Abstract
Our current understanding of glucose homeostasis is centered on glucose-induced secretion of insulin from pancreatic islets and insulin action on glucose metabolism in peripheral tissues. In addition, however, recent evidence suggests that neurocircuits located within a brain-centered glucoregulatory system work cooperatively with pancreatic islets to promote glucose homeostasis. Among key observations is evidence that, in addition to insulin-dependent mechanisms, the brain has the capacity to potently lower blood glucose levels via mechanisms that are insulin-independent, some of which are activated by signals emanating from the gastrointestinal tract. This review highlights evidence supporting a key role for a “gut-brain-liver axis” in control of glucose homeostasis by the brain-centered glucoregulatory system and the implications of this regulatory system for diabetes pathogenesis and treatment.
Introduction
A devastating consequence of the worldwide obesity epidemic is a steady rise in the number of patients with type 2 diabetes (T2D). The global prevalence of T2D is estimated at 347 million currently, and this number is projected to increase to 552 million by 2030 [1]. In the United States alone, 29.1 million individuals (9.3% of the population) are diabetic, with T2D accounting for 90% to 95% of all diagnosed cases [2]. The risk of diabetes complications increases as a function of rising plasma glucose levels; currently, adequate glycemic control is achieved by only approximately 40% of patients with T2D [3]. These observations collectively highlight the need for new strategies to more effectively treat T2D and thereby reduce the enormous toll on human health taken by this disease.
Historically, the primary focus of the diabetes research community has been on pancreatic islet β cells and insulin-sensitive tissues, and this work has revealed an enormous amount with respect to the contribution made by defective insulin secretion or action (or both) to the development of impaired glucose tolerance (IGT) and T2D [4]. However, an increasing body of evidence supports the existence of specific neurocircuits that sense and integrate a variety of afferent signals and in response trigger adaptive changes of glucose metabolism in peripheral tissues, collectively referred to as the brain-centered glucoregulatory system (BCGS). The BCGS appears to act coordinately with pancreatic islets to regulate blood glucose levels via both insulin-dependent and insulin-independent mechanisms [5]. An improved understanding of how BCGS-islet interactions participate in normal and abnormal glucose homeostasis may ultimately facilitate the development of more successful therapeutic regimens.
A role for the brain in glucose homeostasis was first proposed by the French physiologist Claude Bernard on the basis of his observations that glycosuria could be induced in rabbits by lesions directed to the floor of the fourth ventricle [6]. Nearly a century later, Oomura and colleagues [7] and Anand and colleagues [8] confirmed the presence of specialized glucose-sensing neurons within the hypothalamus that could be either excited or inhibited by fluctuations of ambient glucose levels. Subsequent studies identified distinct subsets of these neurons as being either “glucose-excited” or “glucose-inhibited” [9], and new technologies in mouse genetics, viral tracing, optogenetics, and DREADDs (designer receptor exclusively activated by designer drugs) are rapidly accelerating research progress. The goals of this review are to highlight recent advances in our understanding of neural and hormonal connections linking the brain and gastrointestinal tract in the control of glucose homeostasis and how they interact with the islet-centered glucoregulatory system.
The gut-brain-liver axis
After ingestion of a meal, the presence of nutrients in the gastrointestinal tract initiates complex neural and hormonal responses that inform the brain of ongoing changes in nutritional status. The gut is richly supplied with primary visceral afferent nerve fibers from both sympathetic and parasympathetic branches of the autonomic nervous system [10]. Neural signals from the gut are integrated by hindbrain nuclei that project in turn to forebrain regions, including the hypothalamus [11,12]. The gut also informs the brain of current nutritional status by secreting a host of gut peptides from both enteroendocrine cells—ghrelin, cholecystokinin (CCK), and glucagon-like peptide 1 (GLP-1)—and enterocytes (fibroblast growth factor 19, or FGF19). Some of these hormones communicate with the central nervous system (CNS) primarily via effects on nearby afferent nerve fibers supplying the gut, whereas others are secreted from the gut into the circulation, whereupon they enter the brain to mediate their central effects [13,14].
An emerging picture suggests that some of these gut signals are involved a “gut-brain-liver axis” that participates in glucose homeostasis. Increased levels of circulating nutrients (glucose, fatty acids, and amino acids) following a meal generate signals to the CNS regarding short-term energy status and act within the hypothalamus and hindbrain to regulate both food intake and glucose homeostasis [15]. Neurons in these brain areas can be either excited or inhibited in response to glucose, lactate, or other nutrients (for example, oleic acid or leucine), and central infusion of many of these nutrients has been shown to lower circulating glucose levels by inhibiting hepatic glucose production (HGP) [16–19].
The intestinal neuronal network has also been identified as a target for nutrient regulation of glucose homeostasis. Using an intra-intestinal nutrient infusion paradigm in rats, Lam and colleagues report that infusion of lipids into either the duodenum or jejunum reduces HGP, with the specific mechanisms underlying this effect depending on the intestinal segment being targeted [20,21]. The metabolic response to intra-intestinal lipid infusion appears to involve increased concentrations of long-chain fatty acids (LCFAs) within enterocytes and is abolished by either hepatic vagotomy or blockade of hindbrain glutamatergic receptors (vagal afferents communicate with hindbrain neurons via glutamate release). Intraduodenal lipids also stimulate the release of CCK from duodenal I cells, and activation of CCK receptors on vagal afferents is implicated in the mechanism that lowers HGP [22]. The same group reported that, in the jejunum, administration of either glucose or lipids also reduces HGP, and this effect cannot be explained by entry of nutrients into the hepatic portal vein, since equimolar infusion of glucose directly into the portal vein was without effect [23].
The gut hormone GLP-1 is also secreted postprandially from the distal small intestine and acts through a specific G-protein-coupled receptor (GLP1R) expressed on pancreatic β and δ cells, CNS neurons, afferent and efferent enteric neurons, vagal and dorsal root ganglia, vascular smooth muscle, and gastric antrum and pylorus among other sites [24]. GLP-1 is defined as an “incretin” on the basis of its ability to enhance glucose-induced insulin secretion [25]. When administered peripherally, GLP-1 also inhibits glucagon secretion, slows gastric emptying, reduces food intake, and increases insulin-independent glucose disposal [26]. Accordingly, long-acting GLP-1 analogues are widely used in the treatment of T2D, but they also have documented benefit in patients with type 1 diabetes (T1D), despite having no ability to increase insulin secretion in this setting [27]. The mechanisms underlying the insulin-independent action of GLP-1 are unknown, but both peripheral and central sites of action likely contribute. In the periphery, GLP-1 infusion into the hepatic portal vein both increases the firing rate of the vagus nerve and enhances glucose disposal [28,29]. Similarly, centrally administered GLP-1 improves glucose tolerance, whereas intracerebroventricular (ICV) infusion of a pharmacological inhibitor of GLP-1 receptors impairs glucose tolerance [30]. Together, these findings suggest that intact neuronal GLP-1 signaling is required for normal glucose homeostasis, which implies that the glucose-lowering action of GLP-1 involves central as well as peripheral mechanisms. However, the role of CNS or vagal GLP-1 signaling (or both) in glucose homeostasis remains controversial, as the glucose-lowering effects of the long-acting GLP-1 agonist liraglutide are maintained in mice with either vagal- or brain-specific deletion of GLP1R [31].
The enterocyte hormone FGF19 is a member of the FGF hormone family (which includes FGF21 and FGF23) that was discovered in a screen of genes induced by activation of the bile acid receptor, farnesoid X receptor (FXR) [32]. In response to a meal, increased release of bile acids into the intestine is hypothesized to increase FXR signaling in enterocytes and thereby induce FGF19 synthesis and secretion [33]. FGF19 was initially identified as a key regulator of hepatic bile acid homeostasis via its effect to inhibit CYP7A1, the rate-limiting enzyme in hepatic bile acid synthesis, and growing evidence suggests that FGF19 may participate in glucose homeostasis as well. When administered peripherally, FGF19 improves glucose tolerance in both genetically obese (for example, leptin-deficient ob/ob mice) and diet-induced obese (DIO) mice [34]. In the liver, FGF19 reduces HGP via actions that closely resemble those of insulin but are insulin-independent [35]. Moreover, these hepatic actions may be mediated centrally, since ICV FGF19 administration improves glucose tolerance in DIO rats, whereas ICV administration of an FGF-receptor inhibitor has the opposite effect [36]. As for GLP1, available evidence suggests that intact neuronal FGF signaling is required for normal glucose homeostasis.
Indirect control of hepatic glucose production by brain insulin signaling
The fact that insulin inhibits HGP via a direct action on hepatic insulin receptors is well established. Binding of insulin to its receptor on hepatocytes activates the canonical insulin receptor substrate (IRS)-phosphatidylinositol 3-OH (PI3K)-Akt pathway [37]. Among many intracellular targets of the serine-threonine kinase Akt is Forkhead box protein O1 (FoxO1), a transcription factor that potently stimulates hepatic gluconeogenesis [38]. Phosphorylation of FoxO1 by Akt inhibits its transcriptional activity, and this effect is required for insulin to suppress HGP [37]. A role for hepatic insulin action in the physiological control of HGP was established with the demonstration of severe glucose intolerance and insulin resistance in mice with liver-specific knockout of the insulin receptor (LIRKO mice), and a similar phenotype is seen in mice with liver-specific deletion of the two hepatic Akt isoforms (DLKO mice) [39,40]. Not surprisingly, activation of FoxO1 in hepatocytes (owing to reduced Akt signaling) is implicated in the increased HGP characteristic of these mouse models.
In addition to its direct hepatic effect, however, growing evidence suggests that HGP can be inhibited by insulin action at a remote site. Buettner and colleagues [41] demonstrated in mice that although knockdown of hepatic insulin receptors (using insulin receptor anti-sense oligonucleotides) has the expected effect of markedly attenuating hepatic insulin signaling, HGP can nevertheless be inhibited by hyperinsulinemia. Subsequent evidence of an indirect pathway for insulin regulation of HGP comes from comparison of DLKO mice with mice with liver-specific deletion of FoxO1 as well as of the two AKT isoforms (TLKO mice). Unlike glucose intolerance of LIRKO mice, TLKO mice exhibit normal glucose homeostasis and intact inhibition of HGP in response to hyperinsulinemia, although hepatocytes of these mice are insulin-insensitive [39]. These data suggest that (a) insulin can inhibit HGP via both direct and indirect pathways, (b) insulin action via the indirect pathway is sufficient to maintain normal glucose homeostasis, (c) excessive hepatic FoxO1 signaling (as occurs in LIRKO and DLKO mice) can block both direct and indirect pathways.
Although the mechanisms mediating the indirect control of HGP by insulin remain to be elucidated, insulin action on neuronal insulin receptors likely plays a role [42]. In humans, intranasal application of insulin lowers postprandial blood insulin levels [43] and improves whole-body insulin sensitivity in lean patients as assessed by the hyperinsulinemic euglycemic clamp [44]. In rodents, low-dose ICV insulin is reported to increase hepatic insulin sensitivity, whereas infusion into the third ventricle of insulin receptor antibodies or an inhibitor of PI3K decreases hepatic insulin sensitivity and impairs the ability of systemic insulin to suppress HGP [45,46]. These findings suggest that neuronal insulin signaling is required to maintain HGP within normal limits.
A signal transduction mechanism forwarded to explain this insulin effect involves activation of neuronal ATP-sensitive potassium (KATP) channels via the IRS-PI3K pathway. KATP channels are expressed by neurons in the hypothalamus and are composed of an inwardly rectifying potassium ion channel (Kir6.2) subunit and sulfonylurea receptor subunit. When the channel is activated (or “opened”) (for example, in response to the drug diazoxide), potassium ions diffuse down the concentration gradient from the intracellular to the extracellular space, thereby reducing resting membrane potential and the likelihood of firing [47,48]. A similar effect is observed following ICV administration of insulin, which activates KATP channels and thereby decreases the firing rate of insulin-responsive hypothalamic neurons [49]. These neuronal effects of insulin are blocked by ICV co-infusion of either the KATP channel inhibitor tolbutamide or the PI3K inhibitor wortmannin, and these interventions also negate the effect of ICV insulin to lower HGP [48,50]. Interestingly, ICV infusion of diazoxide mimics the glucose-lowering effects of ICV insulin in rats [48], and in humans, systemic administration of diazoxide reduces HGP even when circulating insulin levels are clamped, implying an insulin-independent mechanism [51].
Extra-hypothalamic sites such as the dorsal vagal complex of the hindbrain are also implicated in the indirect control of HGP by insulin, apparently via insulin receptor-mediated activation of extracellular signal-related kinase (ERK) 1/2 rather than PI3K [52]. A distributed network of insulin-responsive neurons may therefore participate in the indirect control of HGP by insulin.
The efferent mechanism(s) linking the brain to indirect control of HGP by insulin may involve vagal efferent fibers, as ablation of efferent, but not afferent, vagal input to the liver prevents the effect of centrally administered insulin to inhibit HGP [48]. Additional mechanisms are likely, however, and going forward, studies to definitively identify mechanisms linking the brain to indirect control of HGP are an important scientific priority.
Brain regulation of insulin-independent glucose disposal
Although there is little doubt that insulin receptor signaling in peripheral tissues plays a key role in glucose homeostasis (especially in the postprandial state), growing evidence suggests that both HGP and tissue glucose uptake can also be controlled via insulin-independent mechanisms. Evidence that the brain can potently induce insulin-independent glucose lowering stems in part from studies of rodents with uncontrolled diabetes. Unger and colleagues [53,54] demonstrated that, when administered systemically at pharmacological doses, the adipocyte hormone leptin normalizes elevated blood glucose levels in rats and mice with uncontrolled diabetes, whether induced by a β cell toxin—streptozotocin (STZ)—or by autoimmune destruction (in non-obese diabetic mice). A role for the brain in this anti-diabetic leptin action was identified initially on the basis of the finding that, in rats with STZ-diabetes, continuous ICV infusion of leptin normalized markedly elevated blood glucose levels at low doses that are ineffective when administered peripherally [55]. Subsequent work in the STZ-diabetic rat model by Morton and colleagues [55] demonstrated that ICV leptin infusion both increases tissue glucose uptake and normalizes HGP despite persistent severe insulin deficiency. One implication of these findings is that, since insulin levels were too low to explain the observed normalization of HGP, the effect of central leptin cannot be attributed to increased hepatic insulin sensitivity. This conclusion implies that the brain has the capacity to normalize HGP and restore euglycemia to diabetic animals even in the absence of insulin.
Although the mechanisms whereby central leptin administration mediates glucose lowering in the absence of insulin remain uncertain, the effect involves both decreased gluconeogenic gene expression in liver and increased whole-body glucose turnover and glucose uptake in peripheral tissues, including skeletal muscle, heart, and brown adipose tissue [56]. Work from the Shulman laboratory [57] recently suggested that the primary mechanism mediating the central action of leptin in diabetic rats involves inhibition of the hypothalamic-pituitary-adrenal (HPA) axis and associated lowering of elevated circulating glucocorticoid levels. Such an effect is plausible since the HPA axis is clearly activated in uncontrolled diabetes and since ICV leptin reverses this effect [55]. Other findings, however, suggest that normalizing HPA axis activity is insufficient to reverse diabetic hyperglycemia [58], suggesting that further work is needed for a comprehensive understanding of the mechanisms by which central leptin signaling normalizes blood glucose in the absence of insulin.
Glucose effectiveness
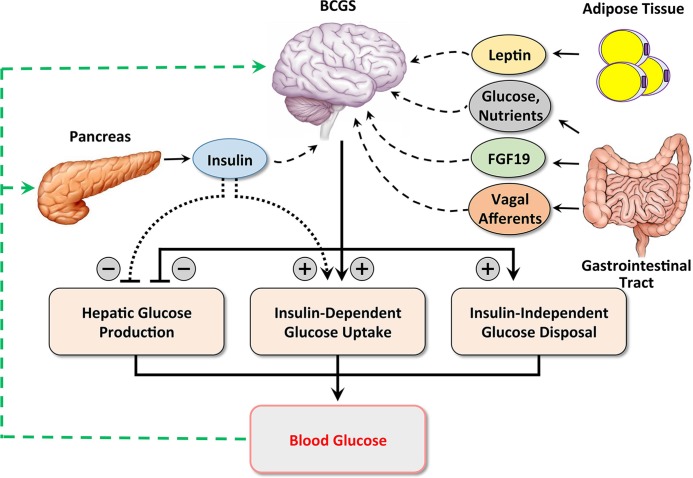
Glucose tolerance measures the efficiency with which glucose is cleared from the bloodstream after a glucose load, and it is determined by both insulin-dependent and -independent mechanisms. The insulin-independent component of glucose tolerance, termed “glucose effectiveness” (GE), can be calculated in humans and rodents on the basis of minimal model analysis of glucose and insulin kinetics during a frequently sampled intravenous glucose tolerance test developed by Bergman and colleagues [59]. Our recent work in ob/ob mice investigated whether the glucose-lowering action of FGF19 in the brain involves an insulin-independent mechanism [60]. The rationale underlying this work lies in part in the fact that insulin-independent mechanisms contribute as much to glucose tolerance as insulin does [61] and that in ob/ob mice GE is reduced by approximately 75% [62]. It was therefore difficult to envision a scenario in which FGF19 could normalize glucose tolerance in these animals (as reported by Fu and colleagues in 2004 [34]) unless GE were to increase. Consistent with this hypothesis, we found that ICV FGF19 markedly improves glucose tolerance in ob/ob mice by potently, rapidly, and selectively increasing GE [60]. These observations support the overarching concept that, in response to neural and hormonal signals generated by nutrient signaling in the gut, the brain can influence glucose homeostasis via both insulin-dependent and -independent mechanisms (Figure 1).
Figure 1. Hypothetical model of combined brain- and islet-centered control of glucose homeostasis.
Glucose homeostasis is hypothesized to involve cooperative and coordinated interactions between brain- and islet-centered regulatory systems. After a meal, nutrients in the gastrointestinal tract are absorbed into the circulation. Rising blood glucose levels trigger pancreatic β cells to secrete insulin, which lowers blood glucose levels both by inhibiting hepatic glucose production and by stimulating glucose uptake into insulin-sensitive tissues (primarily muscle and adipose tissue). Like the islet, the brain-centered glucoregulatory system (BCGS) senses and integrates multiple humoral—nutrients, glucose, leptin, and fibroblast growth factor 19 (FGF19)—and neural (vagal afferent) signals of nutritional status and in response promotes glucose disposal by both insulin-dependent and -independent mechanisms. In this way, coordinated activation of both islet- and brain-centered systems by incoming nutrients serves to maintain blood glucose levels within a narrow physiologic range.
Bariatric surgery
The ineffectiveness of medical therapy for the treatment of obesity has driven the development of multiple bariatric surgical procedures, including Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy. In 1952, Henrikson performed the first surgical intervention for the treatment of obesity, and with subsequent refinement bariatric surgery has emerged as a relatively safe and effective therapeutic option for some obese patients [63]. Unexpectedly, some bariatric surgical procedures also produce an almost immediate glucose-lowering effect that appears to involve mechanisms that are at least partly independent of weight loss. Consequently, although remission of T2D is rare with conventional treatment, it is common (typically, in more than 50% of cases) following RYGB surgery [64].
Although the physiological and molecular mechanisms underlying the metabolic benefits of bariatric surgery remain incompletely understood, a marked increase in the rapidity with which ingested nutrients enter the small intestine appears to be key. In response, a number of profound intestinal adaptations are described, including hypertrophy of intestinal mucosa, increased postprandial secretion of gut hormones (including GLP-1, PYY, and FGF-19), increased serum bile acid levels, reprogramming of intestinal glucose metabolism, and alterations of gut microbiota [65–68]. A growing literature has begun to unravel the contributions made by each of these adaptations to improvements of glucose homeostasis after bariatric surgery, and it seems reasonable to anticipate that metabolic benefit is conferred by a constellation of many of these effects (rather than by a single, overarching mechanism). Nonetheless, Seeley and colleagues [69] recently reported that metabolic benefit arising from vertical sleeve gastrectomy is linked to increased circulating bile acids and is prevented in mice lacking the bile acid receptor, FXR. Interestingly, activation of FXR by bile acids is required for intestinal secretion of FGF19 (FGF15 in rodents), raising the untested possibility that this hormone plays a key role in the glucose-lowering effect of the procedure.
Does the brain play a role in the response to bariatric surgery? In humans, changes in neural activity in brain reward centers in response to food cues can be measured by functional magnetic resonance imaging, and this brain response is modified after RYGB in a manner dissimilar to that observed in patients who underwent gastric banding for obesity [70]. (Unlike RYGB, gastric banding reduces, rather than increases, the rate of intestinal nutrient delivery.) In a rat model, Jiao and colleagues [71] demonstrated that the effect of duodenal-jejunal bypass (DJB) to improve glucose tolerance is prevented by hepatic vagotomy. Moreover, Breen and colleagues [23] reported that DJB is effective in lowering blood glucose levels in rats with STZ-induced uncontrolled diabetes, implicating insulin-independent mechanisms in the effect, and Paranjape and colleagues [72] reported that intact insulin signaling in the ventromedial hypothalamic nucleus (VMN) is necessary for the ability of RYGB to improve hepatic insulin sensitivity in a rat DIO model. The hypothesis that the VMN is involved in the improvement of glucose homeostasis after bariatric surgery is of interest given its known role in the neural control of hepatic glucose metabolism by efferent sympathetic nervous system fibers that reach the liver via the splanchnic nerve [73–75]. Whether the brain plays a major role in the anti-diabetic effect of bariatric surgery in humans is an important unanswered question; if so, interventions that target brain, intestine, and islet together may achieve improvements in glucose homeostasis greater than those that can be achieved by insulin-based therapies alone.
Summary and concluding remarks
A growing body of evidence in animal models and humans supports a key role for the brain in the control of glucose homeostasis. Although the focus of the diabetes research community has traditionally been placed on the islet, clear evidence now indicates that the brain can exert potent and rapid effects on both HGP and glucose uptake and that, in some cases, the effect involves insulin-independent mechanisms. Historically, the delivery of therapeutics to the brain has been hindered both by the challenge imposed by drug delivery across the blood-brain barrier (BBB) and by the potential for worrisome off-target effects. However, the development of novel drug delivery systems designed to circumvent the BBB, including intranasal preparations, nanocarriers, and structural manipulation and targeted use of endogenous transport/carrier systems, holds promising for the future of brain-directed therapies. Similarly, the use of biologicals may reduce unwanted side effects associated with small-molecule drugs, many of which have significant off-target effects. Moving forward, it will be important to better understand how brain and islet interact on a day-to-day basis in the normal and abnormal control of glucose homeostasis. Also important are studies to clarify how the brain promotes insulin-independent glucose disposal and whether such an effect contributes to the improvement of glucose homeostasis in patients with T2D after bariatric surgery. Studies along these lines have important potential to advance our understanding of diabetes pathogenesis and to identify new treatments for diabetes and related disorders.
Acknowledgments
This work was supported by grants to Michael W. Schwartz from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK090320, DK083042, and DK052989), the National Institutes of Health (NIH)-funded Nutrition Obesity Research Center (DK035816), and the Diabetes Research Center (DK17047) at the University of Washington and by an NIH-NIDDK training grant (T325T32DK007742-17).
Abbreviations
- BBB
blood-brain barrier
- BCGS
brain-centered glucoregulatory system
- CCK
cholecystokinin
- CNS
central nervous system
- DIO
diet-induced obesity
- DJB
duodenal-jejunal bypass
- FXR
farnesoid X receptor
- FGF
fibroblast growth factor
- FoxO1
Forkhead box protein O1
- GE
glucose effectiveness
- GLP-1
glucagon-like peptide 1
- GLP1R
G-protein-coupled receptor
- HGP
hepatic glucose production
- HPA
hypothalamic-pituitary-adrenal
- ICV
intracerebroventricular
- IRS
insulin receptor substrate
- KATP
ATP-sensitive potassium
- PI3K
phosphatidylinositol 3-OH
- RYGB
Roux-en Y gastric bypass
- STZ
streptozotocin
- T2D
type 2 diabetes
- VMN
ventromedial hypothalamic nucleus
Disclosures
Michael W. Schwartz has served as a consultant to Novo Nordisk (Bagsværd, Denmark) and Janssen (Beerse, Belgium) within the past year. JMS declares that he has no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/7/12
References
- 1.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–77. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Atlanta, GA: U.S. Department of Health and Human Services; 2014. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States; p. 2014. [Google Scholar]
- 3.Vinik A. Advancing therapy in type 2 diabetes mellitus with early, comprehensive progression from oral agents to insulin therapy. Clin Ther. 2007;29 Spec No:1236–53. doi: 10.1016/j.clinthera.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–83. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz MW, Seeley RJ, Tschöp MH, Woods SC, Morton GJ, Myers MG, D'Alessio D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard C. Lecons De Physiologie Experimentale Appliquee a La Medecine, Faites Au College De France Par M. Claude Bernard. Paris: JB Bailliere and son; p. 1856. [Google Scholar]
- 7.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–4. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 8.Anand BK, Chhina GS, Shatma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–54. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 9.Levin BE, Kang L, Sanders NM, Dunn-Meynell AA. Role of neuronal glucosensing in the regulation of energy homeostasis. Diabetes. 2006;55:S122–S130. doi: 10.2337/db06-S016. [DOI] [Google Scholar]
- 10.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 11.Sumal KK, Blessing WW, Joh TH, Reis DJ, Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- 12.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breen DM, Rasmussen BA, Côté CD, Jackson VM, Lam Tony KT. Nutrient-sensing mechanisms in the gut as therapeutic targets for diabetes. Diabetes. 2013;62:3005–13. doi: 10.2337/db13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayson BE, Seeley RJ, Sandoval DA. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci. 2013;14:24–37. doi: 10.1038/nrn3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol. 2006;95:1491–8. doi: 10.1152/jn.00697.2005. [DOI] [PubMed] [Google Scholar]
- 17.Lam Tony KT, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–7. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- 18.Su Y, Lam Tony KT, He W, Pocai A, Bryan J, Aguilar-Bryan L, Gutiérrez-Juárez R. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes. 2012;61:85–93. doi: 10.2337/db11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–5. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 20.Wang Penny YT, Caspi L, Lam Carol KL, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam Tony KT. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–6. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]; http.//f1000.com/prime/1109058
- 21.Lam Tony KT. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16:392–5. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718770614
- 22.Cheung Grace WC, Kokorovic A, Lam Carol KL, Chari M, Lam Tony KT. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720247601
- 23.Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung Grace WC, Lam Tony KT. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18:950–5. doi: 10.1038/nm.2745. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718770360
- 24.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–33. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718196489
- 25.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–16. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger J. Rationale use of GLP-1 receptor agonists in patients with type 1 diabetes. Curr Diab Rep. 2013;13:663–8. doi: 10.1007/s11892-013-0404-x. [DOI] [PubMed] [Google Scholar]
- 28.Nishizawa M, Moore MC, Shiota M, Gustavson SM, Snead WL, Neal DW, Cherrington AD. Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol Endocrinol Metab. 2003;284:E1027–36. doi: 10.1152/ajpendo.00503.2002. [DOI] [PubMed] [Google Scholar]
- 29.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271:E808–13. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 30.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–54. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725082948
- 31.Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest. 2014;124:2456–63. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718362414
- 32.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1028428
- 34.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan J, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/723616919
- 35.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–4. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/9332958
- 36.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/723611886
- 37.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–95. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718420514
- 40.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. doi: 10.1016/S1097-2765(00)00010-1. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722243930
- 41.Buettner C, Patel R, Muse ED, Bhanot S, Monia BP, McKay R, Obici S, Rossetti L. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306–13. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725281211
- 42.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signaling mechanisms and functional aspects. Prog Neurobiol. 1991;36:343–62. doi: 10.1016/0301-0082(91)90015-S. [DOI] [PubMed] [Google Scholar]
- 43.Coates PA, Ismail IS, Luzio SD, Griffiths I, Ollerton RL, Vølund A, Owens DR. Intranasal insulin: the effects of three dose regimens on postprandial glycaemic profiles in type II diabetic subjects. Diabet Med. 1995;12:235–9. doi: 10.1111/j.1464-5491.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 44.Heni M, Wagner R, Kullmann S, Veit R, Mat-Husin H, Linder K, Benkendorff C, Peter A, Stefan N, Häring H, Preissl H, Fritsche A. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes. 2014;63:4083–8. doi: 10.2337/db14-0477. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718494265
- 45.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–72. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717147829
- 46.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–82. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717147830
- 47.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 48.Pocai A, Lam Tony KT, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–31. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1025406
- 49.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–8. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 50.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang C, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–32. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1091747
- 51.Kishore P, Boucai L, Zhang K, Li W, Koppaka S, Kehlenbrink S, Schiwek A, Esterson YB, Mehta D, Bursheh S, Su Y, Gutierrez-Juarez R, Muzumdar R, Schwartz GJ, Hawkins M. Activation of K(ATP) channels suppresses glucose production in humans. J Clin Invest. 2011;121:4916–20. doi: 10.1172/JCI58035. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725281212
- 52.Filippi BM, Yang CS, Tang C, Lam Tony KT. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012;16:500–10. doi: 10.1016/j.cmet.2012.09.005. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720247166
- 53.Wang M, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813–9. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2669957
- 54.Yu X, Park B, Wang M, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070–5. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1120918
- 55.German JP, Thaler JP, Wisse BE, Oh-I S, Sarruf DA, Matsen ME, Fischer JD, Taborsky GJ, Schwartz MW, Morton GJ. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152:394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perry RJ, Zhang X, Zhang D, Kumashiro N, Camporez JG, Cline GW, Rothman DL, Shulman GI. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20:759–63. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450297
- 58.German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–34. doi: 10.2337/db09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergman RN. Minimal model: perspective from 2005. Horm Res. 2005;64(Suppl 3):8–15. doi: 10.1159/000089312. [DOI] [PubMed] [Google Scholar]
- 60.Morton GJ, Matsen ME, Bracy DP, Meek TH, Nguyen HT, Stefanovski D, Bergman RN, Wasserman DH, Schwartz MW. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123:4799–808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19:1018–30. doi: 10.2337/diacare.19.9.1018. [DOI] [PubMed] [Google Scholar]
- 62.Alonso LC, Watanabe Y, Stefanovski D, Lee EJ, Singamsetty S, Romano LC, Zou B, Garcia-Ocaña A, Bergman RN, O'Donnell CP. Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice. Obesity (Silver Spring) 2012;20:1403–12. doi: 10.1038/oby.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker MT. The history and evolution of bariatric surgical procedures. Surg Clin North Am. 2011;91:1181–201. doi: 10.1016/j.suc.2011.08.002. viii. [DOI] [PubMed] [Google Scholar]
- 64.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717955917
- 65.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–10. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718047684
- 66.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 2013;21:E660–8. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718023467
- 67.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–64. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725152119
- 68.Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery: it's all gastrointestinal factors versus it's all food restriction. Diabetes Care. 2013;36(Suppl 2):S287–91. doi: 10.2337/dcS13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725152074
- 69.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–8. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718078564
- 70.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux Carel W, Goldstone AP. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891–902. doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718078564
- 71.Jiao J, Bae EJ, Bandyopadhyay G, Oliver J, Marathe C, Chen M, Hsu J, Chen Y, Tian H, Olefsky JM, Saberi M. Restoration of euglycemia after duodenal bypass surgery is reliant on central and peripheral inputs in Zucker fa/fa rats. Diabetes. 2013;62:1074–83. doi: 10.2337/db12-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725081231
- 72.Paranjape SA, Chan O, Zhu W, Acharya NK, Rogers AM, Hajnal A, Sherwin RS. Improvement in hepatic insulin sensitivity after Roux-en-Y gastric bypass in a rat model of obesity is partially mediated via hypothalamic insulin action. Diabetologia. 2013;56:2055–8. doi: 10.1007/s00125-013-2952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720357097
- 73.Szabo AJ, Iguchi A, Burleson PD, Szabo O. Vagotomy or atropine blocks hypoglycemic effect of insulin injected into ventromedial hypothalamic nucleus. Am J Physiol. 1983;244:E467–71. doi: 10.1152/ajpendo.1983.244.5.E467. [DOI] [PubMed] [Google Scholar]
- 74.Shimazu T, Ogasawara S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am J Physiol. 1975;228:1787–93. doi: 10.1152/ajplegacy.1975.228.6.1787. [DOI] [PubMed] [Google Scholar]
- 75.Matsushita H, Ishikawa K, Shimazu T. Chemical coding of the hypothalamic neurones in metabolic control. I. Acetylcholine-sensitive neurones and glycogen synthesis in liver. Brain Res. 1979;163:253–61. doi: 10.1016/0006-8993(79)90353-6. [DOI] [PubMed] [Google Scholar]