Abstract
Objective
We created a national policy model to evaluate the projected cost-effectiveness of multiple hospital-based strategies to prevent MRSA transmission and infection.
Design
Cost-effectiveness analysis using a Markov microsimulation model that simulates the natural history of MRSA acquisition and infection.
Patients and setting
Hypothetical cohort of 10,000 adult patients admitted to a U.S. ICU.
Methods
We compared 7 strategies to standard precautions using a hospital perspective: (1) active surveillance cultures (ASC); (2) ASC plus selective decolonization; (3) universal contact precautions (UCP); (4) universal chlorhexidine gluconate (CHG) baths; (5) universal decolonization; (6) UCP + CHG baths; and (7) UCP + decolonization. For each strategy, both efficacy and compliance were considered. Outcomes of interest were: (1) MRSA colonization averted; (2) MRSA infection averted; (3) incremental cost per colonization averted; (4) incremental cost per infection averted.
Results
1,989 cases of colonization and 544 MRSA invasive infections occurred under standard precautions per 10,000 patients. Universal decolonization was the least expensive strategy and was more effective compared to all strategies except UCP + decolonization and UCP + CHG. UCP + decolonization was more effective than universal decolonization, but would cost $2,469 per colonization averted and $9,007 per infection averted. If MRSA colonization prevalence drops from 12% to 5%, ASC plus selective decolonization becomes the least expensive strategy.
Conclusions
Universal decolonization is cost-saving, preventing 44% of cases of MRSA colonization and 45% of cases of MRSA infection. Our model provides useful guidance for decision makers choosing between multiple available hospital-based strategies to prevent MRSA transmission.
Background
Methicillin-resistant Staphylococcus aureus (MRSA) has become a major problem for U.S. healthcare facilities over the past 20 years.1,2 MRSA colonization is associated with increased risk of infection even after hospital discharge,3 worse clinical outcomes,4–8 and higher costs of care.5–9 Healthcare-associated infections (HAI) have been identified as a focus area in Healthy People 2020, with reduction of invasive healthcare-associated MRSA infections named as a top priority.10
Many strategies can be used to reduce the risk of MRSA transmission and infection. Active surveillance cultures (ASC) can be used to screen patients, with isolation of identified MRSA carriers. This strategy can be combined with decolonization of patients with chlorhexidine gluconate (CHG) baths, with or without mupirocin to the nares, to further reduce the risk of transmission and infection within carriers. At the other extreme, depending on factors such as the prevalence of MRSA among patients entering a particular hospital, a more effective strategy might be to simply isolate and/or decolonize all patients who enter a hospital regardless of MRSA status.
Decision-makers are thus faced with a wide variety of strategies while considering the cost and effectiveness of each strategy. Decision analytic models have increasingly been used to evaluate the potential impact of health policy questions,11–13 by comparing the projected outcomes and costs of alternative strategies.14 Models are particularly useful when it is impractical to support multiple clinical trials to compare a range of strategies. In real world settings where healthcare facilities consider a range of strategies under constrained resources, a comprehensive model that brings together all available strategies would aid decision-making at both the local and national levels. We created a national policy model to evaluate the projected cost-effectiveness of multiple hospital-based management strategies to prevent MRSA transmission and infection.
Methods
Natural History Model
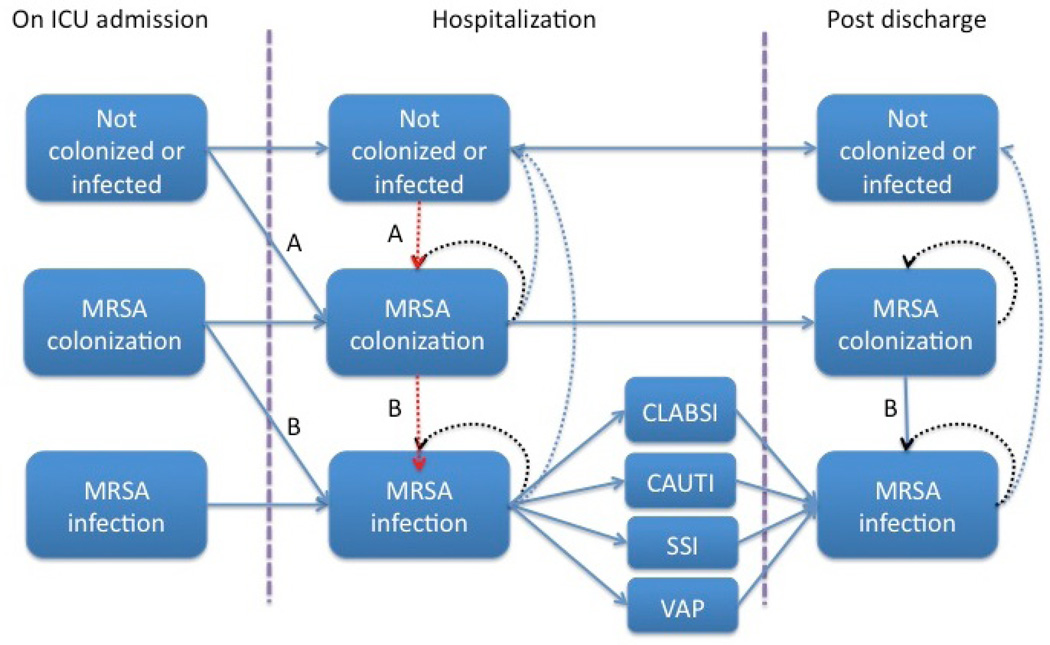
We developed a Markov microsimulation model using TreeAge Pro (TreeAge Software Inc. 2014, Williamstown, MA) for a hypothetical cohort of 10,000 patients admitted to intensive care units (ICUs) in the U.S. The model yields a hospital perspective analysis as the cohort moves through hospitalization and post discharge. We simulate the natural history of MRSA colonization and infection using a sequence of daily transitions among health states (Figure 1) over a 1-year time horizon. MRSA colonization is defined as a health state in which a patient carries the bacteria, but has no clinical infection. MRSA infection occurs when a patient develops an invasive infection.
Figure 1.
Natural History Model
*Acquisition of MRSA colonization and subsequent infection can be interrupted at the transition indicated by A (by contact precautions preventing transmission of MRSA, and/or by decolonization decreasing colonization pressure). MRSA infections can also be interrupted at the transitions indicated by B (by decolonization with mupirocin and/or CHG preventing infections that would have resulted from colonization in already colonized patients.)
Some patients are colonized and/or infected with MRSA at admission (Table 1), while others face a daily risk of acquiring MRSA colonization based on the prevalence of MRSA in the ICU. As overall MRSA colonization prevalence increases, we assume a higher risk of acquiring MRSA colonization among non-colonized patients. In our base case analysis, we assume that the average ICU prevalence is 12% (Table 1); we also consider the impact of lower (5%) and higher (20%) prevalence in alternative analyses.
Table 1.
Model Variables: Baseline Values and Ranges Used in Sensitivity Analyses
| Variable | Base Case | Distribution | Source(s) |
|---|---|---|---|
| Disease costs | |||
| Cost of MRSA colonization | $0 | N/A | |
| Average attributable LOS in days of MRSA infection during hospitalization CAUTI CLABSI SSI VAP |
0 ICU/1 ward 2 ICU/5 ward 3 ICU/4 ward 3 ICU/5 ward |
Log normal |
7,30–34 |
| Average ICU LOS if no MRSA infection | 3 days | Log normal | 35 |
| Average overall LOS if no MRSA infection | 5 days | Log normal | 35 |
| Cost per hospital day (ICU) | $4285 | Log normal | 36 |
| Cost per hospital day (general ward) | $969 | Log normal | Calibrated to model |
| Daily cost of treatment of invasive MRSA infection after hospital discharge |
$248 | Log normal | 37 |
| Total days in treatment course CAUTI CLABSI SSI VAP |
7 14 14 10 |
Log normal |
Expert consensus |
| Cost per episode of MRSA infection in outpatient setting* | $375 | Log normal | 17,38,39 |
| Intervention costs | |||
| Cost of contact precautions per day | $125 per day | Log normal | 40 |
| Cost of ASC test | $13 per test | Log normal | 26,27 |
| Cost of CHG bath per day | $11 per day | Log normal | 17 |
| Cost of decolonization (CHG + mupirocin) per day | $23 per day | Log normal | 17 |
| Intervention estimates | |||
| ASC with selective CP Efficacy for preventing colonization Efficacy for preventing infection Compliance |
(tied to efficacy for universal CP) -- 95% |
Beta |
22,41–43 44 |
| ASC with selective decolonization Efficacy for preventing colonization Efficacy for preventing infection Compliance |
94% -- 80% |
Beta |
23,45 |
| Universal CP Efficacy for preventing colonization Efficacy for preventing infection Compliance |
94% -- 47% |
Beta |
46 21,47–51 |
| Universal CHG Efficacy for preventing colonization Efficacy for preventing infection Compliance |
38% 35% 88% |
Beta |
52 Expert consensus 53,54 |
| Universal decolonization** Efficacy for preventing colonization Efficacy for preventing infection Compliance |
60% 50% 88% |
Beta |
Expert consensus 25 25 |
| ASC characteristics | |||
| Sensitivity of ASC testing*** | 64% | -- | 55,56 and expert consensus |
| Specificity of ASC testing | 96% | -- | 55,56 |
| Prevalence/incidence parameters | |||
| MRSA colonization at ICU admission | 12% | Beta | 21,41,52,57–64 |
| MRSA infection at ICU admission | 0.6% | Beta | 65,66 |
| Risk of acquiring MRSA colonization per hospitalization day among non- colonized patients based on prevalence (prevalence - risk of acquiring MRSA colonization) |
0% – 0.0% 3% – 0.2% 6% – 0.4% 9% – 0.6% 12% – 0.9% 15% – 1.1% 18% – 1.3% 21% – 1.5% |
Log normal | 41,52,57–59 |
| Risk of developing MRSA infection per hospitalization day among colonized patients |
0.6% | Beta | 15,16,59,67 |
| Type of MRSA infection CAUTI CLABSI SSI VAP |
4% 23% 43% 30% |
-- |
19 |
| Cumulative probability of death due to MRSA infection CAUTI CLABSI SSI VAP |
0% 21% 24% 30% |
-- |
5,6,8,18,68–70 |
| Daily probability of death due to other causes General ward ICU |
0.01% 3% |
-- |
Expert consensus |
| Outpatient | |||
| Risk of developing MRSA infection per outpatient day among colonized patients <3 months 3–<6 months 6–<=12 months |
0.15% 0.09% 0.04% |
Triangular |
15,16 |
| Probability of requiring hospitalization if develop an outpatient MRSA infection |
47% |
Triangular |
15,16 |
Based on cost of skin and soft tissue infections
We based the estimates for efficacy for preventing infection and compliance with the intervention from the trial from reference 25 (extrapolating from the effectiveness result presented in the trial). We then used expert consensus to judge the possible efficacy for colonization based on these estimates.
We assume that patients are receiving nares swabs only. Per expert consensus, we took an estimate of 92% from the literature for detection of MRSA from culture under perfect conditions, and multiplied that by 70% to reflect the sensitivity of nares swabs to detect MRSA colonization.
Patients with MRSA infection either die, or remain persistently infected until treatment is complete. If infected patients complete treatment, they are no longer colonized but can be recolonized and reinfected. Any patients who are discharged from the hospital and who are still colonized (either due to persistent colonization or because of recolonization) can remain uninfected or develop an MRSA infection post-discharge.15,16 Patients who develop a post-discharge infection are either treated as an outpatient or may require readmission (for MRSA bacteremia, for example).
Strategies
We assume a baseline approach of standard precautions (including hand hygiene) plus contact precautions for patients known to be MRSA colonized or infected, and compare 7 distinct ICU-based strategies: (1) ASC on ICU admission (includes contact precautions if found to be MRSA colonized); (2) ASC + selective decolonization for identified MRSA carriers; (3) universal contact precautions (UCP); (4) universal CHG baths; (5) universal decolonization (CHG baths and nasal mupirocin); (6) UCP + universal CHG baths; and (7) UCP + universal decolonization. The first 5 strategies were chosen after reviewing the literature and seeking expert guidance to reflect the most common strategies in ICUs. The latter two strategies consist of combinations (UCP + CHG baths; UCP + decolonization) to provide insight into the utility of the most aggressive approaches to MRSA control.
For ASC strategies, cultures are obtained on ICU admission and weekly thereafter as long as the patient remains in the ICU. We assume use of a standard swab of the nasal mucosa plated to chromogenic agar with results available within 1–2 days (to allow time for the lab to receive the swab, process it, and report results). If patients screen positive, t hey are placed on contact precautions (healthcare workers wear gloves and gowns). With selective decolonization, those who screen positive undergo daily CHG baths and twice daily mupirocin ointment applied to the nares for a maximum of 5 days or until discharge from the ICU, whichever occurs first. In contrast, universal strategies are applied to all ICU patients. If the universal CHG strategy is selected, we assume all patients are bathed daily with CHG for the duration of their ICU stay. UCP refers to the practice where gowns and gloves are used routinely for all patients while in the ICU, unless they are known MRSA carriers, which results in the use of contact precautions throughout hospitalization.
For each strategy, both efficacy and compliance are considered. Efficacy is the strategy’s ability to reduce risk of MRSA colonization or infection under ideal circumstances. Less-than-perfect compliance mitigates the overall effectiveness of each intervention, reflecting real-world conditions. We consider 3 types of efficacy in our model (Fig 1). For barrier-based strategies that use contact precautions, we assume a lower risk of transmission from carriers to non16 carriers, which lowers colonization pressure and reduces the risk of MRSA acquisition. Barrier-based strategies are assumed to have no impact on either colonization or infection among individuals with pre-existing colonization. For decolonization-based strategies, we assume a reduction in the probability of ongoing colonization and subsequent infection in previously colonized individuals. We also assume decolonization-based strategies reduce the risk for MRSA acquisition among non-colonized patients by lowering overall MRSA colonization prevalence. For strategies that rely on ASC, we assume that contact precautions with or without selective decolonization are implemented once test results are available. False negative test results and the delay in implementation both lead to lower estimates of efficacy compared to empiric strategies such as universal CP and universal decolonization.
Costs
We consider the following costs in the model: (1) inpatient medical costs attributable to MRSA infection; (2) outpatient medical costs attributable to MRSA infection (both for outpatient treatment of inpatient infection, as well as for infections that occur after hospitalization due to colonization acquired while hospitalized); and (3) intervention costs. We estimated costs and attributable length of stay due to infection based on literature review and the model was calibrated accordingly. If a patient develops a post-discharge infection and is then readmitted, all subsequent hospitals costs are considered attributable to MRSA. Outpatient costs for MRSA infection include the cost of an outpatient visit (average 1.5 visits) and cost of oral antibiotic therapy (trimethoprim-sulfamethoxazole, clindamycin, or doxycycline for an average duration of 7 days). Cost estimates are based on dollar values from 2013 by using the medical care component of the consumer price index to account for inflation.17 Future costs are discounted at an annual rate of 3%.
Outcomes
Outcomes of interest are: (1) cases of colonization averted; (2) cases of infection averted; (3) incremental cost per colonization averted; and (4) incremental cost per infection averted. The number of cases of colonization averted include those patients who were colonized on admission to the ICU and subsequently became decolonized. Cases of infections prevented include those infections avoided within a colonized patient (through decolonization), as well as those prevented by decreasing colonization pressure (through e ither decolonization or contact precautions). The incremental cost per case of colonization or infection averted is calculated as the cost difference for a given strategy compared to the cost of the next least expensive strategy, divided by the difference in the number of cases (of colonization or infection) prevented by the two strategies.
Uncertainty
Estimates used for model parameters were based on the best available estimates in the literature (Table 1). We also sought input from key experts through the CDC Prevention Epicenters program at their meeting on August 18, 2010. We presented estimates for key parameters, including MRSA transmission rates, infection rates, efficacy of interventions, compliance, and costs, and sought their feedback. We also convened a smaller formal expert panel in order to seek further input and validate our estimates.
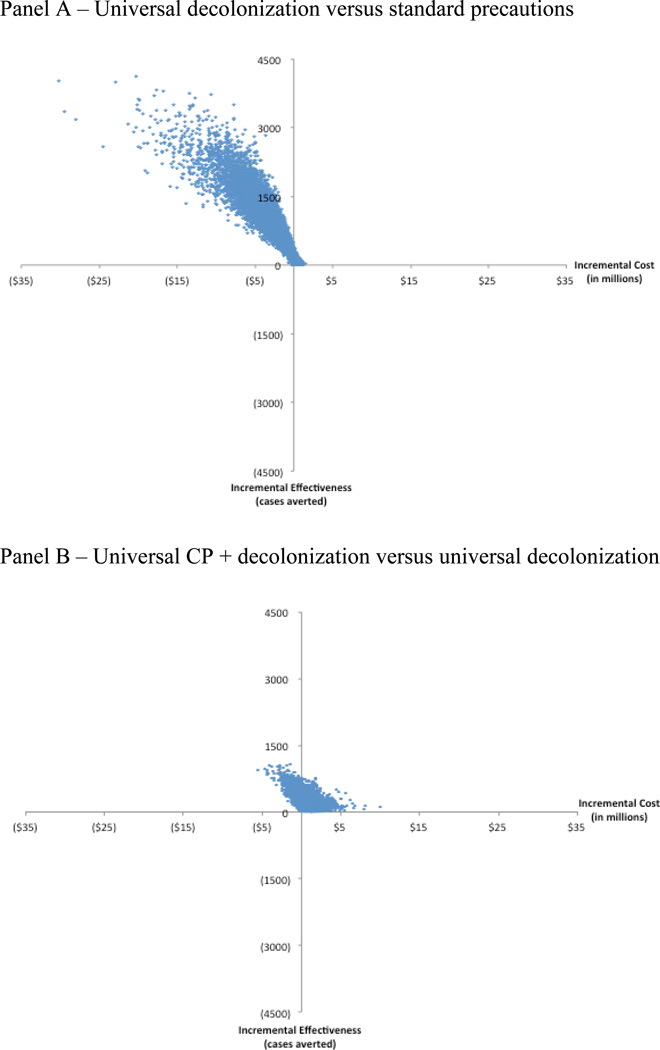
To account for first order uncertainty, we use a microsimulation model to capture the stochastic nature of events among individuals in the cohort. We also consider 2nd order uncertainty, or parameter uncertainty, by using probabilistic sensitivity analysis to vary the following parameters concurrently: (1) probability colonization and infection at admission; (2) probability of acquiring colonization per day if not colonized; (3) attributable length of stay due to infection as well as baseline length of stay in uninfected patients; (4) efficacy of each intervention; (5) risk of post-discharge infection if colonized; (6) cost of infection; and (7) cost of each intervention strategy. We use beta distributions for probabilities, log normal distributions for length of stay and cost, and triangular distributions for risk of post-discharge infection. Our Markov microsimulation model was run for 10,000 cohorts with 10,000 patients each to evaluate the robustness of our findings for the cost-effective strategies. Incremental costs and effectiveness estimates for each of the 10,000 cohorts are depicted on a scatterplot. We also calculated a proportion for how often the strategy of interest was more effective and cost saving compared to the next most expensive strategy.
Results
Base case scenario
In the absence of MRSA prevention strategies, 1,989 cases of colonization and 544 invasive infections occurred in a cohort of 10,000 patients admitted to ICUs. Strategies involving universal contact precautions paired with another intervention were the most effective. UCP + decolonization averted the greatest number of cases of MRSA colonization (1,107, or 56%) and infection (312, or 57%) per 10,000 patients, while UCP + universal CHG was the next most effective strategy (890 cases of colonization and 250 infections averted, or 45% and 46% respectively), when compared to standard precautions (which includes CP for known MRSA carriers) (Table 2).
Table 2.
Incremental cost per colonization and infection averted per 10,000 ICU admissions for alternative strategies using base case assumptions.
| Strategy | Total costs (in millions) |
Disease costs (in millions) |
Intervention costs (in millions) |
Cases of colonization averted |
Cases of infection averted |
Incremental cost per colonization averted |
Incremental cost per infection averted |
|---|---|---|---|---|---|---|---|
| Universal decolonization | $5.35 | $4.57 | $0.78 | 870 | 246 | -- | -- |
| ASC testing plus selective decolonization | $5.36 | $4.83 | $0.54 | 794 | 224 | Dominated | Dominated |
| Universal CHG | $5.91 | $5.65 | $0.26 | 601 | 171 | Dominated | Dominated |
| UCP + decolonization | $5.94 | $3.62 | $2.33 | 1,107 | 312 | $2,469 | $9,007 |
| UCP + universal CHG | $6.30 | $4.49 | $1.81 | 890 | 250 | Dominated | Dominated |
| ASC testing alone | $6.56 | $6.03 | $0.53 | 521 | 144 | Dominated | Dominated |
| UCP alone | $8.15 | $6.58 | $1.57 | 387 | 107 | Dominated | Dominated |
| Standard precautions | $8.16 | $8.16 | -- | -- | -- | Dominated | Dominated |
Under standard precautions, 1,989 and 544 cases of MRSA colonization and infection would occur, respectively.
Strategies without decolonization − meaning ASC + selective contact precautions or UCP alone − were the least effective strategies. Among strategies involving decolonization (with chlorhexidine +/− mupirocin), ASC testing + selective decolonization of MRSA carriers, universal CHG, and universal decolonization prevented more cases of MRSA than standard precautions, but were not the most effective strategies.
The total medical cost associated with MRSA infection in t his hypothetical cohort of 10,000 patients admitted to the ICU was $8.16 million (we assume that colonization itself does not result in higher medical costs in the absence of prevention strategies). Strategies without decolonization − UCP or ASC + selective CP− were the most expensive, with net costs of $8.15 million and $6.56 million respectively. The next most expensive were UCP + CHG, UCP + decolonization, and universal CHG. Universal decolonization alone had the lowest net cost ($5.35 million), including intervention cost ($0.78 million) and costs saved due to MRSA infection prevention ($3.59 million). ASC testing plus selective decolonization resulted in decreased intervention costs but also decreased savings due to less effective disease prevention.
Universal decolonization was cost saving overall and prevented 44% of cases of colonization and 45% of cases of infection. The next best strategy was UCP + decolonization, which resulted in incremental cost-effectiveness ratios of $2,469 per colonization averted and $9,007 per infection averted compared to universal decolonization. All other strategies were dominated (more costly and less effective).
Impact of MRSA colonization prevalence
If MRSA prevalence were lowered to 5%, the least expensive and reasonably effective strategy became ASC + selective decolonization (Appendix A). Universal decolonization was slightly more expensive and could prevent an additional 35 cases of colonization and 8 cases of infection. UCP + decolonization remained the most effective strategy to prevent both colonization and infection, with incremental cost-effectiveness ratios of $834 and $49,724 respectively. At a higher prevalence of 20%, results were very similar to the main model, with universal decolonization again being the least costly strategy (Appendix B). UCP + universal decolonization was the next best strategy with incremental cost-effectiveness ratios of $719 and $2,329 per additional case of colonization and infection prevented. All other strategies were dominated.
Appendix A.
Incremental cost per colonization and infection averted per 10,000 ICU admissions for alternative strategies under assumption that MRSA prevalence is 5%.
| Strategy | Total costs (in millions) |
Disease costs (in millions) |
Intervention costs (in millions) |
Cases of colonization averted |
Cases of infection averted |
Incremental cost per colonization averted |
Incremental cost per infection averted |
|---|---|---|---|---|---|---|---|
| ASC testing plus selective decolonization | $2.06 | $1.65 | $0.41 | 437 | 135 | -- | -- |
| Universal decolonization | $2.34 | $1.50 | $0.84 | 472 | 143 | $8,233 | $36,020 |
| Universal CHG | $2.46 | $2.16 | $0.29 | 303 | 87 | Dominated | Dominated |
| ASC testing alone | $2.70 | $2.30 | $0.40 | 283 | 83 | Dominated | Dominated |
| Standard precautions | $3.27 | $3.27 | -- | -- | -- | Dominated | Dominated |
| UCP + universal CHG | $3.68 | $1.59 | $2.09 | 473 | 133 | Extended dominance | Dominated |
| UCP + universal decolonization | $3.79 | $1.16 | $2.63 | 597 | 172 | $834 | $49,724 |
| UCP alone | $4.20 | $2.39 | $1.80 | 219 | 70 | Dominated | Dominated |
Appendix B.
Incremental cost per colonization and infection averted per 10,000 ICU admissions for alternative strategies under assumption that MRSA prevalence is 20%.
| Strategy | Total costs (in millions) |
Disease costs (in millions) |
Intervention costs (in millions) |
Cases of colonization averted |
Cases of infection averted |
Incremental cost per colonization averted |
Incremental cost per infection averted |
|---|---|---|---|---|---|---|---|
| Universal decolonization | $6.57 | $5.73 | $0.84 | 1,752 | 543 | -- | -- |
| UCP + universal decolonization | $6.81 | $4.17 | $2.64 | 2,079 | 644 | $719 | $2,329 |
| ASC testing plus selective decolonization | $7.68 | $6.97 | $0.71 | 1,513 | 468 | Dominated | Dominated |
| UCP + universal CHG | $8.25 | $6.13 | $2.12 | 1,601 | 513 | Dominated | Dominated |
| Universal CHG | $8.48 | $8.18 | $0.30 | 1,183 | 377 | Dominated | Dominated |
| ASC testing alone | $10.91 | $10.21 | $0.70 | 817 | 261 | Dominated | Dominated |
| UCP alone | $12.94 | $11.08 | $1.86 | 596 | 192 | Dominated | Dominated |
| Standard precautions | $13.95 | $13.95 | -- | -- | -- | Dominated | Dominated |
Probabilistic Sensitivity Analysis
Universal decolonization is more effective and cost saving compared to standard precautions 85% of the time for MRSA colonization and infection (Figures 2 and 3). When UCP + decolonization is compared to universal decolonization, 24% of the model runs result in estimates that are more effective and cost saving for colonization and infection. Furthermore 95% yielded incremental cost-effectiveness ratios that were <$2,500 per colonization averted and <$9,000 per infection averted.
Figure 2.
Probabilistic sensitivity analyses of cost per case of colonization prevented under different strategies
Figure 3.
Probabilistic sensitivity analyses of cost per case of infection prevented under different strategies
Discussion
We developed a national policy model to evaluate the cost-effectiveness of strategies to prevent MRSA colonization and infection. Assuming 4 million ICU visits per year in the U.S., our model estimates that 218,000 MRSA infections would occur in absence of any intervention (i.e. standard precautions) at a cost of approximately $3.3 billion, similar to previously published national estimates.18–20 Our model predicts that universal decolonization would prevent 44% of MRSA infections and reduce overall costs by 34% (i.e. by $1.11 billion). UCP + decolonization was more effective than universal decolonization, and may be worth considering if the willingness-to-pay threshold is at least $2,400 per colonization averted or $9,000 per infection averted. Universal decolonization was more effective and less expensive compared to standard precautions, non-decolonization strategies, or use of universal CHG alone. Decolonization-based strategies that utilize both intranasal mupirocin and CHG baths were preferred over other strategies even when we varied assumptions about disease probabilities, efficacy, and costs.
Our U.S.-based policy model is one of the first to compare all ICU-based strategies head-to-head. Other models have typically evaluated a single intervention,21–23 which limits the utility for decision-makers who may want to optimize their investment among a range of available strategies. A model based in the United Kingdom also evaluated a wide range of strategies and found that universal nasal mupirocin alone was the most cost-effective strategy.24 However, universal decolonization with both mupirocin and CHG was not considered, which is relevant for decision makers in the U.S. We not only consider the costs and outcomes associated with MRSA during the initial hospital admission, but the subsequent impact post-discharge. This is critically important since much of the infection burden following acquisition of MRSA occurs after discharge from the hospital.15,16
Our findings are based on a theoretical model, and are remarkably in line with results from recent trials on MRSA control strategies. Huang et al.25 demonstrated that universal decolonization of ICU patients was the most effective strategy to decrease bloodstream infections (across all pathogens) and reduce MRSA clinical isolates. Our model provides further support to this approach by demonstrating that it is cost-saving in addition.
Though we conducted a robust set of sensitivity analyses, there are several limitations to consider. First, our model applies to an “average” hospital based on average U.S. estimates. Individual healthcare facilities may wish to reconsider the full range of strategies using alternative assumptions about MRSA prevalence, costs, and compliance based on local data. For example, when we changed the MRSA prevalence to 5%, we found that ASC plus selective decolonization became the least expensive strategy. Further, hospitals may be constrained by the resources needed for interventions that may not be directly reimbursed by payers. However, shorter lengths of stay, lower readmission rates and improved outcomes provide a counterbalancing financial incentive from the hospital perspective. The ability of hospitals to invest in prevention efforts may vary depending on their calculated return on investment.
Second, we did not explicitly consider the horizontal impact of these interventions on reducing transmission of other common pathogens, such as vancomycin-resistant enterococci. The added benefit of strategies using CHG in preventing transmission and infection by organisms other than MRSA would improve the overall cost-effectiveness and further incentivize investment of resources for infection prevention.
Third, our model assumes that all patients return to the same hospital for readmissions. In reality, patients are often admitted to other facilities that subsequently benefit from the cost savings of interventions. This also means that we could not formally consider the potential for decolonization-based strategies to reduce spread of MRSA to other healthcare facilities. Such strategies could be tested in community-based models26,27 which capture the synergistic effect of coordinated regional efforts to minimize MRSA transmission.
Fourth, our model did not incorporate mupirocin or CHG resistance. One approach to incorporate the possibility of resistance would be to decrease the overall efficacy of decolonization-based interventions over a longer time horizon. Prolonged mupirocin use would very likely lead to the emergence of resistance, which would mitigate the overall cost-effectiveness of these interventions unless alternative options such as retapamulin became readily available.
Our model did not consider other MRSA infections during the initial admission, such as MRSA bacteremia that was not associated with a central line, which could contribute substantially to morbidity and costs.28,29 However, it did account for post-discharge MRSA bacteremia and subsequent readmissions. Finally, our model was not designed to be a dynamic model of MRSA transmission, though we did incorporate estimates for increased risk of MRSA transmission as hospital-wide prevalence increases.
In conclusion, our national policy model compares multiple strategies to prevent MRSA colonization and infection in an ICU cohort. Universal decolonization is more effective and cost saving when compared to all other strategies, except for UCP + decolonization. UCP + decolonization may be more effective than universal decolonization alone if decision makers are willing-to-pay ~$2,400 per case of MRSA colonization averted or ~$9,000 per case of MRSA infection averted. ICUs with a high prevalence of MRSA colonization that are not currently using strategies to control MRSA beyond standard precautions may wish to consider universal decolonization with or without universal contact precautions, depending on local factors such as the baseline prevalence of MRSA colonization and the availability of resources.
Acknowledgements
This work was funded through CDC grant 1U01CI000344 for the Epicenters Program. The findings and conclusions in this presentation are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This work was also supported by Award Number U54GM088558 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Dr. Huang is conducting a clinical trial in which participating hospitals are receiving contributed product from Sage Products and MoInlycke.
We would like to thank the following individuals for their invaluable assistance with this work: Marc Lipsitch and Joshua Salomon (Harvard School of Public Health), Anthony Harris (University of Maryland), Lauren Hunter (RAND Corporation), Melisa Rett, Michael Murphy, and Julie Lankiewicz (Harvard Pilgrim Health Care Institute). We also thank our colleagues from the CDC Prevention Epicenters who provided input on key estimates.
Footnotes
The work presented in this manuscript was presented in part as a poster at the Gidengil CA, Gay C, Huang SS, Yokoe D, Lee GM. Optimal Strategies to Prevent Methicillin-Resistant Staphylococcus Aureus (MRSA) Transmission in Hospitals. 48th Annual Meeting of the Infectious Diseases Society of America (IDSA), October 21–24, 2010.
The remaining authors have no conflict of interest to report.
References
- 1.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 2.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 5.Engemann JJ, Carmeli Y, Cosgrove SE, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592–598. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, McKinnon PS. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005;52:113–122. doi: 10.1016/j.diagmicrobio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Reed SD, Friedman JY, Engemann JJ, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2005;26:175–183. doi: 10.1086/502523. [DOI] [PubMed] [Google Scholar]
- 9.McHugh CG, Riley LW. Risk factors and costs associated with methicillin-resistant Staphylococcus aureus bloodstream infections. Infect Control Hosp Epidemiol. 2004;25:425–430. doi: 10.1086/502417. [DOI] [PubMed] [Google Scholar]
- 10.Healthcare-Associated Infections - Healthy People. [Accessed January 24, 2012]; at http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicId=17.)
- 11.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. Jama. 2012;307:804–812. doi: 10.1001/jama.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98:774–782. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein M, Fineberg H. Clinical Decision Analysis. Philadelphia: W.B. Saunders Company; 1980. [Google Scholar]
- 15.Datta R, Huang SS. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis. 2008;47:176–181. doi: 10.1086/589241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One. 2011;6:e24340. doi: 10.1371/journal.pone.0024340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Analy$ource. First DataBank Inc.; 2011. [Data accessed 8/31/2012]. © (2011). [Google Scholar]
- 18.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 19.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention; 2009. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention. at http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf.) [Google Scholar]
- 21.Huskins WC, Huckabee CM, O'Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364:1407–1418. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 23.Fraser TG, Fatica C, Scarpelli M, et al. Decrease in Staphylococcus aureus colonization and hospital-acquired infection in a medical intensive care unit after institution of an active surveillance and decolonization program. Infect Control Hosp Epidemiol. 2010;31:779–783. doi: 10.1086/654001. [DOI] [PubMed] [Google Scholar]
- 24.Robotham JV, Graves N, Cookson BD, et al. Screening, isolation, and decolonisation strategies in the control of meticillin resistant Staphylococcus aureus in intensive care units: cost effectiveness evaluation. BMJ. 2011;343:d5694. doi: 10.1136/bmj.d5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BY, Singh A, Bartsch SM, et al. The potential regional impact of contact precaution use in nursing homes to control methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2013;34:151–160. doi: 10.1086/669091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BY, Wong KF, Bartsch SM, et al. The Regional Healthcare Ecosystem Analyst (RHEA): a simulation modeling tool to assist infectious disease control in a health system. Journal of the American Medical Informatics Association : JAMIA. 2013;20:e139–e146. doi: 10.1136/amiajnl-2012-001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA internal medicine. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein L, Kallen AJ, Bellflower R, et al. Risk Factors for Invasive Methicillin-Resistant Staphylococcus Aureus Infection After Discharge from Acute-Care Hospitals - United States, 2011–2013. Centers for Disease Control and Prevention; 63rd Epidemic Intelligence Service Conference; 2014; Atlanta, GA. 2014. [Google Scholar]
- 30.Chaix C, Durand-Zaleski I, Alberti C, Brun-Buisson C. Control of endemic methicillin-resistant Staphylococcus aureus: a cost-benefit analysis in an intensive care unit. Jama. 1999;282:1745–1751. doi: 10.1001/jama.282.18.1745. [DOI] [PubMed] [Google Scholar]
- 31.Kopp BJ, Nix DE, Armstrong EP. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann Pharmacother. 2004;38:1377–1382. doi: 10.1345/aph.1E028. [DOI] [PubMed] [Google Scholar]
- 32.Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect Control Hosp Epidemiol. 1999;20:408–411. doi: 10.1086/501641. [DOI] [PubMed] [Google Scholar]
- 33.The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudmonas aeruginosa on length of hospital stay. Infect Control Hosp Epidemiol. 2002;23:106–108. doi: 10.1086/502018. [DOI] [PubMed] [Google Scholar]
- 34.Kaye KS, Anderson DJ, Sloane R, et al. The effect of surgical site infection on older operative patients. J Am Geriatr Soc. 2009;57:46–54. doi: 10.1111/j.1532-5415.2008.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lilly CM, Zuckerman IH, Badawi O, Riker RR. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest. 2011;140:1232–1242. doi: 10.1378/chest.11-0718. [DOI] [PubMed] [Google Scholar]
- 36.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 37.Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51(Suppl 2):S198–S208. doi: 10.1086/653520. [DOI] [PubMed] [Google Scholar]
- 38.Szumowski JD, Cohen DE, Kanaya F, Mayer KH. Treatment and outcomes of infections by methicillin-resistant Staphylococcus aureus at an ambulatory clinic. Antimicrob Agents Chemother. 2007;51:423–428. doi: 10.1128/AAC.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machlin and Chowdhury. Statistical Brief #318: Expenses and characteristics of physician visits in different ambulatory care settings, 2008. [Accessed 9 June 2011];AHRQ / MEPS Statistical Briefs. 2011 Mar; Available at: http://www.meps.ahrq.gov/mepsweb/data_files/publications/st318/stat318.shtml.
- 40.Muto CA, Giannetta ET, Durbin LJ, Simonton BM, Farr BM. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2002;23:429–435. doi: 10.1086/502080. [DOI] [PubMed] [Google Scholar]
- 41.Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2006;43:971–978. doi: 10.1086/507636. [DOI] [PubMed] [Google Scholar]
- 42.Ellingson K, Muder RR, Jain R, et al. Sustained reduction in the clinical incidence of methicillin-resistant Staphylococcus aureus colonization or infection associated with a multifaceted infection control intervention. Infect Control Hosp Epidemiol. 2011;32:1–8. doi: 10.1086/657665. [DOI] [PubMed] [Google Scholar]
- 43.Larson E, Bobo L, Bennett R, et al. Lack of care giver hand contamination with endemic bacterial pathogens in a nursing home. Am J Infect Control. 1992;20:11–15. doi: 10.1016/s0196-6553(05)80118-x. [DOI] [PubMed] [Google Scholar]
- 44.Pettinger A, Nettleman MD. Epidemiology of isolation precautions. Infect Control Hosp Epidemiol. 1991;12:303–307. doi: 10.1086/646343. [DOI] [PubMed] [Google Scholar]
- 45.Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol. 2007;28:1155–1161. doi: 10.1086/520102. [DOI] [PubMed] [Google Scholar]
- 46.Jernigan JA, Titus MG, Groschel DH, Getchell-White S, Farr BM. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol. 1996;143:496–504. doi: 10.1093/oxfordjournals.aje.a008770. [DOI] [PubMed] [Google Scholar]
- 47.Afif W, Huor P, Brassard P, Loo VG. Compliance with methicillin-resistant Staphylococcus aureus precautions in a teaching hospital. Am J Infect Control. 2002;30:430–433. doi: 10.1067/mic.2002.125174. [DOI] [PubMed] [Google Scholar]
- 48.Dedrick RE, Sinkowitz-Cochran RL, Cunningham C, et al. Hand hygiene practices after brief encounters with patients: an important opportunity for prevention. Infect Control Hosp Epidemiol. 2007;28:341–345. doi: 10.1086/510789. [DOI] [PubMed] [Google Scholar]
- 49.Golan Y, Doron S, Griffith J, et al. The impact of gown-use requirement on hand hygiene compliance. Clin Infect Dis. 2006;42:370–376. doi: 10.1086/498906. [DOI] [PubMed] [Google Scholar]
- 50.Weber DJ, Sickbert-Bennett EE, Brown VM, et al. Compliance with isolation precautions at a university hospital. Infect Control Hosp Epidemiol. 2007;28:358–361. doi: 10.1086/510871. [DOI] [PubMed] [Google Scholar]
- 51.Kim PW, Roghmann MC, Perencevich EN, Harris AD. Rates of hand disinfection associated with glove use, patient isolation, and changes between exposure to various body sites. Am J Infect Control. 2003;31:97–103. doi: 10.1067/mic.2003.32. [DOI] [PubMed] [Google Scholar]
- 52.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 53.Wendt C, Schinke S, Wurttemberger M, Oberdorfer K, Bock-Hensley O, von Baum H. Value of whole-body washing with chlorhexidine for the eradication of methicillin-resistant Staphylococcus aureus: a randomized, placebo-controlled, double-blind clinical trial. Infect Control Hosp Epidemiol. 2007;28:1036–1043. doi: 10.1086/519929. [DOI] [PubMed] [Google Scholar]
- 54.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30:959–963. doi: 10.1086/605925. [DOI] [PubMed] [Google Scholar]
- 55.Flayhart D, Hindler JF, Bruckner DA, et al. Multicenter evaluation of BBL CHROMagar MRSA medium for direct detection of methicillin-resistant Staphylococcus aureus from surveillance cultures of the anterior nares. J Clin Microbiol. 2005;43:5536–5540. doi: 10.1128/JCM.43.11.5536-5540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paule SM, Mehta M, Hacek DM, Gonzalzles TM, Robicsek A, Peterson LR. Chromogenic media vs real-time PCR for nasal surveillance of methicillin-resistant Staphylococcus aureus: impact on detection of MRSA-positive persons. Am J Clin Pathol. 2009;131:532–539. doi: 10.1309/AJCP18ONZUTDUGAQ. [DOI] [PubMed] [Google Scholar]
- 57.Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis. 2007;195:330–338. doi: 10.1086/510622. [DOI] [PubMed] [Google Scholar]
- 58.Ridenour GA, Wong ES, Call MA, Climo MW. Duration of colonization with methicillin-resistant Staphylococcus aureus among patients in the intensive care unit: implications for intervention. Infect Control Hosp Epidemiol. 2006;27:271–278. doi: 10.1086/500649. [DOI] [PubMed] [Google Scholar]
- 59.Warren DK, Guth RM, Coopersmith CM, Merz LR, Zack JE, Fraser VJ. Epidemiology of methicillin-resistant Staphylococcus aureus colonization in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2006;27:1032–1040. doi: 10.1086/507919. [DOI] [PubMed] [Google Scholar]
- 60.Clancy M, Graepler A, Wilson M, Douglas I, Johnson J, Price CS. Active screening in high-risk units is an effective and cost-avoidant method to reduce the rate of methicillin-resistant Staphylococcus aureus infection in the hospital. Infect Control Hosp Epidemiol. 2006;27:1009–1017. doi: 10.1086/507915. [DOI] [PubMed] [Google Scholar]
- 61.Patel M, Weinheimer JD, Waites KB, Baddley JW. Active surveillance to determine the impact of methicillin-resistant Staphylococcus aureus colonization on patients in intensive care units of a Veterans Affairs Medical Center. Infect Control Hosp Epidemiol. 2008;29:503–509. doi: 10.1086/588161. [DOI] [PubMed] [Google Scholar]
- 62.Robicsek A, Beaumont JL, Thomson RB, Jr, Govindarajan G, Peterson LR. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: impact on infection risk. Infect Control Hosp Epidemiol. 2009;30:623–632. doi: 10.1086/597550. [DOI] [PubMed] [Google Scholar]
- 63.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148:409–418. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 64.Fishbain JT, Lee JC, Nguyen HD, et al. Nosocomial transmission of methicillin-resistant Staphylococcus aureus: a blinded study to establish baseline acquisition rates. Infect Control Hosp Epidemiol. 2003;24:415–421. doi: 10.1086/502224. [DOI] [PubMed] [Google Scholar]
- 65.Muller A, Talon D, Potier A, Belle E, Cappelier G, Bertrand X. Use of intranasal mupirocin to prevent methicillin-resistant Staphylococcus aureus infection in intensive care units. Crit Care. 2005;9:R246–R250. doi: 10.1186/cc3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim T, Oh PI, Simor AE. The economic impact of methicillin-resistant Staphylococcus aureus in Canadian hospitals. Infect Control Hosp Epidemiol. 2001;22:99–104. doi: 10.1086/501871. [DOI] [PubMed] [Google Scholar]
- 67.Muder RR, Wagener MM, Yu VL. Methicillin-resistant Staphylococcus aureus in nursing homes. Ann Intern Med. 1992;116:267. [PubMed] [Google Scholar]
- 68.Ben-David D, Novikov I, Mermel LA. Are there differences in hospital cost between patients with nosocomial methicillin-resistant Staphylococcus aureus bloodstream infection and those with methicillin-susceptible S. aureus bloodstream infection? Infect Control Hosp Epidemiol. 2009;30:453–460. doi: 10.1086/596731. [DOI] [PubMed] [Google Scholar]
- 69.Shorr AF, Tabak YP, Gupta V, Johannes RS, Liu LZ, Kollef MH. Morbidity and cost burden of methicillin-resistant Staphylococcus aureus in early onset ventilator-associated pneumonia. Crit Care. 2006;10:R97. doi: 10.1186/cc4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ibelings MM, Bruining HA. Methicillin-resistant Staphylococcus aureus: acquisition and risk of death in patients in the intensive care unit. Eur J Surg. 1998;164:411–418. doi: 10.1080/110241598750004210. [DOI] [PubMed] [Google Scholar]