Abstract
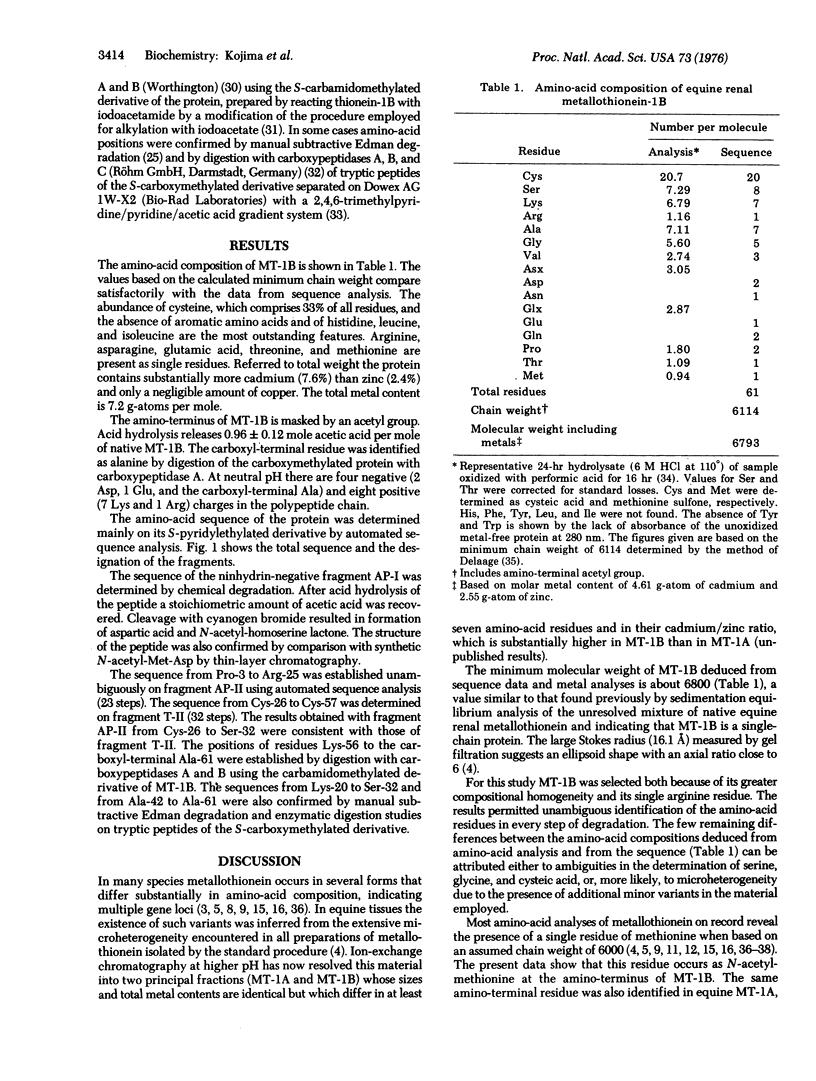
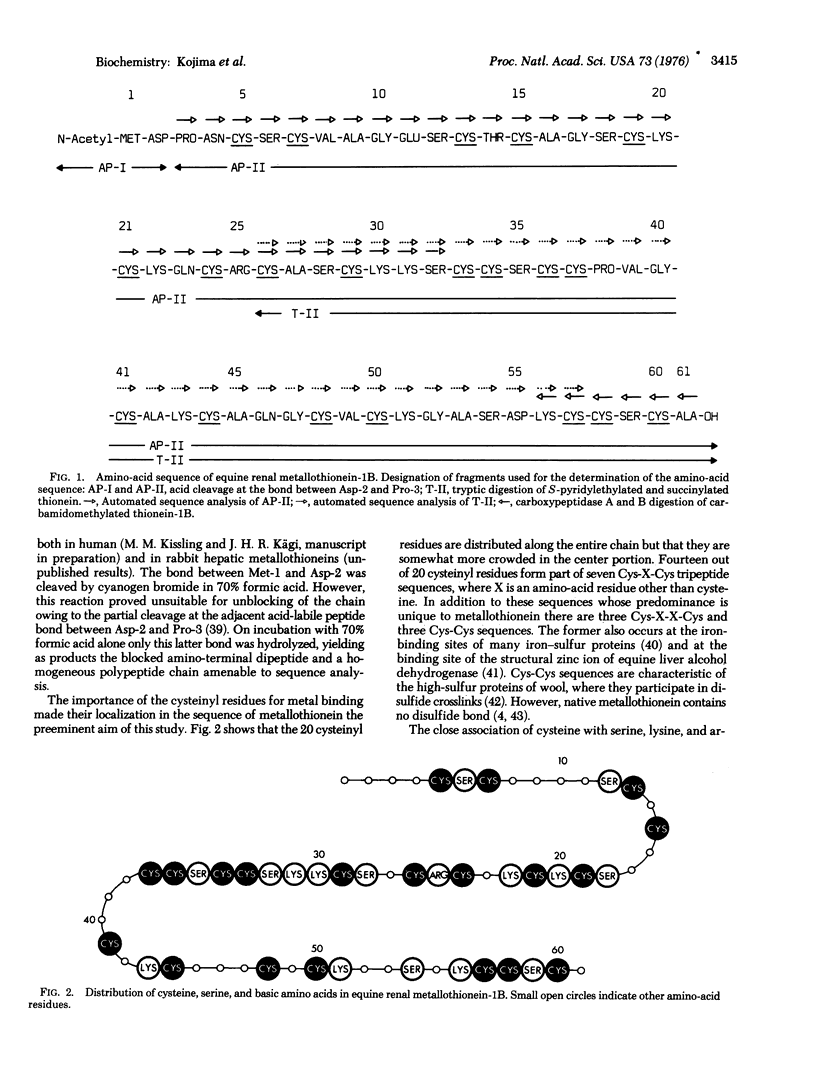
The amino-acid sequence of a metallothionein is reported. Metallothionein is a widely distributed, extremely cysteine-rich, low-molecular-weight protein containing large amounts of cadmium and/or zinc. Metallothionein-1B is one of the two prinicipal variants occurring in equine kidney cortex. The single-chain protein contains 61 amino acids and has the composition Cys20 Ser8Lys7Arg1Ala7Gly5Val3Asp2Asn1-Glu1Gln2Pro2Thr1Met1(Cd + Zn)7. Its amino-terminal residue is N-acetylmethionine. The sequence shows distinct clustering of the twenty cysteinyl residues into seven groups separated by stretches of at least three other residues. Within these groups the cysteines occur seven times in alternating Cys-X-Cys sequences and three times each in Cys-Cys and Cys-X-X-Cys sequences, where X is an amino acid other than cysteine. Another unique feature is the close association of serine and the basic amino acids with cysteine, as manifested by the occurrence of seven Ser-Cys, four Cys-Lys, one Cys-Arg, and three Lys-Cys sequences. These findings are in agreement with the previous suggestion that metallothionein has structurally defined metal-binding sites, most of which contain three cysteinyl residues as the principal metal-binding ligands. The charge difference between the metal-free and the metal-containing protein is consistent with the formation of negatively charged trimercaptide complexes with cadmium and/or zinc ions. The possible additional involvement of serine and the basic amino acids in metal binding is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- BANASZAK L. J., WATSON H. C., KENDREW J. C. THE BINDING OF CUPRIC AND ZINC IONS TO CRYSTALLINE SPERM WHALE MYOGLOBIN. J Mol Biol. 1965 May;12:130–137. doi: 10.1016/s0022-2836(65)80287-x. [DOI] [PubMed] [Google Scholar]
- Bremner I., Davies N. T. The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J. 1975 Sep;149(3):733–738. doi: 10.1042/bj1490733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner I., Marshall R. B. Hepatic copper- and zinc-binding proteins in ruminants. 2. Relationship between Cu and Zn concentrations and the occurrence of a metallothionein-like fraction. Br J Nutr. 1974 Sep;32(2):293–300. doi: 10.1079/bjn19740082. [DOI] [PubMed] [Google Scholar]
- Bremner I., Marshall R. B. Hepatic copper-and zinc-binding proteins in ruminants. 1. Distribution of Cu and Zn among soluble proteins of livers of varying Cu and Zn content. Br J Nutr. 1974 Sep;32(2):283–291. doi: 10.1079/bjn19740081. [DOI] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Freer S. T., Xuong N. H., Alden R. A., Kraut J. Structure of the iron-sulfur cluster in the Chromatius iron protein at 2.25 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:381–385. doi: 10.1101/sqb.1972.036.01.049. [DOI] [PubMed] [Google Scholar]
- Cotton F. A., Bier C. J., Day V. W., Hazen E. E., Jr, Larsen S. Some aspects of the structure of staphylococcal nuclease. I. Crystallographic studies. Cold Spring Harb Symp Quant Biol. 1972;36:243–249. doi: 10.1101/sqb.1972.036.01.032. [DOI] [PubMed] [Google Scholar]
- Delaage M. Sur la recherche du poids moléculaire le plus cohérent avec l'analyse des acides aminés d'une protéine. Biochim Biophys Acta. 1968 Dec 3;168(3):573–575. [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Brändén C. I. The structure of horse liver alcohol dehydrogenase. FEBS Lett. 1974 Aug 25;44(2):200–204. doi: 10.1016/0014-5793(74)80725-8. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Kimura M., Otaki N., Yoshiki S., Suzuki M., Horiuchi N. The isolation of metallothionein and its protective role in cadmium poisoning. Arch Biochem Biophys. 1974 Nov;165(1):340–348. doi: 10.1016/0003-9861(74)90172-6. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Kägi J. H., Himmelhoch S. R., Whanger P. D., Bethune J. L., Vallee B. L. Equine hepatic and renal metallothioneins. Purification, molecular weight, amino acid composition, and metal content. J Biol Chem. 1974 Jun 10;249(11):3537–3542. [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Quiocho F. A., Reeke G. N., Jr The structure of carboxypeptidase A. IX. The x-ray diffraction results in the light of the chemical sequence. Proc Natl Acad Sci U S A. 1969 Sep;64(1):28–35. doi: 10.1073/pnas.64.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafante E. Binding of mercury and zinc to cadmium-binding protein in liver and kidney of goldfish (Carassius auratus L.). Experientia. 1976 Feb 15;32(2):149–150. doi: 10.1007/BF01937734. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Weaver L. H. Binding of lanthanide ions to thermolysin. Biochemistry. 1974 Apr 9;13(8):1719–1725. doi: 10.1021/bi00705a025. [DOI] [PubMed] [Google Scholar]
- Nordberg G. F., Nordberg M., Piscator M., Vesterberg O. Separation of two forms of rabbit metallothionein by isoelectric focusing. Biochem J. 1972 Feb;126(3):491–498. doi: 10.1042/bj1260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISCATOR M. OM KADMIUM I NORMALA MAENNISKONJURAR SAMT REDOGOERELSE FOER ISOLERING AV METALLOTHIONEIN UR LEVER FRAN KADMIUMEXPONERADE KANINER. Nord Hyg Tidskr. 1964;45:76–82. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J., Brewer H. B., Jr Advances in the gas chromatographic analysis of amino acid phenyl- and methylthiohydantoins. Anal Biochem. 1972 Jan;45(1):43–59. doi: 10.1016/0003-2697(72)90006-1. [DOI] [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1173–1178. doi: 10.1016/0006-291x(70)90918-6. [DOI] [PubMed] [Google Scholar]
- Prinz R., Weser U. A naturally occurring Cu-thionein in Saccharomyces cerevisiae. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):767–776. doi: 10.1515/bchm2.1975.356.s1.767. [DOI] [PubMed] [Google Scholar]
- Pulido P., Kägi J. H., Vallee B. L. Isolation and some properties of human metallothionein. Biochemistry. 1966 May;5(5):1768–1777. doi: 10.1021/bi00869a046. [DOI] [PubMed] [Google Scholar]
- RUDLOFF V., BRAUNITZER G. [On hemoglobin. VI. A method for the preparative production of natural peptides. The isolation of tryptic cleavage products of human hemoglobin A with Dowex 1X2 using a ninhydrin-negative volatile buffer]. Hoppe Seylers Z Physiol Chem. 1961 May 3;323:129–144. doi: 10.1515/bchm2.1961.323.1.129. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Influence of parenteral zinc and actinomycin D on tissue zinc uptake and the synthesis of a zinc - binding protein. Bioinorg Chem. 1975 Apr;4(3):215–224. doi: 10.1016/s0006-3061(00)80104-0. [DOI] [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh Z. A., Lucis O. J. Isolation of cadmium-binding proteins. Experientia. 1971 Sep 15;27(9):1024–1025. doi: 10.1007/BF02138857. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Sokolowski G., Weser U. Formation, circular dichroism and x-ray photoelectron spectroscopy of hepatic Zn-thionein. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1715–1726. doi: 10.1515/bchm2.1975.356.2.1715. [DOI] [PubMed] [Google Scholar]
- Suda T., Horiuchi N., Ogata E., Ezawa I., Otaki N. Prevention by metallothionein of cadmium-induced inhibition of vitamin D activation reaction in kidney. FEBS Lett. 1974 May 15;42(1):23–26. doi: 10.1016/0014-5793(74)80270-x. [DOI] [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B. The amino acid sequence of an extracellular nuclease of Staphylococcus aureus. I. Linear order of the fragments produced by cleavage with cyanogen bromide. J Biol Chem. 1966 Oct 10;241(19):4366–4385. [PubMed] [Google Scholar]
- Tschesche H., Kupfer S. C-terminal-sequence determination by carboxypeptidase C from orange levels. Eur J Biochem. 1972 Mar 15;26(1):33–36. doi: 10.1111/j.1432-1033.1972.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Waara I., Lövgren S., Liljas A., Kannan K. K., Bergstén P. C. Functional aspects of the three-dimensional structure of the active site of carbonic anhydrase. Adv Exp Med Biol. 1972;28:169–187. doi: 10.1007/978-1-4684-3222-0_13. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K. D., Sieker L. C., Herriott J. R., Jensen L. H. The structure of a non-heme iron protein: rubredoxin at 1.5 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:359–367. doi: 10.1101/sqb.1972.036.01.047. [DOI] [PubMed] [Google Scholar]
- Webb M. Binding of cadmium ions by rat liver and kidney. Biochem Pharmacol. 1972 Oct 15;21(20):2751–2765. doi: 10.1016/0006-2952(72)90023-8. [DOI] [PubMed] [Google Scholar]
- Weser U., Donay F., Rupp H. Cadmium-induced synthesis of hepatic metallothionein in chicken and rats. FEBS Lett. 1973 May 15;32(1):171–174. doi: 10.1016/0014-5793(73)80764-1. [DOI] [PubMed] [Google Scholar]
- Weser U., Rupp H., Donay F., Linnemann F., Voelter W., Voetsch W., Jung G. Characterization of Cd, Zn-thionein (metallothionein) isolated from rat and chicken liver. Eur J Biochem. 1973 Nov 1;39(1):127–140. doi: 10.1111/j.1432-1033.1973.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Premakumar R., Rajagopalan K. V. Metal-induced formation of metallothionein in rat liver. Arch Biochem Biophys. 1975 Sep;170(1):242–252. doi: 10.1016/0003-9861(75)90115-0. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Rajagopalan K. V. Purification and some properties of Cd-binding protein from rat liver. Arch Biochem Biophys. 1972 Dec;153(2):755–762. doi: 10.1016/0003-9861(72)90395-5. [DOI] [PubMed] [Google Scholar]