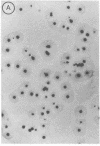
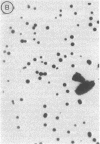
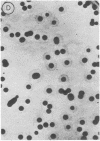
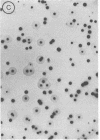
Abstract
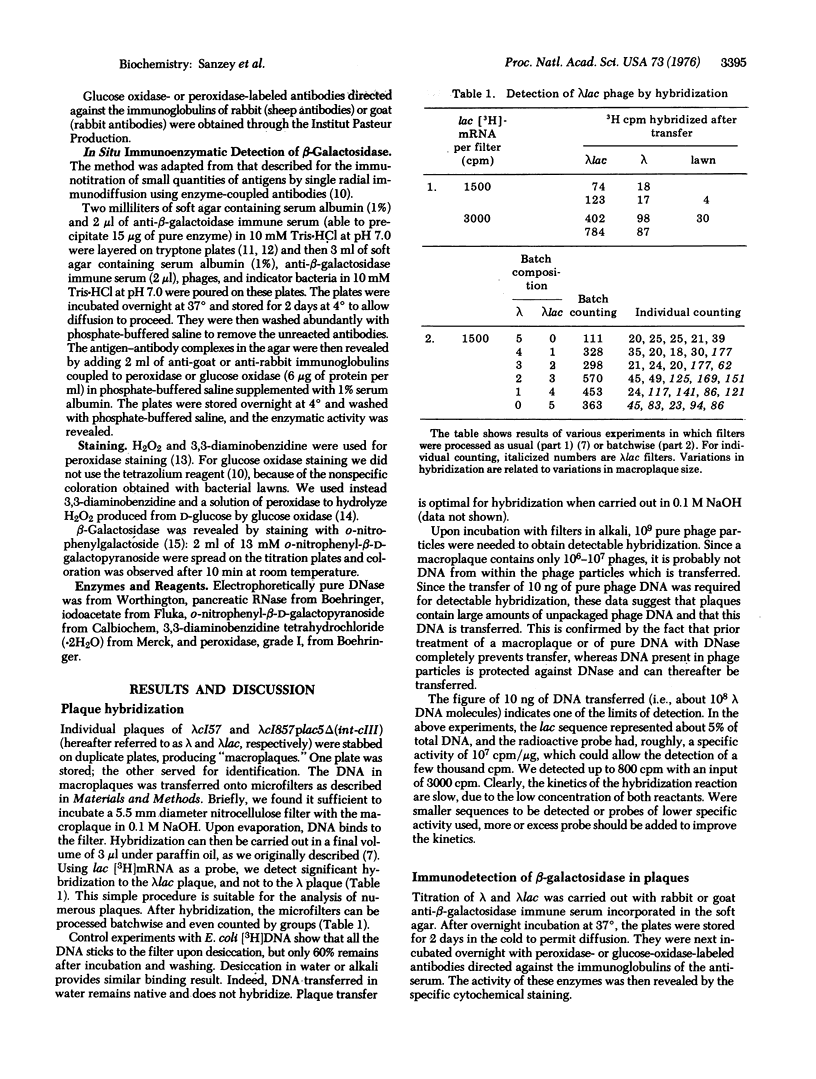
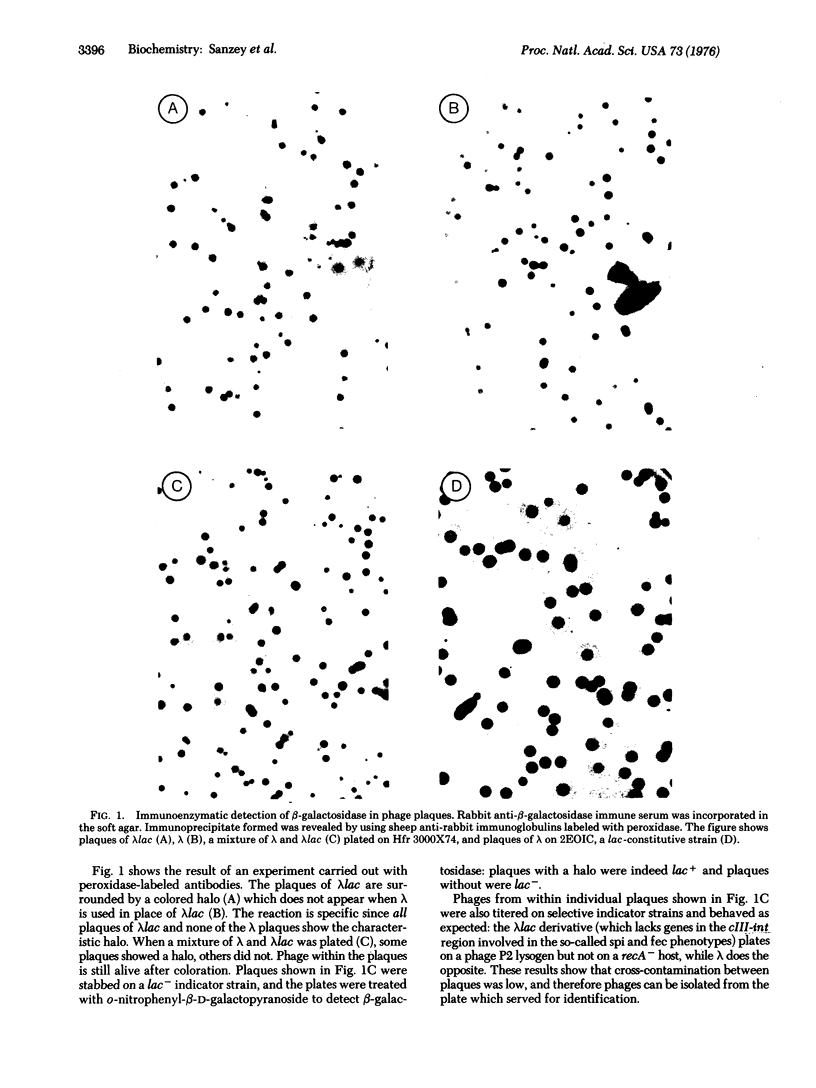
Two methods are described which allow the screening of a large number of phage plaques for a specific DNA sequence carried by the phage or a specific antigen produced within the phage plaque. These methods were set up with lambda and lambdalac phages. Phage plaques were transferred onto nitrocellulose filters by desiccation in 0.1 M NaOH, and the lac sequence was detected by hybridization to radioactive lac mRNA. Beta-Galactosidase was detected by reaction with anti-beta-galactosidase immune serum included in the soft agar of the titration plates; the precipitate thus formed was revealed by means of enzyme-coupled antibodies and in situ coloration. These methods are potentially useful for the identification of lambda transducers, including those which are generated by in vitro recombination with eukaryotic DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOK A., LEDERBERG J. Recombination studies of lactose nonfermenting mutants of Escherichia coli K-12. Genetics. 1962 Oct;47:1335–1353. doi: 10.1093/genetics/47.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Panasenko S. M., Lehman I. R., Davis R. W. In vitro construction of bacteriophage lambda carrying segments of the Escherichia coli chromosome: selection of hybrids containing the gene for DNA ligase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3416–3420. doi: 10.1073/pnas.72.9.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Biochemical construction and selection of hybrid plasmids containing specific segments of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4361–4365. doi: 10.1073/pnas.72.11.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L., Avrameas S. An immunoenzymatic method for measuring low concentrations of antigens by single radial diffusion. Immunochemistry. 1974 Sep;11(9):595–598. doi: 10.1016/0019-2791(74)90251-1. [DOI] [PubMed] [Google Scholar]
- Ippen K., Shapiro J. A., Beckwith J. R. Transposition of the lac region to the gal region of the Escherichia coli chromosome: isolation of lambda-lac transducing bacteriophages. J Bacteriol. 1971 Oct;108(1):5–9. doi: 10.1128/jb.108.1.5-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. W., Murray K. A procedure for detection of heterologous DNA sequences in lambdoid phage by in situ hybridization. J Mol Biol. 1975 Aug 15;96(3):455–460. doi: 10.1016/0022-2836(75)90172-2. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Luzzati D. Etude, par sédimentation de zone, des produits de transcription des fonctions "précocs" de lambda. J Mol Biol. 1967 Apr 28;25(2):357–361. doi: 10.1016/0022-2836(67)90148-9. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Mercereau O., Gros D., Tremblay G. Hybridization of filters with competitor DNA in the liquid phase in a standard and a micro-assay. Biochimie. 1974;56(9):1215–1221. doi: 10.1016/s0300-9084(74)80014-3. [DOI] [PubMed] [Google Scholar]
- Kuhlmann W. D., Avrameas S. Glucose oxidase as an antigen marker for light and electron microscopic studies. J Histochem Cytochem. 1971 Jun;19(6):361–368. doi: 10.1177/19.6.361. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Spiegelman W. G., Reichardt L. F., Yaniv M., Heinemann S. F., Kaiser A. D., Eisen H. Bidirectional transcription and the regulation of Phage lambda repressor synthesis. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3156–3160. doi: 10.1073/pnas.69.11.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]