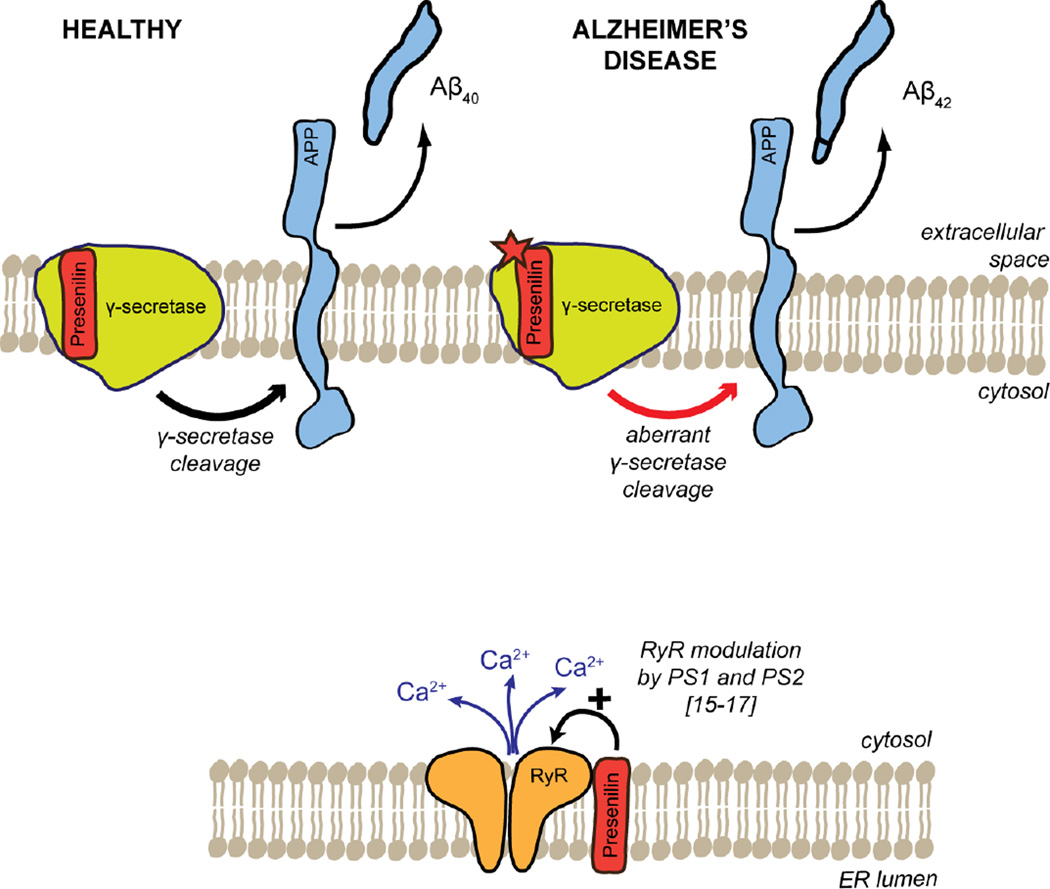
Ca2+ dyshomeostasis is a critical causative mechanism underlying the functional impairment of the central nervous system seen in ‘healthy’ aging[1], specifically in normal brain aging processes in the absence of disease-causing mutations or external causes. It is generally thought that such impairments result from the gradual accumulation of small changes at the molecular level, such as oxidative damage to the structure and function of synapses, that ultimately cause the loss of biological function[2]. Alzheimer’s disease (AD) is a multifactorial pathology directly related to aging and with age as a primary risk factor[3]. Neurons affected by AD have an elevated cytosolic calcium ion concentration[4]. It has not yet been determined if the observed elevation of cytosolic calcium is contributing to or results from AD pathology, but there is mounting evidence in support of the Calcium Hypothesis of brain aging[4–9]. The two presenilin genes found in vertebrates are ubiquitously expressed as the transmembrane proteins presenilin-1 (PS1) and presenilin-2 (PS2), respectively, in the endoplasmic reticulum (ER). They are part of the γ-secretase enzyme complex catalyzing the processing of amyloid precursor protein (APP), which - if aberrant under disease conditions - can result in the generation of neurotoxic amyloid-beta (Aβ) peptides[10] (Fig. 1). Approximately 80% of the mutations linked to familial AD have been found in PS1[11] and clinically relevant PS1 mutations exert effects on cytosolic calcium concentrations[10, 12]. While both presenilins affect cytosolic calcium concentrations, the PS1 isoform binds to several proteins essential to the regulation of intracellular calcium signaling, e.g. the inositol 1,4,5-trisphosphate receptor (IP3R)[13], the N-methyl-D-aspartate (NMDA) receptor (NMDAR)[14], and the ryanodine receptor (RyR)[15–17]. Here, the interaction of presenilins with RyRs will be reviewed.
Figure 1. Role of PS proteins in synaptic signaling.
PS proteins form the enzymatic core of the γ-secretase complex, which cleaves its target, amyloid precursor protein (APP) to generate β-amyloid (Aβ) peptide (top panel, left; indicated by the green arrow). Mutations in the genes encoding PS proteins, PSEN1 and PSEN2, have been linked to familial AD, causing aberrant processing of APP resulting in excess generation of the pathologic and potentially pathogenic Aβ42 peptide (top panel, right; indicated by the red arrow). More recently, a novel role for PS proteins as regulators of intracellular Ca2+ signaling has been discovered and is the focus of this review. Specifically, PS is expressed ubiquitously in the ER, where it interacts directly with the RyR via its cytosolic N-terminal domain (lower panel; indicated by the black arrow).
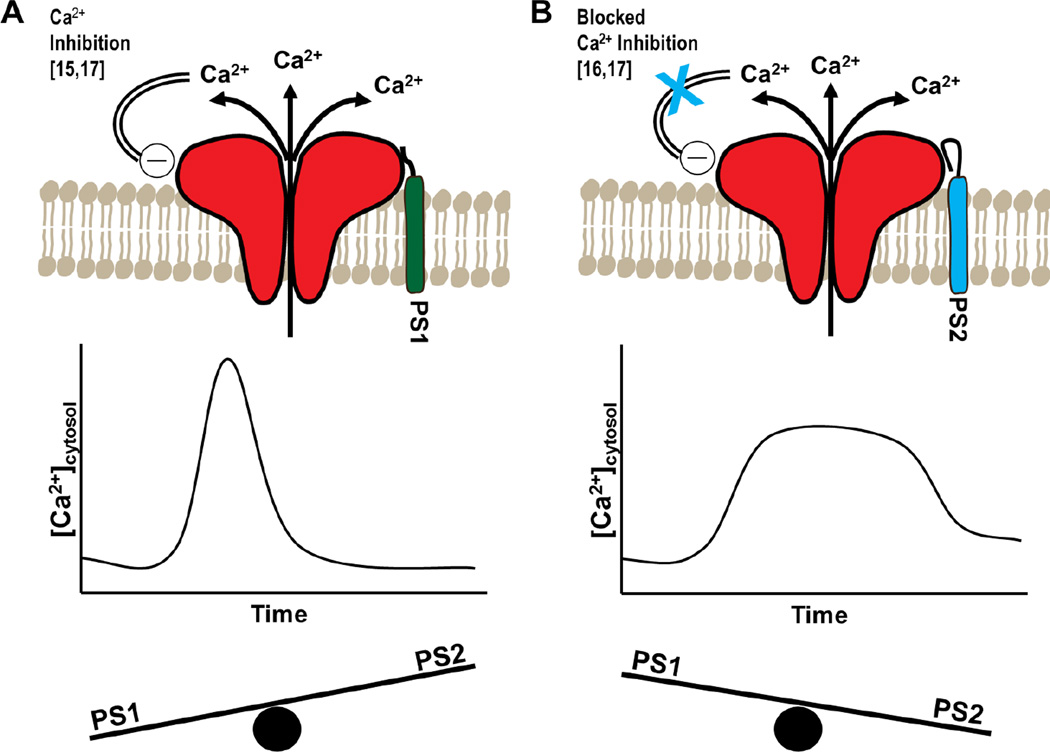
The PS-RyR interaction was first observed in single channel electrophysiological recordings of mouse brain microsomes using recombinant PS N-termini. The cytosolic domain of the N-terminus of both PS1 (residues 1–82; PS1NTF) and PS2 (residues 1–87; PS2NTF), i.e. soluble proteins without the transmembrane domains, bind the large cytoplasmic face of the RyR to control channel gating without the involvement of the PS transmembrane domains. PS1 and PS2 regulate RyR channel activity each with distinct kinetics. PS1NTF increased RyR2 channel open probability and the mean Ca2+ current over the physiological cytosolic calcium range of 10 nM to 1 µM Ca2+ but did not alter channel properties at high, cytosolic Ca2+ concentrations (> 10 µM Ca2+) that inhibit RyR at their low affinity inhibitory Ca2+ binding site[15]. On the other hand, PS2NTF had less effect at physiological calcium concentrations, but increased the RyR channel open frequency and mean Ca2+ current at elevated calcium concentrations (10 µM – 1 mM Ca2+) that can contribute to neurotoxicity[16]. Thus, PS1 has the capacity to facilitate the transition of the RyR ion channel from the closed to the full open state, bypassing subconductance states potentially contributing to Ca2+ leaking from the ER, while at high cytosolic calcium concentrations PS2 blocked the inhibition of the RyR channel by its low affinity inhibitory Ca2+ binding site (Fig. 2).
Figure 2. Presenilins regulate ryanodine receptor-mediated Ca2+ release differentially.
Representations of an individual RyR and its interaction with PS are shown in the top panels. Corresponding graphs below illustrate characteristics of Ca2+ transients mediated by RyR activity. Whole-cell cytosolic calcium concentrations (ordinate) are plotted over time (abscissa) to show the changes in the kinetics of Ca2+ transients dependent on PS binding to RyR. Seesaws depict a predominant effect of PS1 over PS2 (A) or PS2 over PS1 (B), as seen in young and aged animals, respectively[19]. A, Binding of the PS1 N-terminal fragment to RyR increases open probability and results in heightened calcium release and fast channel inhibition by calcium at the RyR’s inhibitory low affinity Ca2+ binding site. B, Binding of PS2 to the RyR blocks inhibition at the low affinity Ca2+ binding site resulting in an increased duration of the Ca2+ transient.
Subsequent studies investigating this new mechanism of cytosolic calcium control focused on the interaction of PS with RyR in live cells. The physiological relevance of the PSNTF-RyR interaction was measured in SH-SY5Y neuroblastoma cells, a cell line with neuronal phenotype, low expression levels of IP3Rs and RyR-driven intracellular calcium release[17]. Live cell calcium imaging of cells transfected with PS1NTF or PS2NTF confirmed the differential regulation of RyR by Ps1 and PS2 identified in the electrophysiology findings. Neither PS N-termini were sufficient to cause RyR activation at sub-threshold Ca2+ concentrations but, with addition of the RyR allosteric agonist caffeine, calcium signaling dynamics specific for each presenilin were observed. The maximum amplitude and total amount of RyR Ca2+ release was decreased in the PS1NTF cells, resulting from PS1NTF bound RyRs rapidly gating to the full open state to release a strong burst of Ca2+ leading to rapid channel inhibition as the cytosolic volume near the RyR rapidly reached inhibitory Ca2+ concentrations (Fig. 2A)[17]. Conversely, and as expected based on the electrophysiology data, PS2NTF transfected cells did not differ from wild type (WT) SH-SY5Y cells since inhibition of the RyR channel by high cytosolic calcium concentrations was prevented (Fig. 2B)[17].
Sequence alignment of PS1 and PS2 reveals a high homology in all regions except for the cytosolic N-termini and the third ER loop regions proximal to the endogenous cleavage sites[17]. One significant difference between the cytosolic N-termini is that PS2NTF has four redox sensitive cysteines while PS1NTF has none. In silico analysis of the PS2NTF cysteines indicated the likely presence of two cysteine bridges, suggesting that the observed presenilin-mediated regulation of RyR channel activity could be dependent on redox sensitive protein confirmations[17]. To test the effect of redox sensitive confirmations of PS on RyR activity, site-directed mutagenesis was performed to exchange the cysteines present in the PS2NTF (C14A, C31D, C56Q, C65Q) with the complementary residues of PS1NTF and vice versa, resulting in constructs named PS2NTF-ADQQ and PS1NTF-CCCC, respectively[17]. Cells expressing the redox insensitive PS2NTF-ADQQ construct recapitulated the regulatory effect that PS1NTF exerts on RyR by decreasing both the maximum amplitude and total amount of calcium released from the ER by RyR[17]. Cells expressing PS1NTF-CCCC displayed an intermediate response, with the calcium release maxima between the ones for the PS2NTF expressing or WT cells[17]. The ability of these mutations to exchange the respective PS variants’ regulation of the RyR suggests a specific and isoform dependent mechanism of ER calcium release by PS1 and PS2 that is directly related to the oxidation state of the cytosol.
Given this potent modulation of Ca2+ signaling by PS proteins[14–17], the question arises whether PS expression is altered in the aged brain either as a pathological cause or consequence of cognitive and/or motor impairments. One early study investigated PS mRNA and protein levels in the cortex and found decreased PS1 and increased PS2 expression in 15 months old compared to 6 months old mice[18]. A more recent study focused on both forebrain and cerebellar PS expression levels in both young, 6 months old, and aged, 24 months old, mice that had been behaviorally characterized to provide a detailed quantification of their cognitive and motor performance[19]. In rodent models, cognitive and motor impairment can be quantified using a variety of well-established behavioral paradigms including the swim maze test and the bridge walking task[20, 21]. PS protein expression was highly correlated with performance in behavioral paradigms for motor function, memory, and learning[19]. Specifically, PS1 levels were decreased while PS2 levels were increased in aged mice compared with young controls and with increasing impairment of their cognitive and motor performance[19]. This study confirmed and expanded on prior results providing strong evidence for the differential expression of PS proteins in a non-genetic model for aging and age-related cognitive impairment. The proposed overall increase of the PS2 to PS1 ratio in the aged brain represents a novel mechanism underlying molecular and functional changes during normal aging and implicates the group of PS proteins directly with synaptic dysfunction in aging and neurodegenerative disease in general adding to the already established mechanism that genetic mutations of presenilins cause familial forms of AD.
Consequently, a novel mechanism should be considered, wherein cytosolic calcium concentrations are regulated by PS binding to RyR. In a healthy neuron, the PS1-RyR interaction predominates and leads to a highly controlled temporal and spatial regulation of calcium release from the ER (Fig. 2). Aging, oxidative damage, and downregulation of PS1 expression contribute to increased RyR-mediated Ca2+ leak and hence potentially chronically increased cytosolic calcium concentrations. Once cytosolic calcium reaches a threshold level, the PS2 interaction with RyR becomes physiologically relevant, blocking the inhibition of the RyR by calcium at its inhibitory low affinity Ca2+ binding site. The resulting increasing cytosolic calcium loads potentially feed forward into age-related functional deficiencies and AD pathologies, such as loss of cognitive function, improper APP cleavage, and neuronal loss. This novel mechanism represents an alternative pathway for the pathogenesis of age-related dysfunction of the CNS and AD thereby contributing significantly to aspects of the calcium hypothesis of brain aging and AD pathology.
Acknowledgements
This publication was supported by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, a Challenge Grant from Research to Prevent Blindness and the Vision Research Foundation of Kansas City (PK) is gratefully acknowledged. The authors thank Margaret, Richard and Sara Koulen for generous support and encouragement.
References
- 1.Sama DM, Norris CM. Calcium dysregulation and neuroinflammation: discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing research reviews. 2013;12(4):982–995. doi: 10.1016/j.arr.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayflick L. Biological Aging Is No Longer an Unsolved Problem. Annals of the New York Academy of Sciences. 2007;1100(1):1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 3.2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Khachaturian ZS. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol Aging. 1987;8(4):345–346. doi: 10.1016/0197-4580(87)90073-x. [DOI] [PubMed] [Google Scholar]
- 5.Khachaturian ZS. Calcium, membranes, aging, and Alzheimer's disease. Introduction and overview. Ann N Y Acad Sci. 1989;568:1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x. [DOI] [PubMed] [Google Scholar]
- 6.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6(3):307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226(4678):1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 8.Landfield PW. 'Increased calcium-current' hypothesis of brain aging. Neurobiol Aging. 1987;8(4):346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- 9.Landfield PW. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiol Aging. 1988;9(5–6):571–579. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- 10.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3(11):862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 11.Elder GA, Gama Sosa MA, De Gasperi R, Dickstein DL, Hof PR. Presenilin transgenic mice as models of Alzheimer's disease. Brain Struct Funct. 2010;214(2–3):127–143. doi: 10.1007/s00429-009-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowburn RF, Popescu BO, Ankarcrona M, Dehvari N, Cedazo-Minguez A. Presenilin-mediated signal transduction. Physiol Behav. 2007;92(1–2):93–97. doi: 10.1016/j.physbeh.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 13.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, et al. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58(6):871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, et al. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460(7255):632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40(1):84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44(5):507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Payne AJ, Gerdes BC, Naumchuk Y, McCalley AE, Kaja S, Koulen P. Presenilins regulate the cellular activity of ryanodine receptors differentially through isotype-specific N-terminal cysteines. Experimental neurology. 2013;250:143–150. doi: 10.1016/j.expneurol.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakur MK, Ghosh S. Age and sex dependent alteration in presenilin expression in mouse cerebral cortex. Cellular and molecular neurobiology. 2007;27(8):1059–1067. doi: 10.1007/s10571-007-9214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaja S, Sumien N, Shah VV, Puthawala I, Maynard AN, Khullar N, et al. Loss of Spatial Memory, Learning, and Motor Function During Normal Aging Is Accompanied by Changes in Brain Presenilin 1 and 2 Expression Levels. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier TJ, Coleman PD. Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging. 1991;12(6):685–693. doi: 10.1016/0197-4580(91)90122-z. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen PV. Comparative plasticity of brain synapses in inbred mouse strains. The Journal of experimental biology. 2006;209(Pt 12):2293–2303. doi: 10.1242/jeb.01985. [DOI] [PubMed] [Google Scholar]