; Supplemental Figure 5). We found that GST-FRI did not coprecipitate with C-terminal truncations of LRB1 and LRB2 (MBP-C-LRB1 and MBP-C-LRB2) (Figure 5D; Supplemental Figure 5).
Figure 5.
FRI Interacts with CUL3A and LRB1 in Vitro.
(A) Schematic diagrams of fusion proteins used. All fusion proteins carry tags at their N terminus. FRI was fused with GST; CUL3A and LRB1 were fused with MBP. The regions of site-directed mutagenesis in CUL3A are highlighted.
(B) In vitro pull-down assay of the interaction between FRI and CUL3A. GST, GST-FRI, GST-N-FRI, or GST-C-FRI was used as the bait and incubated with 1 mg of the indicated full-length or truncated prey protein. These experiments were repeated three times with similar results.
(C) In vitro pull-down assay of the effect of site-directed mutagenesis on the interaction between FRI and CUL3A. GST, GST-FRI, GST-N-FRI, or GST-C-FRI was used as the bait and incubated with 1 mg of the indicated prey protein. These experiments were repeated three times with similar results.
(D) In vitro pull-down assay of the effect of site-directed mutagenesis on the interaction between FRI and LRB1. GST, GST-FRI, GST-N-FRI, or GST-C-FRI was used as the bait and incubated with 1 mg of the indicated prey protein. These experiments were repeated three times with similar results.
[See online article for color version of this figure.]
We further examined the interaction between FRI and CUL3A or LRB1/2 in planta via bimolecular fluorescence complementation (BiFC), wherein the full length, N terminus, or C terminus of FRI was fused with the C-terminal half of yellow fluorescent protein (cYFP), while the full length, N terminus, or C terminus of CUL3A or LRB1/2 was fused with the N-terminal half of YFP (nYFP). Coexpression of full-length FRI with CUL3A or N-CUL3A resulted in strong yellow fluorescence, as did coexpression of C-FRI with N-CUL3A (Figures 6A and 6B). To localize FRI and CUL3A in the nucleus, we prepared transgenic Arabidopsis expressing the nuclear marker H2B-GFP (van Zanten et al., 2011). We found that YFP fluorescence localized within the region exhibiting GFP fluorescence by coexpression of CUL3A or N-CUL3A with FRI in living Arabidopsis protoplast cells expressing the H2B-GFP nuclear marker, suggesting that the interaction between FRI and CUL3A occurs inside the nucleus (Figure 6B). By contrast, coexpression of full-length FRI or C-FRI with C-CUL3A did not result in a fluorescence signal (Figures 6A and 6B). We next performed a coimmunoprecipitation (co-IP) analysis to test these interactions further by transiently expressing FRI and CUL3A, harboring GFP or 6xhemagglutinin (HA) tags, respectively, in tobacco (Nicotiana benthamiana) leaf cells. We found that CUL3A-6HA and N-CUL3A-6HA were coimmunoprecipitated by an anti-GFP antibody when they were coexpressed with either FRI-GFP or C-FRI-GFP, whereas no co-IP signal was produced by coexpressing FRI-GFP with C-CUL3A-6HA, or N-FRI-GFP with CUL3A-6HA. These results indicate that the C-terminal region of FRI and the N-terminal region of CUL3A interact in living cells (Figures 6C and 6E).
Figure 6.
FRI Interacts with CUL3A in Vivo.
(A) Schematic diagrams of fusion proteins used. Full-length or truncated CUL3A was fused to the N-terminal region of nYFP, and full-length or truncated FRI was fused to the C-terminal region of cYFP.
(B) BiFC assay of the interaction between FRI and CUL3A in planta. The indicated versions of FRI and CUL3A were cotransformed into living Arabidopsis protoplast cells expressing the H2B -GFP nuclear marker, and BIFC signal (yellow) was monitored. GFP signal reflects the nuclear localization. The data on the left represent the percentage of cells exhibiting BiFC signals, determined by counting the number of protoplasts with YFP fluorescence from 100 randomly selected protoplasts with H2B-GFP fluorescence. Data are the means ± sd of triplicate experiments. Bar = 10 μm.
(C) and (D) Co-IP of GFP-tagged FRI with HA-tagged CUL3A protein in vivo. Crude protein (input) was extracted from tobacco leaves transiently cotransformed with the indicated version of GFP-tagged FRI and HA-tagged CUL3A and immunoprecipitated (IP) with GFP antibody. Total and immunoprecipitated proteins were analyzed by immunoblotting using anti-GFP or anti-HA antibody. These experiments were repeated three times with similar results.
We then tested the interaction between FRI and LRB1/2 in vivo by BIFC and co-IP. As shown in Figures 7A and 7B, yellow fluorescence was produced upon coexpression of cYFP-FRI with truncated nYFP-LRB1/2-T1 or nYFP-LRB1/2-T2 in living Arabidopsis protoplast cells expressing the H2B-GFP nuclear marker. We also found an overlap between YFP and GFP fluorescence, suggesting that FRI and the N-terminal region of LRB1/2 interact inside the nucleus (Figure 7B). No signal was produced when cYFP-FRI was coexpressed with nYFP-LRB1/2-T3 (Figures 7A and 7B). The co-IP results also showed that LRB1/2-T1 were coimmunoprecipitated with FRI, whereas LRB1-T3 was not (Figures 7C and 7D). These results indicate that the N-terminal region of LRB1 or LRB2 is necessary for the interaction with FRI in vivo.
Figure 7.
FRI Interacts with LRB1 and LRB2 in Vivo.
(A) Schematic diagrams of fusion proteins used. Full-length or truncated LRB1/2 was fused to the N-terminal region of nYFP, and full-length FRI was fused to the C-terminal region of cYFP.
(B) BiFC assay showing the interaction between FRI and LBR1/2 in planta. The indicated versions of FRI and LRB1/2 were cotransformed into living Arabidopsis protoplasts expressing the H2B-GFP nuclear marker, and BIFC signal (yellow) was monitored. GFP signal reflects nuclear localization, and the data on the left indicate the percentage of cells exhibiting BiFC. Data are the means ± sd of triplicate experiments. Bar = 10 μm.
(C) and (D) Co-IP of HA-tagged FRI with Myc-tagged LRB1 (C) or LRB2 (D) protein in vivo. Crude protein (input) was extracted from tobacco leaves transiently cotransformed with the indicated version of GFP-tagged FRI and HA-tagged LRB1/2 and immunoprecipitated (IP) with GFP antibody. Total and immunoprecipitated proteins were analyzed by immunoblotting using anti-GFP or anti-HA antibody. These experiments were repeated three times with similar results.
FRI Is Degraded via the Ubiquitination-26S Proteasome Pathway
We used a cell-free degradation assay to monitor FRI turnover in the presence or absence of MG132, a specific inhibitor of the 26S proteasome. Recombinant GST-FRI protein was purified from transgenic Escherichia coli and then incubated with crude protein extracted from Arabidopsis leaves after 3 weeks of cold treatment. GST-FRI was rapidly degraded (Figure 8A); however, when these protein extracts were incubated with free GST protein, we did not observe degradation of GST, suggesting that FRI protein degradation is specific. Induction of GST-FRI degradation by the crude proteins extracted from Arabidopsis leaves after cold treatment could be completely suppressed by the 26S proteasome inhibitor MG132. As a control, GST-FRI degradation remained nearly unchanged throughout incubation in the solvent DMSO without MG132 (Figure 8B). These data demonstrate that the 26S proteasome degrades FRI specifically after cold stress.
Figure 8.
Degradation of FRI during Cold Stress.
(A) Cell-free analysis of cold-induced GST-FRI degradation in vivo. Purified GST or GST-FRI protein from transgenic E. coli was incubated with cell lysates from 2-week-old Arabidopsis leaves after 3 weeks of cold stress. At different indicated times of incubation, the level of GST or GST-FRI was monitored by immunoblot using anti-GST antibody. Anti-ACTIN was used as the loading control. These experiments were repeated three times with similar results.
(B) Cell-free analysis of the effect of proteasome inhibitor MG132 on GST-FRI degradation in vivo. The purified GST-FRI from E. coli was incubated with cell lysates from 2-week-old Arabidopsis leaves after 3 weeks of cold stress, in the presence of 50 μM MG132, or the solvent 2% DMSO as the control. At different times, the level of GST-GFP was monitored by immunoblot analysis using anti-GST antibody. Anti-ACTIN was used as the loading control. These experiments were repeated three times with similar results.
(C) The degradation of FRI was suppressed in the cul3a or lrb1 lrb2 mutants. Purified GST-FRI was incubated with cell lysates from 2-week-old wild-type Arabidopsis, the cul3a mutant, or the lrb1 lrb2 mutant after 3 weeks of cold stress. At different time points, the level of GST-FRI was monitored by immunoblot using anti-GST antibody. Anti-ACTIN was used as the loading control. These experiments were repeated three times with similar results.
(D) Cold stress induced FRI-GFP degradation in vivo. Two-week-old wild-type, cul3a, and lrb1lrb2 lines were treated with cold for various periods of time, the total protein was extracted from the while seedlings, and FRI-GFP was monitored by immunoblot analysis using anti-GFP antibody. Anti-ACTIN was used as the loading control. These experiments were repeated three times with similar results.
(E) and (F) Detection of in vivo polyubiquitinated FRI-GFP in the wild-type, cul3a, or lrb1/lrb2 background after cold treatment. The crude protein extract from the whole seedlings of 2-week-old FRI-GFP and FRI-GFP cul3a (E) or FRI-GFP lrb1 lrb2 (F) lines was incubated with ubiquitin binding p62 resin to enrich for ubiquitinated protein and then analyzed by immunoblot using antibody against GFP. Anti-ACTIN was used for the input loading control. These experiments were repeated three times and similar results were obtained.
To investigate the possible roles of CUL3A and LRB1/2 in proteasome-mediated FRI degradation, we searched the SIGNAL T-DNA insertion population and identified mutants in CUL3A (SALK_ 046638, cul3a), LRB1 (SALK_145146, lrb1), and LRB2 (SALK_001013, lrb2). We also crossed lrb1 with lrb2 to obtain the double mutant line lrb1 lrb2. RT-PCR analysis of homozygous lines indicated that all three mutants lacked their corresponding transcripts, confirming that they are null mutants (Supplemental Figures 6 and 7). Degradation of GST-FRI was markedly retarded upon incubation with the crude proteins extracted from the cul3a or lrb1 lrb2 lines after 3 weeks of cold stress compared with those from wild-type lines (Figure 8C). These data suggest that CUL3A and LRB1/2 are necessary for GST-FRI degradation. We also determined the FRI-GFP degradation among the cul3a mutant, lrb1 lrb2 double mutant, and wild-type Col-0 lines after cold treatment and found that FRI-GFP degradation was markedly suppressed in the cul3a and lrb1 lrb2 mutant lines compared with the Col-0 line (Figure 8D).
Based on the findings that a CUL3A-, LRB1/2-, and proteasome-dependent process mediates FRI degradation, we then measured the in vivo ubiquitylation (Ub) status of FRI. For this assay, Ub-conjugated protein was enriched from the FRI-GFP line using Ub binding p62 resin and the resulting protein was probed with anti-GFP antibody. Immunoblots showed a marked increase in polyubiquitinated FRI-GFP bands with increasing time of cold treatment of the FRI-GFP line, indicating that cold stress induces FRI-GFP polyubiquitination (Figures 8E and 8F). By contrast, FRI-GFP polyubiquitination was markedly decreased in the cul3a or lrb1 lrb2 background (Figures 8E and 8F), consistent with CUL3A and LRB1/2 being required for FRI polyubiquitination and degradation after cold stress. The FRI-GFP polyubiquitination status in the lrb1 or lrb2 single mutant background was similar to that in the Col-0 background (Supplemental Figure 8), again demonstrating the functional redundancy of LRB1 and LRB2.
CUL3A-Mediated FRI Degradation Modulates Flowering Time via Long Noncoding RNA (ColdAIR) and Histone Methylation
Previous studies showed that the cul3A mutant is late flowering (Thomann et al., 2009), indicating a possible role for the CUL3 protein complex in controlling flowering time. Our findings suggest that CUL3A deficiency delayed flowering time in the FRI-GFP cul3a line under basal conditions, as well as following 3 weeks of cold treatment (Figure 9A). In accordance with these data, the FRI-GFP in the cul3a background was upregulated in the FRI-GFP line and was further increased in the FRI-GFP cul3a mutant following a 3-week cold treatment but not under basal conditions (Figure 9A).
Figure 9.
CUL3A-Dependent FRI-GFP Degradation Affects ColdAIR Generation and H3K4me3 Levels at FLC Chromatin.
(A) Flowering time and FLC transcript level of Col-0, cul3a, FRI-GFP, and FRI-GFP cul3a transgenic lines with or without vernalization. Data are the means ± sd for three biological replicates; for each line, 20 plants were scored. Bars with different letters are significantly different at P < 0.05 (Tukey’s test). NV, without vernalization; 21DV, with 21 d of vernalization.
(B) Quantitative RT-PCR analysis of long noncoding RNA ColdAIR transcript in Col-0, FRI-GFP, FRI-GFP cul3a, and FRI-GFP lrb1 lrb2 transgenic lines with or without vernalization. Means ± sd of quantitative RT-PCR data relative to ACTIN2 are shown (n = 10). 14DV, with 14 d of vernalization.
(C) Effect of COLDAIR level on flowering time during vernalization. Two-week-old transgenic FRI-GFP, FRI-GFP cul3a, one representative COLDAIR RNAi knockdown line (COLDAIR-RNAi-4#, left panel), and one representative COLDAIR overexpression line (COLDAIR-OX-7#, right panel) were treated with 14 or 21 d of vernalization or were not subjected to vernalization treatment, and the flowering times were determined. Data are the means ± sd for three biological replicates; for each line, 20 plants were scored. Bars with different letters are significantly different at P < 0.05 (Tukey’s test).
(D) ChIP assay of the relative levels of trimethylation (H3K4me3) in FLC chromatin. DNA fragments obtained by ChIP using H3K4me3 antibody were analyzed by quantitative PCR. The primer pairs used in PCR are shown as bars below the FLC gene; exons are shown as blue boxes and introns as black lines. Data are the means ± sd of triplicate experiments. Bars with different letters are significantly different at P < 0.05 (Tukey’s test).
[See online article for color version of this figure.]
A long intronic noncoding RNA, ColdAIR, modulates the Arabidopsis vernalization response at the epigenetic level, and suppression of ColdAIR generates delays in flowering time after vernalization treatment (Heo and Sung, 2011). We found that cold treatment for 21 d increased the level of ColdAIR in wild-type and FRI-GFP transgenic plants (Figure 9B), which is consistent with previous results (Heo and Sung, 2011). To address the biological function of ColdAIR in CUL3A-regulated flowering time, we used RNA interference (RNAi) to knock down the expression of ColdAIR in FRI-GFP or FRI-GFP cul3a lines. In agreement with previous results, we found that several of the ColdAIR-RNAi lines in the FRI-GFP background (termed FRI-GFP RNAi) showed later flowering times (Supplemental Figure 9A) and that the COLDAIR RNAi knockdown line in the FRI-GFP cul3a background (termed FRI-GFP cul3a RNAi) also showed later flowering after 14 or 21 d of vernalization (Figure 9C). By contrast, we upregulated the expression of ColdAIR by overexpressing ColdAIR under the control of a cauliflower mosaic virus (CaMV) 35S promoter in the FRI-GFP background (termed FRI-GFP OX) or in FRI-GFP cul3a lines (termed FRI-GFP cul3a OX). We found that both the FRI-GFP OX and FRI-GFP cul3a OX lines presented early flowering time compared with the FRI-GFP and FRI-GFP cul3a lines, respectively, with or without vernalization treatment (Supplemental Figure 9B; Figure 9C).
We then measured the level of histone H3Lys4 trimethylation (H3K4me3), a histone mark associated with active FLC chromatin. Consistent with the FLC transcript patterns, the H3K4me3 level in the FLC-1, FLC-2, and FLC-3 regions was increased in the transgenic FRI-GFP lines without cold treatment and rapidly decreased after cold treatment for 14 and 21 d. However, this decline was less pronounced in the FRI-GFP cul3a line than in the FRI-GFP line, suggesting that CUL3A influences FRI-mediated flowering time regulation at the epigenetic level (Figure 9D).
The lrb1 lrb2 double mutant showed delayed flowering (Christians et al., 2012). We introduced FRI-GFP into the lrb1 lrb2 double mutant (referred to as FRI-GFP lrb1 lrb2) and found that the flowering time of FRI-GFP lrb1 lrb2 was insensitive to cold treatment, unlike the FRI-GFP line (Supplemental Figure 10). In addition, less ColdAIR small interfering RNA was present in the FRI-GFP lrb1 lrb2 line compared with the FRI-GFP line after 3 weeks of cold treatment (Figure 9B), suggesting that LRB1/2 play a role in the epigenetic modification of FLC transcripts during cold stress.
WRKY34 Modulates CUL3A Transcription by Binding to Its Promoter Region
To further examine the role of FRI in flowering time, we used the FRI-GFP line to set up an activation tag pool by randomly inserting the 4X35S strong promoter into the genome of FRI-GFP lines using a previously reported method (Weigel et al., 2000). Since the FRI-GFP line showed earlier flowering after cold treatment, we focused on one line (named FRI-sup1) that still exhibited later flowering after cold treatment (Supplemental Figure 11A). Using the thermal asymmetric interlaced-PCR approach described before (Weigel et al., 2000), we localized the T-DNA tag to the first exon of WRKY34 in the FRI-sup1 line; the insertion inactivated WRKY34 transcription in the FRI-sup1 line (Supplemental Figures 11B and 11C). To confirm the phenotype of the FRI-sup1 mutant, we obtained a wrky34 T-DNA mutant (SALK_133019; Supplemental Figure 12), which was allelic to FRI-sup1. We crossed wrky34 into FRI-GFP and found that FRI-GFP wrky34 was later flowering, like FRI-sup1 (Supplemental Figure 11A). Furthermore, we crossed FRI-GFP wrky34 with FRI-sup1 and found that the heterozygous F1 generation of FRI-GFP wrky34/FRI-sup1 still showed later flowering after vernalization. However, we crossed FRI-GFP wrky34 or FRI-sup1 with wild-type Col-0 and found, like the FRI-GFP line, that the heterozygous F1 generation of FRI-GFP wrky34/Col or FRI-sup1/Col showed earlier flowering time after vernalization treatment (Supplemental Figure 13), suggesting that WRKY34 loci determine FRI-mediated flowering time regulation during vernalization.
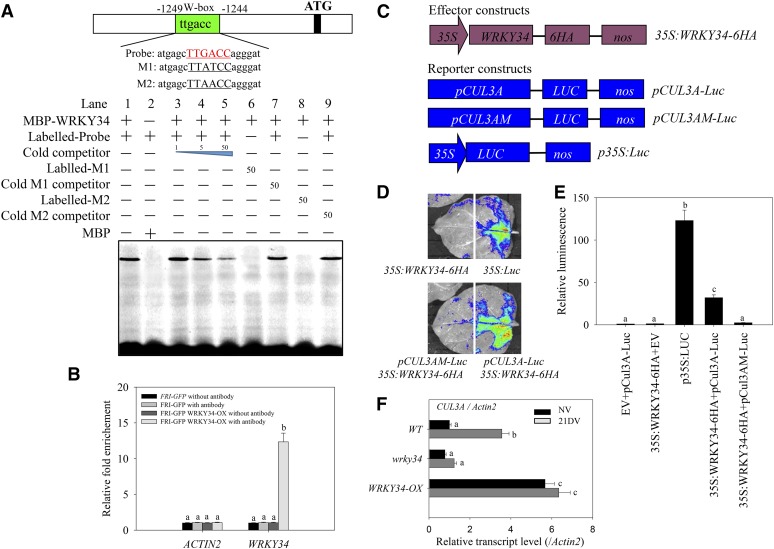
The WRKY proteins belong to a transcription factor superfamily, with up to 72 representatives in Arabidopsis. Phylogenetic analysis showed that WRKY34 is closely related to WRKY2 (Supplemental Figure 14), which, like WRKY34, contains two WRKY domains. For most WRKY transcription factors, the binding site is the W-box (C/T)TGAC(T/C), an element found in the promoters of many stress-related plant genes (Rushton et al., 2010). Using promoter sequence analysis, we identified one W-box (TTGACC) in the promoter region of CUL3A. An electrophoretic mobility shift assay (EMSA) revealed that the W-box (TTGACC) was bound by a WRKY34-MBP fusion protein, but not by MBP alone (Figure 10A). An unlabeled W-box probe competitively blocked binding of the labeled W-box to WRKY34-MBP (Figure 10A). The mutated W-box probe (TTATCC or TTAACC) was not bound by WRKY34-MBP, confirming the specificity of the interaction between the WRK34 transcription factor and the W-box region in the promoter of CUL3A. We then generated a transgenic line, WRKY34-OX, which constitutively expressed WRKY34-6HA under the control of the CaMV 35S promoter. Significant WRKY34 protein accumulation was detected in this transgenic line using a commercial HA-tag antibody (Supplemental Figure 11D). Using this line, chromatin immunoprecipitation (ChIP) assays revealed that HA-tagged WRKY34 bound to the CUL3A promoter (Figure 10B).
Figure 10.
WRKY34 Binds to the Promoter Region of CUL3A.
(A) In vitro EMSA showing that the WRKY34-MBP fusion protein, but not MBP alone, binds to the CUL3A promoter (Probe). The diagram depicts the promoter sequence of CUL3A. The red letters represent the putative W-box binding element (TTGACC). “Competitor” indicates 1-, 5-, and 50-fold excess of unlabeled competitor probe added to compete with the labeled probe. M1, TTGACC was mutated to TTATCC; M2, TTGACC was mutated to TTAACC. Either labeled M1/M2 or their cold competitor was added in 50-fold excess to test their specific binding to WRKY34-MBP.
(B) ChIP enrichment showing the in vivo binding ability of WRKY34 to the W-box region in the promoter of CUL3A. The ChIP results were normalized to input chromatin, and a fragment in the ACTIN2 promoter was used as the negative control. Data are the means ± sd of triplicate experiments. Bars with different letters are significantly different at P < 0.05 (Tukey’s test).
(C) Schematic diagrams of effector and reporter constructs used in the tobacco leaf and Arabidopsis protoplast transcription system.
(D) Transient expression of the p35S:WRKY34-6HA effector construct with pCUL3A-LUC and pCUL3AM-LUC reporter constructs in tobacco leaves. Representative bioluminescence images are shown. 35S:Luc was used as the positive control.
(E) Expression activity of the p35S:WRKY34-6HA effector construct with pCUL3A-LUC and pCUL3AM-LUC reporter constructs in Arabidopsis protoplasts. Transformation with 35S:GUS was used as an internal control. Data are the means ± sd of triplicate experiments. Bars with different letters are significantly different at P < 0.05 (Tukey’s test). EV, empty vector.
(F) CUL3A transcript levels in wild-type (WT), WRKY34-null (wrky34), and transgenic WRKY34-OX (WRKY34-OX) lines with or without vernalization for 3 d. Means ± sd of quantitative RT-PCR data relative to ACTIN2 are shown (n = 10). Bars with different letters are significantly different at P < 0.05 (Tukey’s test). 21DV, with 21 d of vernalization.
To confirm that the binding of WRKY34 to the W-box region was required for CUL3A transcription, we employed a transient expression system in which luciferase (LUC) was under the control of the CUL3A promoter. We measured LUC activity after coinjecting the effector construct 35S:WRKY34-6HA with the pCUL3A-LUC reporter construct or a mutated pCUL3AM-LUC construct, which had TTATCC in place of TTGACC in the W-box region (Figure 10C), into tobacco and Arabidopsis leaf protoplasts. We found that WRKY34 expression resulted in strong LUC activity (Figure 10D) and increased the expression of LUC (Figure 10E) as driven by the native CUL3A promoter but not by the mutated CUL3A promoter. In the WRKY34-OX line, CUL3A transcript accumulation was substantially increased with or without cold treatment compared with the wild type or the wrky34 mutant (Figure 10F). Thus, these results suggest that WRKY34 binds the putative W-box in the CUL3A promoter to increase its transcription.
WRKY34 Overexpression Accelerates FRI Degradation and Advances Flowering Time
The WRKY34 transcript was rapidly induced by cold stress, reaching a maximum level after 3 d of cold treatment, and was maintained at a high level for at least 3 weeks (Figure 11A). Cold stress also increased CUL3A transcript levels, but gradually suppressed FLC transcripts (Figure 11A). In the wrk34 mutant, cold-induced transcription of CUL3A was impaired (Figure 11A). Given that our results demonstrated that WRKY34 could activate CUL3A transcription by binding its W-box and that CUL3A modulated FRI degradation and flowing time, we wanted to establish how WRKY34 might affect FRI protein stability and flowering time. We first obtained the following lines by crossing FRI-GFP cul3a, FRI-GFP wrky34, FRI-GFP WRKY34-OX, and WRKY34-OX FRI-GFP cul3a (hereafter termed Triple). FRI-GFP expression was validated in these lines (Figure 11B). Without vernalization treatment, FRI-GFP protein accumulation in the FRI-GFP line was higher than in the FRI-GFP WRKY34-OX line but still lower than in the FRI-GFP wrky34, FRI-GFP cul3a, and Triple lines (Figure 11B). FRI-GFP was markedly degraded after 21 d of vernalization in the FRI-GFP line, and its degradation kinetics were accelerated in the WRKY34-OX FRI-GFP line, where FRI-GFP could not be detected even after 21 d of vernalization treatment. These data suggest that the WRKY34-OX FRI-GFP line has an increased capacity to degrade FRI-GFP compared with the FRI-GFP line. To test this possibility, we incubated an equal amount of FRI-GFP protein with crude proteins extracted from FRI-GFP or WRKY34-OX FRI-GFP without cold treatment. FRI-GFP remained stable when incubated with the FRI-GFP extract but exhibited some degradation when incubated with WKRY34-OX FRI-GFP extract (Supplemental Figure 15). In the Triple line, as in the FRI-GFP cul3a and FRI-GFP wkry34 lines, FRI-GFP protein degradation was markedly delayed after 21 d of vernalization compared with the FRI-GFP line (Figure 11B).
Figure 11.
WRKY34 Promotes CUL3A Expression to Modulate FRI-GFP Degradation and Flowering Time.
(A) Quantitative RT-PCR analysis of WRKY34, CUL3A, and FLC transcripts in FRI-GFP (left) and FRI-GFP wrky34 (right) after exposure to cold stress for the indicated periods. Two-week-old FRI-GFP or FRI-GFP wrky34 lines were subjected to vernalization for the indicated periods of time, and the transcript levels of WRKY34, CUL3A, and FLC were measured by quantitative RT-PCR using RNA extracted from whole seedlings. Data are the means ± sd of triplicate experiments.
(B) Immunoblot analysis of FRI-GFP protein accumulation in FRI-GFP, FRI-GFP wrky34, FRI-GFP cul3a, FRI-GFP WRKY34-OX, and FRI-GFP WRKY34-OX cul3a (Triple) lines without vernalization or with 14 and 21 d of vernalization treatment. Two-week-old FRI-GFP, FRI-GFP wrky34, FRI-GFP cul3a, FRI-GFP WRKY34-OX, and FRI-GFP WRKY34-OX cul3a (Triple) lines were subjected to 14 or 21 d of vernalization, total protein was extracted from the whole seedlings, and FRI-GFP accumulation was determined using anti-GFP antibody. Anti-ACTIN was used as the loading control. The ratio of FRI-GFP to ACTIN protein signal is listed. These experiments were repeated three times with similar results. NV, without vernalization; 14DV, with 14 d of vernalization; 21DV, with 21 d of vernalization.
(C) The flowering time and FLC transcripts in wild-type Col-0, WRKY34-OX, FRI-GFP wrky34 FRI-GFP, FRI-GFP WRKY34-OX, and FRI-GFP WRKY34-OX cul3a lines without or with 21 d vernalization treatment. Data are the means ± sd of three biological replicates; for each line, 20 plants were scored. Bars with different letters are significantly different at P < 0.05 (Tukey’s test).
We then compared flowering time among these lines. The WRKY34-OX line showed earlier flowering compared with the wild-type Col-0 under a long-day photoperiod, but vernalization treatment did not significantly accelerate the time to flowering (Figure 11C). The FRI-GFP wrky34 line had similar late flowering to that of the FRI-GFP line without vernalization, possibly due to a saturation effect of FRI-GFP. However, the FRI-GFP wrky34 line still presented later flowering after vernalization treatment compared with the FRI-GFP line (Figure 11C). Flowering was markedly earlier in the FRI-GFP WRKY34-OX line than in the FRI-GFP line without vernalization, although they presented similar early flowering phenotypes after vernalization. The Triple line showed markedly later flowering relative to the FRI-GFP WRKY-34-OX line and also presented later flowering after vernalization treatment (Figure 11C). Consistent with the observed flowering time, FLC transcript levels in the FRI-GFP WRKY34-OX lines were lower than those of the wild-type Col-0 line, and the FRI-GFP line exhibited a high level of FLC transcript before vernalization. The vernalization treatment substantially suppressed FLC transcript accumulation. By comparison, Triple exhibited increased accumulation of FLC transcript after vernalization treatment. Unlike the FRI-GFP WRKY-34-OX line, which had a low level of FLC transcript before or after vernalization, the Triple line had a relatively high level of FLC transcript before or after vernalization (Figure 11C). These data suggest that vernalization-induced FRI degradation and earlier flowering depend on both WRKY34 and CUL3A.
DISCUSSION
Vernalization Promotes Proteasome-Dependent FRI Degradation
Sequence variation in FRI is responsible for different Arabidopsis responses to vernalization (Johanson et al., 2000). Here, we found that expressing a GFP fusion of FRI derived from a winter-annual accession of Arabidopsis significantly delayed flowering in the rapid-cycle Arabidopsis accession Col-0 (Figure 1). This system enabled us to investigate dynamic changes of FRI abundance in response to cold stress using a GFP-specific antibody. FRI acts as a scaffold to recruit a series of protein components, including DNA binding proteins, chromatin remodelers, and transcriptional activators, which collectively stimulate FLC expression in winter-annual Arabidopsis accessions (Geraldo et al., 2009; Choi et al., 2011). In particular, FRI regulates the epigenetic structure of FLC by modulating H3 histone methylation and acetylation to silence FLC transcription during vernalization (Bastow et al., 2004; He and Amasino, 2005; Coustham et al., 2012). However, a potential role for FRI turnover in the control of flowering time has received little attention, possibly because of the absence of an available antibody against FRI. In our FRI-GFP transgenic lines, we observed strong GFP fluorescence in the nucleus that colocalized with the Cajal body, a nuclear organelle containing high concentrations of splicing proteins and other RNA-processing factors (Li et al., 2006). Our colocalization results for FRI-GFP with the Cajal body suggest a role for FRI in mRNA splicing or long noncoding RNA modification (Figure 2B). Recent studies identified small Cajal body-specific RNAs that catalyze the methylation and pseudouridylation of small nuclear RNAs within Cajal bodies (Li et al., 2006). In agreement with this finding, we also found that FRI was involved in histone H3Lys4 trimethylation (Figure 9D) and ColdAIR generation (Figures 9B and 9C).
A previous study reported that the E3 ligase HOS1 mediates cold-responsive ICE1 degradation through ubiquitination and the 26S proteasome pathway during cold stress (Dong et al., 2006; Jeon and Kim, 2013). Here, several pieces of independent evidence show that cold treatment induces proteasome-dependent FRI degradation: (1) cold treatment led to reduced accumulation of FRI-GFP (Figures 3A to 3C), without a change in FRI-GFP transcription; (2) the proteasome inhibitor MG132 stabilized FRI-GFP after cold stress (Figure 8B); (3) ubiquitination of FRI was detected and prolonged cold treatment increased the degree of FRI polyubiquitination (Figures 3A, 8E, and 8F). Together, our results demonstrate that vernalization-induced FRI-GFP degradation results in early flowering, as suppressing FRI-GFP turnover by MG132 treatment or the absence of CUL3A delayed the flowering time under cold conditions.
CUL3-based E3 ubiquitin ligases are important regulators of diverse developmental processes and environmental responses in eukaryotic organisms (Figueroa et al., 2005). CUL3 can associate with RBX1 and BTB proteins to form functional E3 ligases (Weber et al., 2005). Here, we provide both in vitro and in vivo evidence that FRI interacts with CUL3A and LRB1/2 containing a BTB domain and is targeted in Arabidopsis by a CRL3LRB1/2 complex (Figures 4 to 7). It was previously demonstrated that LRB1/2 affect the stability of phyB and phyD, although LRB1/2 do not interact with phyB/D (Christians et al., 2012). Our findings establish that FRI is a target of LRB1/2, which are required for destabilization of FRI after cold stress (Figures 4, 5, and 7). This is also supported by the stabilization of FRI-GFP in the lrb1 lrb2 background (Figures 8C and 8D), which occurred along with increased levels of FLC transcript and later flowering after vernalization (Figures 9A and 9B). Our data also suggest that LRB1/2, like other BTB proteins, assemble with CUL3A through their C-terminal region and with FRI through their N-terminal region. LRB1/2 assemble with CUL3A to modify red light signaling in Arabidopsis (Christians et al., 2012), indicating that LRB1/2-mediated red light signaling might play a role in regulating FRI degradation and controlling flowering time during vernalization. A previous study showed that CUL3 interacts with LRB1 and LRB2 (Christians et al., 2012); here, we found that CUL3A also interacts with FRI and that the C terminus of FRI and the N terminus of CUL3A are necessary for this interaction (Figures 4, 5, and 7). This is consistent with a previous report that the C terminus of CUL3A plays a role in its interaction with RUB protein (Figueroa et al., 2005). We further confirmed that CUL3A and LRB1/2 are required for proteasome-mediated FRI degradation (Figures 8E and 8F; Supplemental Figures 8). Overall, our data show that LRB1/2 assemble CUL3 and FRI to promote proteasome-dependent FRI degradation during vernalization.
FRI Degradation Promotes Flowering through Histone Modification and Long Noncoding RNA ColdAIR
Previous investigations demonstrated that FRI acts as a scaffold protein that interacts with FRL1, FES1, SUPPRESSOR OF FRI4 (SUF4), FLC EXPRESSOR, and CBC (nuclear cap binding complex) proteins, including CBP20 and CBP80, to form transcriptional activator complex FRI-C (Choi et al., 2011). FRI-C can recruit transcription factors, such as the TAF14 (transcription initiation factor) homolog, and chromatin modification factors, such as the SWR1 complex and the SET2 homolog, to catalyze histone H2B ubiquitination/deubiquitination, and H3K4 and H3K36 methylation (He et al., 2004; Kim et al., 2005; Choi et al., 2011). Here, we found a clear reduction in the level of H3K4me3, a histone marker associated with active chromatin, at FLC chromatin in FRI-GFP transgenic lines after cold stress (Figure 9D). This reduction was compromised in the cul3a mutant or lrb1 lrb2 mutant background, which suggests that proteasome-dependent FRI degradation results in a low level of H3K4me3 at FLC chromatin, which might suppress FLC expression. This may reflect the fact that reduced levels of FRI fail to recruit sufficient interacting partners under cold stress to process FLC chromatin modifications.
A cold-induced long noncoding RNA, ColdAIR, is thought to mediate the epigenetic silencing of FLC in Arabidopsis (Heo and Sung, 2011; Kim and Sung, 2012). We also detected a change in ColdAIR, which is derived from the first intron of nascent FLC transcript. Consistent with a previous study (Heo and Sung, 2011), ColdAIR was clearly induced by cold treatment in FRI-GFP transgenic lines to repress FLC epigenetically (Figure 9B). However, in the cul3a mutant or lrb1 lrb2 mutant background, the level of ColdAIR rebounded after cold treatment, and the H3K4me3 level also increased in these mutants (Figure 9D). These results indicate interplay between ColdAIR and FRI stabilization, and it is possible that cold treatment promotes the degradation of FRI, which then cannot recruit enough CBC complex proteins, such as CBP20 and CBP80, to increase the proportion of FLC transcripts lacking a 5′-cap. Absence of the FRI partner CBP20 results in very low FLC mRNA levels and increases the proportion of unspliced FLC transcripts (Geraldo et al., 2009). It is possible that FRI protein degradation led to the production of more uncapped nascent FLC transcripts, providing a stable pool for ColdAIR generation.
WRKY34-Dependent CUL3A Accumulation Regulates FRI Protein Homeostasis to Modulate Flowering during Vernalization
Ubiquitin protein-ligase (E3) enzymes specify target substrates, and thus have an important role in protein turnover (Hua and Vierstra, 2011). FRI contains a coiled-coil domain that is important for protein interactions or the formation of a protein scaffold (Choi et al., 2011), and we found that FRI interacted with the CUL3A E3 ubiquitin ligase. The FRI-GFP cul3a lines had delayed flowering compared with the FRI-GFP line (Figure 9A), and consistent with FRI being a target of CUL3A, the FRI-GFP cul3a line exhibited slower FRI-GFP degradation during cold stress (Figures 8C and 8E). At the same time, the FRI-GFP cul3a line also had delayed flowering and lower H3K4me3 at the FLC locus and ColdAIR accumulation relative to the FRI-GFP line (Figures 9A to 9D), suggesting that CUL3-dependent FRI degradation plays an important role in vernalization-induced repression of FLC expression.
WRKY transcription factors specifically bind the W-box (TTGACY) in the promoter region of target genes and play an important role in plant responses to environmental stress and developmental processes (Rushton et al., 2010). WRKY transcription factors can bind the W-box region of the promoter of the disease resistance gene NPR1 to initiate its transcription (Eulgem and Somssich, 2007), and the CUL3-dependent proteasome degradation of NPR1 showed a dual role in regulating plant immunity (Spoel et al., 2009). The HECT E3 ubiquitin ligase degrades WRKY53 to regulate Arabidopsis leaf senescence (Miao and Zentgraf, 2010), indicating that there is interplay between WRKY and E3 ubiquitin ligases. Here, our data suggest that WRKY34 efficiently binds the W-box in the CUL3A promoter region (Figure 10A), and WRKY34 overexpression clearly increased CUL3A transcription (Figure 10F). Previous studies demonstrated that cold treatment induces the transcription of WRKY34 (Rushton et al., 2010; Zou et al., 2010). We propose that cold stress triggers the binding of WRKY34 to the promoter region of CUL3A to accelerate CUL3A protein accumulation and that this subsequently leads to FRI-GFP degradation. In agreement with this finding, the FRI-GFP cul3a and FRI-GFP wrky34 lines, as well as WRKY34-OX FRI-GFP cul3a plants, showed later flowering times and insensitivity to vernalization accompanied by increased FLC transcript levels (Figure 11C) and increased FRI-GFP stability after cold treatment (Figure 11B). Furthermore, the WRKY34-OX FRI-GFP line exhibited earlier flowering compared with the FRI-GFP plants, even in the absence of vernalization (Figure 11C). These data therefore support the idea of interplay between WRKY34 and CUL3A in proteasome-mediated FRI degradation during the response to vernalization in Arabidopsis.
In conclusion, our findings suggest a mechanism by which FRI modulates flowering time under vernalization. On the basis of our data, we present a working model for the regulation of proteasome-mediated FRI degradation in response to vernalization (Figure 12). In the absence of cold stress, FRI acts as a scaffold protein to recruit CBP20, CBP80, FRL1, FES1, SUF4, and RNA Polymerase II (POLII) to increase the level of H3K4me3 in the chromatin of FLC and increase FLC transcript levels. After vernalization, cold stress activates WRKY34 accumulation and potentiates its binding to the CUL3A promoter region to enhance its accumulation. Cold stress also facilitates FRI protein recruitment to CUL3A-based ubiquitin ligase to promote FRI degradation, resulting in the loss of the FRI scaffold, which impairs the interactions of its partner proteins with FLC mRNA. Less CBP20/80 binding to FLC mRNA causes more unspliced and uncapped mRNA to accumulate, from which more ColdAIR is generated, which subsequently reduces the H3K4me3 level in FLC chromatin and suppresses FLC transcription. The degradation of FRI also impairs the ability of POLII to bind to FLC and initiate its transcription. In summary, our study reveals a role for proteasome-mediated protein homeostasis in the modulation of flowering time.
Figure 12.
Proposed Model in Which WRKY34-Driven and CUL3A-Dependent FRI-GFP Degradation Modulates Arabidopsis Flowering in Response to Vernalization.
In the absence of cold stress, FRI acts as a scaffold protein to recruit FRL1, FES1, SUF4, and POLII to increase the level of H3K4me3 in the chromatin of FLC and increases FLC transcript levels. Upon vernalization, cold stress activates WRKY34 accumulation and potentiates its binding to the CUL3A promoter region to enhance its accumulation and further facilitates FRI protein degradation. Loss of the FRI scaffold impairs the interaction of its partner protein with FLC and suppresses FLC transcription, which is accompanied by increased production of ColdAIR small interfering RNA.
METHODS
Materials, Plant Growth Conditions, and Plant Transformation
Wild-type and mutant plants used in this study were in the Col-0 genetic background. The mutants used in this study are cul3a (Salk_046638), lrb1 (Salk_145146), lrb2 (Salk_001013), and wrky34 (SALK_133019). Arabidopsis thaliana (Col-0) seeds were sown in plastic pots under a 16-h-light/8-h-dark photoperiod (photosynthetically active radiation, 9.5 μmol m−2 s−1; red-far red ratio, 3.9). Nonvernalized plants were stratified for 2 d under the same conditions. To generate pFRI:FRI-GFP transgenic plants, the 2.0-kb FRI promoter region and full-length functional FRI cDNA were subcloned into the modified pEGAD vector, in which the CaMV 35S promoter was deleted, using the In-Fusion cloning system (Clontech). To generate 35S:WRKY34-6HA (WKRY34-OX) and 35S:COLDAIR (COLDAIR-OX) transgenic lines, full-length WRKY34 or COLDAIR cDNA was amplified and cloned into the XhoI/EcoRI site of the pOE-6HA binary vector containing a 35S promoter and HA tag at the C terminus. To generate the RNAi transgenic line with downregulated expression of COLDAIR (COLDAIR-RNAi), COLDAIR cDNA was cloned into the NcoI/SwaI and BamHI/XbaI sites of the pFGC5941 binary vector. The PCR primers used for subcloning are listed in Supplemental Data Set 1. Agrobacterium tumefaciens-mediated Arabidopsis (Col-0) transformation was performed according to a modified floral dip method (Clough and Bent, 1998). Seeds from Agrobacterium-transfected Arabidopsis were screened on one-half-strength Murashige and Skoog medium containing 10 mg/L Basta or 35 mg/L kanamycin. The Arabidopsis plants were grown at 22°C under long-day (16 h light/8 h dark) conditions. For vernalization, seeds were germinated and pregrown for 7 d under standard conditions (16 h light/8 h darkness, 22°C), transferred to cold conditions (16 h light/8 h darkness, 4°C) for 2, 3, or 4 weeks, and then returned to standard conditions. The time to flowering (measured as the time until stem elongation [bolting] was observed) was determined from the day of germination but did not include the vernalization period or was recorded as the number of leaves when the floral bolt was 1 cm high (Sheldon et al., 2000; Swiezewski et al., 2009).
Y2H Screen
The cDNA of functional FRI, LRB1, LRB2, and CUL3A was cloned into the pMD18 vector. For the Y2H studies, full-length or truncated FRI, LRB1, LRB2, and CUL3A were cloned into the pGBKT7 bait vector and the pGADT7 prey vector, respectively, using the In-Fusion cloning system (Clontech). Two-hybrid screening was performed using the mating protocol described in the Matchmaker Gold Yeast Two-Hybrid user manual (Clontech).
RNA Extraction and PCR
Total RNA was extracted from Arabidopsis seedlings using the TRIzol reagent (Invitrogen). Quantitative RT-PCR was performed as described (Zhang et al., 2013). Briefly, first-strand cDNA was synthesized from 1.5 mg DNase-treated RNA in a 20-mL reaction volume using M-MuLV reverse transcriptase (Fermentas) with oligo(dT)18 primer. For the PCR, the cDNA samples were diluted to 2 to 10 ng/mL, and PCR was performed using SYBR Green I Master Mix on a Roche LightCycler 480 real-time PCR machine according to the manufacturer’s instructions. At least three biological replicates for each sample were used for the quantitative RT-PCR analysis, and at least two technical replicates were analyzed for each biological replicate. ACTIN2 was used as an internal gene expression control. The gene-specific primers used to detect the transcripts are listed in Supplemental Data Set 1.
Transient Transformation and BiFC Assays
For the BiFC assay, full-length or truncated FRI, LRB1, LRB2, or CUL3A was individually cloned into the pDONR207 vector (Invitrogen) through the Gateway reaction (Invitrogen) and subsequently recombined into the binary YFP BiFC vectors (Nakagawa et al., 2007) so that they were fused with the N- or C-terminal fragment of YFP (nYFP or cYFP). Protoplasts were isolated from Arabidopsis leaves as reported (Yoo et al., 2007), and the resulting nYFP- or cYFP-tagged constructs, plus the 35S:H2B-RFP construct, which is used as a nucleus marker, were transiently cotransformed into Arabidopsis protoplasts by polyethylene glycol-mediated transformation (Yoo et al., 2007). The YFP fluorescence was analyzed 48 h after cotransformation under a confocal laser scanning microscope (Olympus), and RFP fluorescence was observed to monitor nuclear localization.
Immunoblot Assay
Total proteins were prepared by grinding seedlings on ice in extraction buffer (50 mM Tris, 5% glycerol, 4% SDS, 1% polyvinylpolypyrrolidone, and 1 mM phenylmethylsulfonyl fluoride, pH 8.0), followed by centrifugation at 4°C and 14,000g for 15 min. A 15-μg aliquot of protein was separated by electrophoresis on a 12% SDS-polyacrylamide gel and blotted onto polyvinylidene difluoride membranes, which were then probed with the appropriate primary anti-GFP (1:3000; Clontech) or anti-actin (1:1000; Sigma-Aldrich) antibody and horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:3000; Promega). Signals were detected using a ONE-HOUR IP-Western Kit (Genescript).
In Vitro Pull-Down, Co-IP, and Ubiquitination Analysis
The full-length or truncated coding sequence of FRI was cloned into the EcoRI/XhoI sites of pGEX-4T-1 to generate GST-FRI, GST-N-FRI, or GST-C-FRI. The full-length or truncated coding region of LRB1, LRB2, and CUL3A was cloned into the EcoRII/SalI sites of pMAL-C2 to generate MBP-LRB1 (or LRB2), MBP-LRB1 (or LRB2)-T1, MBP-LRB1 (or LRB2)-T2, MBP-LRB1 (or LRB2)-T3, MBP-CUL3A, MBP-N-CUL3A, or MBP-C-CUL3A, respectively, using the In-Fusion cloning technique (Clontech; Supplemental Data Set 1). The Escherichia coli BL21 (DE3) strain harboring expression vectors was incubated at 37°C for 2 h, shifted to 22°C, and incubated for an additional 12 h after induction with 1 mM isopropyl β-d-1-thio-galactopyranoside. GST- or MBP-tagged recombinant proteins were purified using glutathione- or MBP-Sepharose according to the manufacturers’ protocols (GE Healthcare for GST-tagged protein and NEB for MBP-tagged protein). For in vitro pull-down assays, 1 to 2 mg of target protein was incubated with 2 mg of glutathione-Sepharose 4B beads (25 μL) in PBS buffer (1× PBS, 1× protease inhibitor cocktail [Roche], and 0.1% Nonidet P-40) for 2 h at 4°C. The pulled-down proteins were extensively washed with buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.6% Triton X-100) before the samples were resolved on 8% SDS-PAGE gels and analyzed by protein gel blot analysis using anti-MBP (1:5000; NEB) followed by a mouse secondary antibody (1:5000; Promega).
For the in vivo co-IP using FRI-GFP as bait, the FRI-GFP transgenic seedlings with or without vernalization treatment were ground under liquid nitrogen. Proteins were extracted into MOPS buffer (100 mM MOPS, pH 7.6, 150 mM NaCl, 0.1% Nonidet P-40, 1% Triton X-100, 20 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride, 2 μg/L aprotinin, 5 μg/L leupeptin, 1 μg/L pepstatin, 2× Complete Protease Inhibitor Cocktail, and PhosStop Cocktail from Roche), centrifuged at 13,000 rpm under 4°C, and filtered through two layers of Miracloth. Supernatant (1.0 mL) was incubated with anti-GFP coupled to Protein G Sepharose beads overnight under gentle rotation. Beads were washed four times with wash buffer (50 mM Tris, pH 8.0, 150 mM NaCl, and 0.1% [v/v] Triton X-100), and the proteins were eluted at 95°C for 10 min in 2× loading buffer (100 mM Tris-HCl, pH 6.8, 200 mM DTT, 4% SDS, 20% glycerol, and 0.2% bromophenol blue), and analyzed by immunoblotting with anti-GFP (1:3000; Clontech) or anti-HA (1:2000; Roche) monoclonal antibody.
For the in vivo detection of ubiquitinated FRI protein, 2-week-old FRI-GFP transgenic seedlings in the Col-0, CUL3A, or lrb1 lrb2 background were pretreated with 50 μM proteasome inhibitor MG132 or with vernalization. Isolation of ubiquitinated proteins was performed as described previously with slight modification (Manzano et al., 2008). Briefly, proteins were extracted from plants subjected to different treatments using buffer BI (50 mM Tris-HCl, pH 7.5, 20 mM NaCl, 0.1% Nonidet P-40, and 5 mM ATP) plus Plant Protease Inhibitor Cocktail (Sigma-Aldrich), 1 mM phenylmethylsulfonyl fluoride, 50 μM MG132, 10 nM Ub aldehyde, and 10 mM N-ethylmaleimide. Protein extracts were incubated in 40 mL prewashed p62 agarose (Enzo Life Sciences) at 4°C for 4 h. The beads were then washed two times with 1 mL BI buffer and once more with 1 mL BII buffer (BI plus 200 mM NaCl), and proteins were eluted by boiling in 50 μL SDS loading buffer. The eluted proteins were separated by SDS-PAGE and analyzed by immunoblotting using anti-Ub antibody (1:3000; Boston Biochem) to detect the presence of ubiquitinated proteins or anti-GFP antibody (1:2000; Roche) for FRI-GFP.
Transient Expression of FRI-GFP in Tobacco Leaves and Cell-Free Degradation Assay
GFP-tagged versions of FRI were transiently expressed in tobacco (Nicotiana benthamiana) leaves following previously described procedures (Wang et al., 2009). The FRI-GFP protein was enriched from the tobacco leaves via the Chromtek-GFP Trap following the manufacturer’s instructions (Allele). GST-FRI recombination proteins were expressed in the E. coli BL21 (DE3) strain harboring the pGEX-4T-FRI expression vector and purified using glutathione-Sepharose beads according to the manufacturer’s protocol (GE Healthcare for GST-tagged protein). For the cell-free degradation assay, Arabidopsis seedlings with or without cold treatment were harvested and ground into a fine powder in liquid nitrogen. Total proteins were subsequently extracted in degradation buffer containing 25 mM Tris-HCl, pH 7.5, 10 mM NaCl, 10 mM MgCl2, 4 mM phenylmethylsulfonyl fluoride, 5 mM DTT, and 10 mM ATP as previously described (Wang et al., 2009). Cell debris was removed by two 10-min centrifugations at 17,000g and 4°C. The supernatant was collected and protein concentration was determined by the Bio-Rad protein assay. The total protein extracts prepared from cul3a, lrb1 lrb2, and the corresponding wild-type control seedlings were adjusted to equal concentrations in the degradation buffer for each assay. Purified GST-GFP from E. coli or enriched GFP-FRI from tobacco leaves was incubated at 22°C with the Arabidopsis extracts in degradation buffer. Samples were taken at the indicated intervals for determination of GFP protein abundance by immunoblots. For treatments in the presence of proteasome inhibitor, 50 µM MG132 (Calbiochem), or the solvent 1% DMSO (control), was incubated with Arabidopsis crude protein extracts for 20 min before conducting the cell-free degradation assay.
EMSA
The EMSA was performed as described (Zhang et al., 2013). Briefly, oligonucleotide probes were synthesized, annealed, and labeled using the Biotin 3′-DNA labeling kit (Pierce). E. coli BL21 cells that had been transformed with pMAL-WRKY34 and the pMAL-C2 empty plasmid were induced by isopropyl β-d-1-thiogalactopyranoside as previously described (Zhang et al., 2013). Cells were lysed in lysate buffer (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, and 100 mM NaCl) with an ultrasonic cell crusher. After centrifugation, the supernatant was purified by amylose resin affinity chromatography (NEB). The binding reactions were performed according to the manufacturer’s protocol. The chemiluminescence of biotin-labeled DNA was detected using the Light Shift Chemiluminescent EMSA Kit (Pierce).
In Vivo Analysis of CUL3A Promoter Activation by WRKY34
This transient expression assay was performed in tobacco leaves or Arabidopsis mesophyll protoplasts as previously described (Yoo et al., 2007; Sun et al., 2012). The 1850-bp native CUL3A promoter region was amplified from genomic DNA. In addition, mutated CUL3A promoter fragments were generated using a QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene). These promoter fragments were then fused with the luciferase reporter LUC gene through Gateway reactions in the plant binary vector pGreenII-0800-LUC using the In-Fusion cloning system (Clontech) to generate the pCUL3A-LUC or pCUL3AM-LUC construct (Supplemental Data Set 1). Luminescence in the tobacco leaf transient assay and LUC activity in the protoplast transformation assay were measured as previously described (Sun et al., 2012). The experiments were repeated at least three times, yielding similar results.
ChIP Assay
The ChIP assay was performed as described (Zhang et al., 2013) using 100-mg (fresh weight) samples of seedlings following different treatments. Immunoprecipitation was performed with rabbit polyclonal antibodies against HA (1:1000; clone 3F10; Roche) and trimethyl H3K4 (1:300; 17-614; Millipore). FLC regions were detected using the PCR primers as described in Supplemental Data Set 1. ChIP products were analyzed by quantitative PCR. Data from the ChIP experiments are expressed as the mean ± sd of three biological replicates.
Phylogenetic Analyses
The amino acid sequences of 71 Arabidopsis WRKY proteins were downloaded from the TAIR database (http://www.arabidopsis.org/). The WRKY domains of all proteins were then identified using the Pfam website (http://pfam.sanger.ac.uk/search). Twelve of the proteins have two WRKY domains and the N-terminal and C-terminal domain. All 83 WRKY domains were aligned using ClustalX version 2.0 and manually adjusted using BioEdit version 7.0.5 (Hall, 1999) as described in Supplemental Data Set 2. An unrooted maximum likelihood tree was constructed using RAxML software with the empirical Jones-Taylor-Thornton substitution model (Stamatakis, 2006). The bootstrap values (>50%) are given at nodes in the phylogenetic tree based on 1000 replications.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: CUL3A, AT1G26830; LRB1, AT2G46260; LRB2, AT3G61600; WRKY34, AT4G26440; FLC, AT5G10140; and ACTIN2, At3g18780.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. FRI-GFP Localization in Transgenic FRI-GFP Arabidopsis Plants.
Supplemental Figure 2. Quantitative RT-PCR Analysis of FRI-GFP Transcript Levels in the FRI-GFP Line with or without Vernalization Treatment.
Supplemental Figure 3. Prediction of the Functional Domains of LRB1/2 and FRI Using SMART Software.
Supplemental Figure 4. FRI Interacts with the N-Terminal Portion of LRB2 (LRB2-T1, LRB2-T2) but Not the C-Terminal Region of LRB2.
Supplemental Figure 5. In Vitro Pull-Down Assay Showing the Interaction between FRI and LRB2.
Supplemental Figure 6. Identification of the cul3a Mutant and FRI-GFP cul3a Line.
Supplemental Figure 7. Identification of lrb1 and lrb2 Single Null Mutants and the lrb1 lrb2 Double Mutant.
Supplemental Figure 8. Detection of in Vivo Polyubiquitinated FRI-GFP in the Wild Type, lrb1, or lrb2 Background after Cold Treatment.
Supplemental Figure 9. Regulation of Flowering Time by COLDAIR siRNA.
Supplemental Figure 10. Flowering Time and FLC Transcript Levels in the FRI-GFP, lrb1, or lrb2 Background.
Supplemental Figure 11. Identification of FRI-sup1 and FRI wrky34 Lines.
Supplemental Figure 12. Identification of the wrky34 Null Mutant Line and Related Transgenic Line.
Supplemental Figure 13. The Differential Flowering Time in the FRI-GFP and F1 Generation among FRI-GFP, FRI-GFP wrky34, and FRI-sup1.
Supplemental Figure 14. Phylogenetic Tree of Arabidopsis WRKY Domains.
Supplemental Figure 15. Cell-Free Analysis of FRI-GFP Degradation.
Supplemental Data Set 1. Primers Used in This Study.
Supplemental Data Set 2. Alignment Data Used to Produce the Phylogenetic Tree Shown in Supplemental Figure 14.
Supplementary Material
Acknowledgments
We thank the ABRC for providing the seeds stocks, Nakagawa Tsuyoshi (Shimane University) for the BiFC vector, Hao Yu (National University of Singapore) for the pOE-6HA vector, and Qi Xie (Institute of Genetics and Development Biology) for the anti-ubiquitin antibody. We thank Caroline Dean’s lab for their help at the beginning of this project and Kathleen Farquharson for English polishing. We also thank the anonymous reviewers for their instructive advice. This article was supported by the Young Academic and Technical Leader Raising Foundation of Yunnan Province (No. 2012HB041), the Major State Basic Research Development Program (2010CB951700 and 2010CB951704), the Special Fund for Forest Scientific Research in the Public Welfare (No. 201304103) and the National Science Foundation of China (No. 31100204, No. 31170256, and No. 31470348). X.K. was supported by the West Light Foundation of the Chinese Academy of Sciences.
AUTHOR CONTRIBUTIONS
X.H. designed the research. X.K., C.W., L.M., J.Z., J.W., and X.Z. performed the research. X.K., G.J.L., J.H., Y.Y., and X.H. analyzed the data. T.Z. performed the bioinformatics analysis. G.J.L. and X.H. wrote the article.
Glossary
- Col-0
Columbia-0
- Y2H
yeast two-hybrid
- BiFC
bimolecular fluorescence complementation
- co-IP
coimmunoprecipitation
- CaMV
cauliflower mosaic virus
- EMSA
electrophoretic mobility shift assay
- DAPI
4′,6-diamidino-2-phenylindole
- ChIP
chromatin immunoprecipitation
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Bastow R., Mylne J.S., Lister C., Lippman Z., Martienssen R.A., Dean C. (2004). Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427: 164–167. [DOI] [PubMed] [Google Scholar]
- Chen L., Lee J.H., Weber H., Tohge T., Witt S., Roje S., Fernie A.R., Hellmann H. (2013). Arabidopsis BPM proteins function as substrate adaptors to a cullin3-based E3 ligase to affect fatty acid metabolism in plants. Plant Cell 25: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Ohta M., Kanrar S., Lee B.H., Hong X., Agarwal M., Zhu J.-K. (2003). ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Kim J., Hwang H.-J., Kim S., Park C., Kim S.Y., Lee I. (2011). The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 23: 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians M.J., Gingerich D.J., Hua Z., Lauer T.D., Vierstra R.D. (2012). The light-response BTB1 and BTB2 proteins assemble nuclear ubiquitin ligases that modify phytochrome B and D signaling in Arabidopsis. Plant Physiol. 160: 118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Collier S., Pendle A., Boudonck K., van Rij T., Dolan L., Shaw P. (2006). A distant coilin homologue is required for the formation of cajal bodies in Arabidopsis. Mol. Biol. Cell 17: 2942–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustham V., Li P., Strange A., Lister C., Song J., Dean C. (2012). Quantitative modulation of polycomb silencing underlies natural variation in vernalization. Science 337: 584–587. [DOI] [PubMed] [Google Scholar]
- Dennis E.S., Peacock W.J. (2007). Epigenetic regulation of flowering. Curr. Opin. Plant Biol. 10: 520–527. [DOI] [PubMed] [Google Scholar]
- Dong C.-H., Agarwal M., Zhang Y., Xie Q., Zhu J.-K. (2006). The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 103: 8281–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Somssich I.E. (2007). Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 10: 366–371. [DOI] [PubMed] [Google Scholar]
- Figueroa P., Gusmaroli G., Serino G., Habashi J., Ma L., Shen Y., Feng S., Bostick M., Callis J., Hellmann H., Deng X.W. (2005). Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 17: 1180–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldo N., Bäurle I., Kidou S., Hu X., Dean C. (2009). FRIGIDA delays flowering in Arabidopsis via a cotranscriptional mechanism involving direct interaction with the nuclear cap-binding complex. Plant Physiol. 150: 1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Nezames C.D., Sheng L., Deng X., Wei N. (2013). Cullin-RING ubiquitin ligase family in plant abiotic stress pathways(F). J. Integr. Plant Biol. 55: 21–30. [DOI] [PubMed] [Google Scholar]
- Hall T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41: 95–98. [Google Scholar]
- He Y., Amasino R.M. (2005). Role of chromatin modification in flowering-time control. Trends Plant Sci. 10: 30–35. [DOI] [PubMed] [Google Scholar]
- He Y., Doyle M.R., Amasino R.M. (2004). PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis. Genes Dev. 18: 2774–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.B., Sung S. (2011). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331: 76–79. [DOI] [PubMed] [Google Scholar]
- Hua Z., Vierstra R.D. (2011). The cullin-RING ubiquitin-protein ligases. Annu. Rev. Plant Biol. 62: 299–334. [DOI] [PubMed] [Google Scholar]
- Jeon J., Kim J. (2013). Cold stress signaling networks in Arabidopsis. J. Plant Physiol. 56: 69–76. [Google Scholar]
- Johanson U., West J., Lister C., Michaels S., Amasino R., Dean C. (2000). Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347. [DOI] [PubMed] [Google Scholar]
- Kim D.-H., Doyle M.R., Sung S., Amasino R.M. (2009). Vernalization: winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 25: 277–299. [DOI] [PubMed] [Google Scholar]
- Kim E.-D., Sung S. (2012). Long noncoding RNA: unveiling hidden layer of gene regulatory networks. Trends Plant Sci. 17: 16–21. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., He Y., Jacob Y., Noh Y.-S., Michaels S., Amasino R. (2005). Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell 17: 3301–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Xiong L., Gong Z., Ishitani M., Stevenson B., Zhu J.-K. (2001). The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo—cytoplasmic partitioning. Genes Dev. 15: 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.F., Pontes O., El-Shami M., Henderson I.R., Bernatavichute Y.V., Chan S.W.-L., Lagrange T., Pikaard C.S., Jacobsen S.E. (2006). An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 126: 93–106. [DOI] [PubMed] [Google Scholar]
- Manzano C., Abraham Z., Lopez-Torrejon G., Del Pozo J.C. (2008). Identification of ubiquitinated proteins in Arabidopsis. Plant Mol. Biol. 68: 145–158 [DOI] [PubMed] [Google Scholar]
- Miao Y., Zentgraf U. (2010). A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J. 63: 179–188. [DOI] [PubMed] [Google Scholar]
- Michaels S.D., Bezerra I.C., Amasino R.M. (2004). FRIGIDA-related genes are required for the winter-annual habit in Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 3281–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. [DOI] [PubMed] [Google Scholar]
- Ni W., Xu S.L., Tepperman J.M., Stanley D.J., Maltby D.A., Gross J.D., Burlingame A.L., Wang Z.Y., Quail P.H. (2014). A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344: 1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D., Pedmale U.V., Morrow J., Sachdev S., Lechner E., Tang X., Zheng N., Hannink M., Genschik P., Liscum E. (2011). Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 23: 3627–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton P.J., Somssich I.E., Ringler P., Shen Q.J. (2010). WRKY transcription factors. Trends Plant Sci. 15: 247–258. [DOI] [PubMed] [Google Scholar]
- Schmitz R.J., Hong L., Michaels S., Amasino R.M. (2005). FRIGIDA-ESSENTIAL 1 interacts genetically with FRIGIDA and FRIGIDA-LIKE 1 to promote the winter-annual habit of Arabidopsis thaliana. Development 132: 5471–5478. [DOI] [PubMed] [Google Scholar]
- Sheldon C.C., Rouse D.T., Finnegan E.J., Peacock W.J., Dennis E.S. (2000). The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc. Natl. Acad. Sci. USA 97: 3753–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel S.H., Mou Z., Tada Y., Spivey N.W., Genschik P., Dong X. (2009). Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137: 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Sun J., Qi L., Li Y., Chu J., Li C. (2012). PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating arabidopsis hypocotyl growth. PLoS Genet. 8: e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiezewski S., Liu F., Magusin A., Dean C. (2009). Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462: 799–802. [DOI] [PubMed] [Google Scholar]
- Thomann A., Lechner E., Hansen M., Dumbliauskas E., Parmentier Y., Kieber J., Scheres B., Genschik P. (2009). Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanisms. PLoS Genet. 5: e1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten M., Koini M.A., Geyer R., Liu Y., Brambilla V., Bartels D., Koornneef M., Fransz P., Soppe W.J. (2011). Seed maturation in Arabidopsis thaliana is characterized by nuclear size reduction and increased chromatin condensation. Proc. Natl. Acad. Sci. USA 108: 20219–20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Zhu D., Huang X., Li S., Gong Y., Yao Q., Fu X., Fan L.M., Deng X.W. (2009). Biochemical insights on degradation of Arabidopsis DELLA proteins gained from a cell-free assay system. Plant Cell 21: 2378–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Hellmann H. (2009). Arabidopsis thaliana BTB/POZ-MATH proteins interact with members of the ERF/AP2 transcription factor family. FEBS J. 276: 6624–6635. [DOI] [PubMed] [Google Scholar]
- Weber H., Bernhardt A., Dieterle M., Hano P., Mutlu A., Estelle M., Genschik P., Hellmann H. (2005). Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol. 137: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., et al. (2000). Activation tagging in Arabidopsis. Plant Physiol. 122: 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li B., Xu Y., Li H., Li S., Zhang D., Mao Z., Guo S., Yang C., Weng Y. (2013). The cyclophilin CYP20-2 modulates the conformation of BRASSINAZOLE-RESISTANT1, which binds the promoter of FLOWERING LOCUS D to regulate flowering in Arabidopsis. Plant Cell 25: 2504–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C., Jiang W., Yu D. (2010). Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J. Exp. Bot. 61: 3901–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.