Abstract
Spinal muscular atrophy (SMA) is the most frequent genetic cause of death in infants and toddlers. All cases of spinal muscular atrophy result from reductions in levels of the survival motor neuron (SMN) protein, and so SMN upregulation is a focus of many preclinical and clinical studies. We examine four issues that may be important in planning for therapeutic success. First, neuromuscular phenotypes in the SMNΔ7 mouse model closely match those in human patients but peripheral disease manifestations differ, suggesting that endpoints other than mouse lifespan may be more useful in predicting clinical outcome. Second, SMN plays important roles in multiple central and peripheral cell types, not just motor neurons, and it remains unclear which of these cell types need to be targeted therapeutically. Third, should SMN-restoration therapy not be effective in all patients, blocking molecular changes downstream of SMN reduction may confer significant benefit, making it important to evaluate therapeutic targets other than SMN. Lastly, for patients whose disease progression is slowed, but who retain significant motor dysfunction, additional approaches used to enhance regeneration of the neuromuscular system may be of value.
Introduction
SMA is the most frequent genetic cause of death in infants and toddlers. It was first described by Werdnig in 1891, when he observed two infant brothers with the onset of progressive proximal leg weakness at 10 months of age [1]. Hoffman, between 1893 and 1900, described an additional seven patients from three families [2–4]. Although these cases were of intermediate severity, Sylvestre in 1899 and Beevor in 1903 presented the first cases of severe SMA: two infants with flaccid paralysis of limbs and trunk muscles at birth [5–7]. These infants were from two families in which 7 out of 14 total children were affected, and all affected children died within 6 months of age. Over half a century later, Wohlfart, Eliasson, and Fex in 1955 and Kugelberg and Welander in 1956 described the mild ambulant form of SMA in two case series [7–9]. The clinical presentation was similar to a limb-girdle muscular dystrophy, but electromyography and a muscle biopsy documented neurogenic changes, leading to speculation that this represented a mild form of the disease described by Werdnig and Hoffman. Early descriptions of intermediate and severe forms of SMA all recognized a progressive and symmetric weakness involving the proximal extremities, axial muscles, and intercostal muscles, with prominent sparing of the diaphragm [7]. The accompanying pathological studies described degeneration of the motor neurons in the anterior horn of the spinal cord, the neurons through which the brain triggers contraction of skeletal muscle.
Despite this stereotyped pattern of neuromuscular weakness and motor neuron loss at autopsy, these early studies highlighted marked differences in age of onset, rate of progression, and overall severity of SMA. Thus, for over a century, it was unclear if SMA was one disease with a broad spectrum of severity or represented multiple diseases. This spectrum of phenotypes was formally classified in 1991, based on the age of clinical onset and maximum motor function achieved [10]. Type I SMA, the most common subtype, is characterized by disease onset within 6 months of age and death within 2 years. The onset of Type II SMA occurs between 6 and 18 months of age, and patients gain the ability to sit upright but not walk. Type III SMA presents after 18 months of age and patients achieve ambulation, at least temporarily [10–15].
The fact that these are different manifestations of a single disease was demonstrated by the finding that 95 % of all cases of SMA are caused by homozygous loss of the survival motor neuron 1 (SMN1) gene [16]. SMN is a ubiquitously expressed protein involved in multiple aspects of RNA metabolism, including RNA splicing. Complete loss of SMN would be embryonic lethal, but the absence of SMN1 is compensated for by variable copy numbers of the hypomorphic gene paralog survival motor neuron 2 (SMN2). SMN2 potentially encodes for the same protein as SMN1, but a single nucleotide transition in exon seven leads to the skipping of exon seven in the majority of transcripts, and production of a truncated SMN protein that is rapidly degraded [17–19]. On average, the higher the copy number of SMN2 the milder the phenotype, but copy number is not fully prognostic since SMN2 is not the sole disease modifier [20]. SMA has an incidence of approximately 1/11,000 live births and a pan-ethnic carrier frequency of 1/54 [21,22].
Outstanding questions
Human genetics and preclinical studies have provided clear proof of concept for SMN upregulation as a therapeutic strategy potentially applicable to all patients [23,24]. For this reason, clinical trials of several approaches using antisense oligonucleotides to correct the missplicing of SMN2, or viral vectors and small molecules to increase SMN levels, are underway or planned (Figure 1; [25]). This is, therefore, an exciting period for SMA therapeutics and the outcome of the trials is eagerly awaited. These approaches raise important questions about the molecular functions of SMN and the timing of SMN restoration, which have been reviewed elsewhere [23,26] and so are not covered here. Instead, we focus on four questions concerning the relationship of preclinical and clinical studies, which may be important in refining future therapeutic strategies: (a) how predictive is the SMNΔ7 mouse model on which most preclinical data are based?; (b) in which cell types does SMN function need to be restored?; (c) will SMN upregulation be sufficient, or should we target other downstream targets in parallel? and (d) what is the role of regenerative medicine in restoring function of the neuromuscular system?
Figure 1. Therapeutic strategies in spinal muscular atrophy.
The predominant therapeutic approach in spinal muscular atrophy (SMA) is to increase the amount of full-length survival motor neuron (SMN) protein, by promoting greater inclusion of exon seven in transcripts from Smn2 or by over-expressing full-length SMN complementary DNA. Both approaches have been shown to provide striking rescue of neuromuscular phenotype and survival when applied early in preclinical mouse models. To prepare for the possibility that SMN-targeted therapies may not prove fully effective in all patients, other strategies are being evaluated in parallel. One involves correcting the downstream effects of SMN deficiency, such as splicing defects in specific transcripts required for neuromuscular integrity. Another, which was recently reported to show benefit in SMA patients, is to identify neuroprotective agents that can prevent or slow motor neuron death in an SMN-independent manner or stimulate the regeneration of motor circuits. It is likely that a combination of such approaches will be required to completely address the needs of all SMA patients.
Abbreviations: AAV, adeno-associated virus; ANT, adenine nucleotide translocator; ASO, antisense oligonucleotide; CypD, cyclophilin D; SMN, survival motor neuron; SMN2, survival motor neuron 2; SNARE, soluble NSF-attachment protein (SNAP) receptors; VDAC, voltage-gated anion channel.
In addition to the points highlighted below, we refer the reader to several recent reviews that treat some of these questions in more detail [19,23,25,26].
How predictive is the SMNΔ7 mouse?
Inactivation of the Smn gene in mice, which have no equivalent of SMN2, results in massive cell death during embryonic development [27]. However, mice bearing a human SMN2 transgene on an Smn-null background exhibit a progressive neuromuscular phenotype that, in many ways, mimics the human pathology, including motor neuron loss and muscle denervation [28,29]. Moreover, as in humans, phenotypic severity is inversely correlated with SMN levels: introducing two copies of SMN2 produces a severe SMA phenotype and death within 5 days, whereas eight copies of SMN2 essentially rescue the mice. The addition of an SMN transgene lacking exon seven (SMNΔ7), together with two copies of SMN2, further extends lifespan to ~13 days [30–32]. Other models have been created using distinct but comparable strategies [33]. Nevertheless, the SMNΔ7 model has been the most widely utilized for evaluation of candidate SMA therapeutics and so we focus on it here. How close is it to the human disease, and how predictive are positive outcomes in this model?
SMA patients exhibit dramatic differential vulnerability of motor units that innervate different muscles. Despite widespread motor neuron loss and flaccid paralysis, SMA patients retain normal eye movements and external sphincter continence and relatively normal facial expressions [34–36]. Additionally, preserved function of the diaphragm in conjunction with degeneration of the intercostal muscles that support the thoracic cavity produces a “bell-shaped chest” that is virtually pathognomonic for SMA [35,37–39]. Using muscle denervation as a quantitative readout for disease progression, SMNΔ7 mice also show a high degree of differential motor unit vulnerability [32]. Moreover, by comparing the SMNΔ7 model with post-mortem samples from Type I SMA patients on a muscle-by-muscle basis, we have shown a remarkable degree of overlap between the mouse and human neuromuscular phenotypes [40,41]. Therefore, despite the severe phenotype of the SMNΔ7 mouse, the exquisite selectivity of the human disease at the neuromuscular level is modeled with very high fidelity.
There is a less perfect match in terms of other aspects of pathology that also affect the lifespan of the mice. For example, SMNΔ7 mice exhibit cardiac defects [42,43] and distal tissue necrosis [29,44–46] that are not characteristic features of the human condition [34]. It has been suggested that human SMA is a multi-system disorder, including congenital heart disease and vascular perfusion abnormalities [47,48]. However, in the largest study to date, congenital heart defects were observed only in Type 0 SMA with one copy of SMN2, totaling three out of four Type 0 patients that exhibited a prenatal onset of weakness, contractures, and respiratory distress at birth. None of the 61 Type I SMA patients examined had congenital heart defects, with the exception of a very small number of patients with common, minor cardiac anomalies that resolved spontaneously [49]. Two additional studies examining cardiac involvement in approximately 80 SMA patients with Types I, II, and III concluded that heart dysfunction is not a feature of SMA [50,51]. Case studies have reported ulcerations and necrosis in the distal extremities but, to our knowledge, this is limited to four reported patients with clinical descriptions suggesting Type 0 SMA [49,52]. In the two patients that were kept alive on mechanical ventilation for an extended period of time, all lesions resolved without recurrence [52]. We conclude that multi-organ dysfunction, including cardiac and vascular defects, is not a general feature of human SMA. These findings call for caution when interpreting some published data in SMA mice, as therapeutics that rescue the neuromuscular phenotype but do not ameliorate the underlying cardiac pathology may not produce a commensurate improvement in gross phenotype or survival [53], whereas they might be effective in patients. Endpoints based on quantitative evaluation of neuromuscular pathology may be of greater predictive value.
Overall, therefore, the SMNΔ7 model mimics some aspects of SMA pathology with remarkable precision, but also exhibits differences that may be species-specific and need to be taken into account in comparing therapeutic outcomes.
In which cell types does SMN need to be restored?
SMA shares a major characteristic with all neurodegenerative disorders: selective degeneration of a limited subset of neurons in response to dysfunction or deletion of a ubiquitously expressed protein, in this case SMN. An abundant literature based on the SMNΔ7 mouse indicates that, although motor neuron dysfunction and degeneration underlie the principal clinical phenotypes, loss of SMN function in other cell types contributes in important ways. Here, we review the effects of reductions in SMN in different cell types.
Motor neurons
SMA in humans is characterized by extensive loss of spinal motor neurons [41,54–56], and human induced pluripotent stem cells (iPSC)-derived motor neurons from SMA patients show an intrinsic survival deficit in vitro [57–59]. However, the fact that many motor neuron subpopulations survive intact at end-stage in the SMNΔ7 mouse, perhaps due to its limited lifespan, has led the field to question the contribution of motor neuron death to the overall phenotype. Nevertheless, motor neurons of the median motor column, which innervate the proximal muscles that are most strongly affected in patients, do show significant cell death at early stages in SMNΔ7 mice [41,54]. Therefore, motor neurons in mouse models, as in human patients, are selectively vulnerable to low SMN. One potential explanation is that normal motor neurons express markedly lower levels of full-length SMN from the SMN2 gene than other cell populations in the spinal cord do, due to particularly inefficient splicing of exon seven [60]. The splicing defect is further exacerbated by the depletion of SMN in the SMNΔ7 mouse, generating a negative feedback loop that may underlie some aspects of motor neuron vulnerability [60,61].
Other studies in mice have examined the effect of modulating SMN specifically within motor neurons. Genetic knockdown of SMN in motor neuron progenitors, using Olig2-Cre-driven recombination of mouse Smn on a background of two copies of SMN2, produced an SMA-like phenotype with motor neuron degeneration and neuromuscular weakness [62]. Despite this demonstration of a cell-autonomous requirement for SMN in motor neurons, the phenotype was markedly less severe than in SMNΔ7 mice, which have reduced SMN in all tissues: approximately 70% of mice with SMN selectively depleted in motor neurons survived to 12 months of age, while SMNΔ7 mice, with ubiquitous SMN reduction, survived an average of 13 days [62]. Moreover, the “reverse” experiment, selective restoration of SMN in the motor neurons of SMNΔ7 mice using a Cre-inducible Smn allele under control of the choline acetyltransferase (ChAT) promoter, fully prevented synaptic dysfunction at the neuromuscular junction, but only partially reduced motor neuron death, and had a relatively modest effect on overall neuromuscular phenotype and death [63]. Collectively, these experiments suggest that SMN reduction in cell types other than motor neurons also contribute substantially to the pathogenesis of SMA.
Other neuronal classes
The motor circuitry is a critical mediator of the firing, and thus the functional output, of motor neurons. Given the severe impairments in motor behavior in SMA mice, such as an impaired righting reflex as early as P1 in SMNΔ7 mice, the modest changes in motor neuron loss and transmission at the neuromuscular junction are somewhat surprising. Thus, it has been hypothesized that motor circuit dysfunction contributes to the SMA phenotype. Indeed, studies in the SMNΔ7 mice demonstrated a loss of number and function of synapses onto motor neurons that mediate proprioceptive reflexes, which are important for refining the output of the motor system through feedback signals [54,64]. Loss of these afferent synapses precedes motor neuron loss and even occurs in embryonic SMNΔ7 mice, suggesting this is an early pathological event that contributes to functional impairment in SMA [54,65]. Additionally, it has been demonstrated that the SMA phenotype in Drosophila results primarily from dysfunction in the motor circuit, not the motor unit [66]. However, increasing SMN levels within motor neurons (though perhaps also other cell types) in SMNΔ7 mice, and in a more severe inducible SMA mouse, improves electrophysiological deficits and loss of sensory-motor synapses, indicating that low SMN in motor neurons may also contribute to motor circuit dysfunction [53,63]. The H-reflex, which measures motor unit firing in response to the stimulation of proprioceptive 1a afferents, is reportedly absent in many Type I SMA patients [67]. However, the interpretation of these results is complicated by neuromuscular denervation and motor neuron loss, so this merits further investigation. Overall, there are functional consequences of low SMN in multiple elements of the spinal circuitry, but the full extent of the contribution of each cell type to SMA pathogenesis remains to be fully determined, particularly in human patients.
Other neuronal phenotypes have been reported in mouse models of SMA, including loss of corticospinal neurons in the SMNΔ7 mouse and reduced cell proliferation and neurogenesis in the hippocampus in the severe Smn-/-; SMN2+/+ mouse [56,68]. More studies are required to determine whether pathology in these and other neuronal cell types is a previously unappreciated aspect of the human disease.
Muscle
Given the close trophic and functional interactions between motor neurons and the muscles they innervate, much work has been performed to delineate the potential contribution of intrinsic skeletal muscle abnormalities to the SMA phenotype. Early co-culture experiments indicated that extracts of muscle biopsies from SMA patients, but not aged-matched controls, inhibited the trophic effect of neonatal chick muscle on embryonic chick spinal neurons [69]. A later study found that myofibers formed by fused muscle satellite cells from severely affected SMA patients degenerated within 3 weeks of innervation by rodent spinal cord explants, whereas myofibers from mildly affected SMA patients or controls survived for several months [70]. Cultures of SMA satellite cells from severe SMA mice and primary myoblasts from SMNΔ7 mice exhibit an altered expression of MyoD and myogenin, two key muscle developmental factors, and myotube formation deficits [71,72]. Additionally, cultured muscle cells from SMA patient biopsies are smaller than those from control patients and have significantly disrupted expression of myogenic genes critical for muscle development [73,74].
Myofibers in SMNΔ7 mice fail to grow during early postnatal development, producing a severe and uniform reduction in muscle size [75]. These defects may either be muscle-intrinsic or may be secondary to abnormalities in neuromuscular transmission observed even in the presence of fully innervating motor axons [31,76,77]. Moreover, key developmental events at the neuromuscular junction, such as expression of critical myosin isoforms and maturation of the motor endplate, are severely delayed in SMNΔ7 mice [75]. In order to determine the origin of these changes, investigators have modulated SMN expression in SMA mice selectively in muscle.
An early study that increased SMN in muscle did not find significant improvements in motor phenotype or lifespan [78]. However, this study utilized the human skeletal actin (HSA) promoter, which is not expressed in satellite cells or myoblasts. Satellite cells, located between the sarcolemma and basal lamina of muscle fibers, are muscle stem cells responsible for neonatal muscle growth and maintenance and repair of adult muscle; they constitute the major regenerative population in muscles [79,80]. A body of literature has suggested that SMN-deficient satellite cells may contribute to muscle pathology in SMA [70,81–85]. Selective restoration of SMN levels by 50% in muscle satellite cells, on a background of complete Smn deletion in mature myofibers, markedly improved the phenotype, with an extension in median survival from 1 month to approximately 8 months of age [82]. This improvement is likely due to the enormous regenerative capacity of muscle satellite cells. A more recent study selectively restored SMN in early muscle progenitors using the MyoD and Myf5 promoters and found a complete rescue of myofiber growth and an improvement in motor phenotype and survival, but no effect on neuromuscular junction deficits or central synapses. Selective restoration in motor neurons with the ChAT promoter, in contrast, produced only a partial rescue of myofiber growth but restored neuromuscular junction transmission [63]. In conclusion, SMN appears to have cell-autonomous functions in muscle fiber growth and/or maintenance independently of the rest of the motor unit in both human and mouse SMA and may contribute to disease pathogenesis. In particular, SMN in muscle satellite cells appears to be critically important for the regenerative capacity of muscle in response to chronic SMA pathology.
Glial cells
Astrocytes execute critical functions in normal motor neuron physiology, including buffering extracellular ions and neurotransmitters, modulating synaptic structure and function, and the release of neurotrophic factors [86,87]. They also play a pathogenic role in a variety of neurodegenerative diseases, including the motor neuron disease amyotrophic lateral sclerosis (ALS). In preclinical models of familial ALS, astrocytes expressing mutated SOD1 contribute to motor neuron death in a non-cell autonomous manner, likely mediated by the release of a neurotoxic factor [88–91]. This raises the possibility that astrocytes similarly contribute to SMA pathogenesis. Studies using the SMNΔ7 mouse and iPSCs from SMA patients found morphological and cellular changes consistent with activation, including the upregulation of glial fibrillary acidic protein and decreased length of cellular processes [92]. Furthermore, SMA iPSC-derived astrocytes exhibited functional alterations, with an increase in baseline Ca2+ levels and a reduced Ca2+ response to ATP [92]. These changes, which precede motor neuron loss in vivo, indicate that astrocyte dysfunction may contribute to SMA pathology.
Schwann cells around peripheral motor axons form the myelin sheath, which is critical for axonal integrity and fast axon potential conduction [93]. Peripheral nerve abnormalities have been observed in human SMA patients, including reduced conduction velocities, altered membrane conductance, and disruption in myelin [94–97]. It was recently reported that Schwann cells isolated from SMA mice failed to express key myelin proteins during differentiation in vitro, a phenotype that was reversible with restoration of SMN protein. Moreover, defective myelin protein expression and myelination of neurites was observed in co-cultures of SMA-derived Schwann cells and healthy neurons [98]. Alterations in myelination in human and mouse SMA are difficult to interpret, since the motor neurons and their axons also have reduced SMN protein. However, this study raises the intriguing possibility that intrinsic defects in Schwann cells also contribute to SMA pathogenesis.
Consequences of cell-type specificity for therapeutic strategies based on SMN
The data above indicate a role for SMN in multiple central nervous system (CNS) cell types related to motor neuron function, or muscles, to which motor units form connections (Figure 2). This implies that, for optimal clinical efficacy, therapeutic restoration of SMN should occur in the whole nervous system and perhaps the periphery (i.e. muscles). Is SMN required in any other peripheral organs, and to what degree is this backed up by preclinical data in the SMNΔ7 mouse?
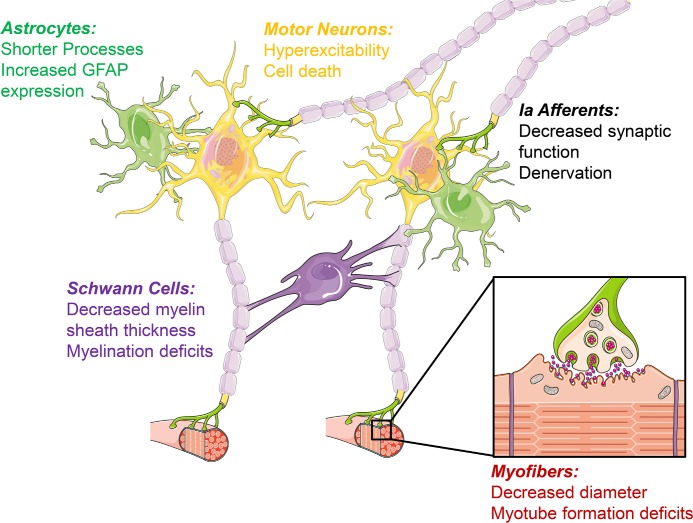
Figure 2. Cell type diversity of requirement for survival motor neuron.
Although spinal muscular atrophy (SMA) has long been considered a disease affecting primarily motor neurons, studies in animal models have demonstrated that multiple cell types are affected and may contribute to SMA pathogenesis. Selective restoration of survival motor neuron (SMN) in specific cell types in SMA mouse models has been a powerful tool used to determine the cell type-specific effects of low SMN. Restoring SMN expression selectively in motor neurons provides a relatively modest benefit, while restoring SMN in all neurons provides a dramatic phenotypic rescue. Additionally, it has been demonstrated that loss of proprioceptive afferents precedes motor neuron loss and may induce electrophysiological deficits in motor neurons. These experiments suggest that non-cell autonomous disease mechanisms within the motor circuit contribute substantially to SMA pathogenesis. In addition to synaptic deficits, altered myelination and reactive astrocytes may contribute to motor neuron loss in SMA. An important consideration in the development of potential therapies for SMA is to design treatments that target the cell types most relevant to disease progression.
Abbreviations: GFAP, glial fibrillary acidic protein.
Increasing SMN levels in the CNS of SMNΔ7 mice with antisense oligonucleotides (ASOs) provides dramatic improvement in the neuromuscular pathology, gross behavior, and lifespan of mice [55,99–104]. Use of morpholino chemistry provides marked rescue of the SMNΔ7 mouse with a single intracerebroventricular (ICV) injection, from ~2 weeks to over 14 weeks [101,102,104]; comparable rescue was achieved using peripheral administration [104]. In contrast, a study using 2’-O-methoxyethyl (MOE) chemistry in another severe SMA mouse model found modest rescue with a single ICV injection but dramatic rescue to over 100 days using high doses peripherally, suggesting that low SMN in peripheral organs contributes significantly to the overall SMA phenotype [100]. This effect was attributed, at least in part, to the correction of an SMA-related decrease in liver production of insulin-like growth factor 1 (IGF-1), which can act as a neurotrophic factor and is important for normal postnatal growth and cardiac development and function [105,106]. However, caution is required when interpreting these studies, since the blood-brain barrier is open during this stage of development and peripheral administration produced substantial increases in full-length SMN in the CNS [100]. A recent study examined the effect of peripheral administration of IGF-1 with adeno-associated virus (AAV) serotype 1 in severe SMA mice and found improvements in neuromuscular pathology, behavioral deficits, and life span. Unexpectedly, these improvements appeared to result from IGF-1-mediated increases in SMN protein centrally and peripherally [107]. Thus, much of the phenotypic improvement in peripheral versus central administration of MOE oligonucleotides may be due to IGF-1-mediated increases in SMN, further complicating interpretation of the cell type-specific SMN requirements and the implications for human therapy.
The use of AAV vectors, which achieve long-term transgene expression in non-dividing cells, represents another powerful method for restoring SMN in targeted cell types in mouse models of SMA. Indeed, a number of groups have reported dramatic results with self-complementary AAV serotype 9 expressing SMN (scAAV9-SMN), with increases in median survival in SMNΔ7 mice from ~15 days to over 150 days [45,46,108–110]. Peripheral vein or intramuscular scAAV9-SMN administration led to widespread SMN expression in the CNS and periphery, including muscle and liver. Direct CNS injection with scAAV8-SMN, by contrast, appeared to transduce the spinal cord and brain without detectable expression in muscle, although other peripheral organs, such as the liver, were not examined [111]. CNS-restricted SMN expression achieved comparable phenotypic rescue to studies that also transduced peripheral tissue, with an increase in median lifespan from 15 to 157 days [111]. The only study to compare CNS and peripheral administration of scAAV9-SMN found a greater phenotypic rescue in the CNS-injected cohort [110]. However, this study used relatively low titer virus and did not normalize viral dose for route of administration, resulting in significantly lower SMN expression in the spinal cord with peripheral injection. Thus, despite the ability of scAAV8-SMN (and presumably scAAV9-SMN) to selectively target the CNS with intraparenchymal injection, studies to date have not compared these routes of administration in a manner that sheds light on the tissue-specific requirements of SMN restoration.
In conclusion, interpretation of studies using ASOs and AAV vectors to determine the potential contribution of low SMN in the periphery to the SMA phenotype are complicated by a number of factors. Different ASOs have different chemistries (morpholino versus MOE) and the central versus peripheral biodistribution is difficult to predict, especially considering the relative immaturity of the blood-brain barrier in neonatal mice. Moreover, the finding that increasing SMN in the periphery, specifically the liver, may lead to IGF-1-mediated increases in SMN both peripherally and centrally, complicates delineation of a possible SMN-independent contribution of the liver and IGF-1 signaling to SMA pathogenesis [107]. Moreover, as discussed previously, there are deficits in SMA mouse models that are not present in human patients. Thus, the organ-specific and cell type-specific requirements for SMN require more study before the cellular basis of the benefits of restoration of SMN can be fully defined.
Therapeutic targets other than SMN and the role of regenerative medicine
There is rightly much excitement about the ongoing and anticipated trials of SMN-restoring drugs in patients. If the results reflect those obtained using mouse models, they will provide significant benefit for patients. Nevertheless, given general problems in transitioning from mouse to man, as well as specific challenges linked to the stage of SMA at which each agent can be used, it seems reasonable to plan complementary strategies in parallel. These fall into three main categories: (a) drugs that target molecular or cellular elements of the disease pathway downstream of SMN reduction; (b) neuroprotective treatments that, independently of the disease mechanism, prevent or slow further motor unit loss; and (c) approaches to enhance regeneration of a neuromuscular system that is stabilized but functioning at suboptimal strength.
Downstream therapeutic targets
Recent studies have identified molecular steps in the downstream pathway, or candidate modifier genes, that extend survival in SMA mice and are of potential interest [112–121]. However, it remains to be demonstrated that modulation of any of these candidate therapeutic targets can provide protection in the SMNΔ7 mouse comparable with that of SMN restoration. Since the only molecularly defined role for SMN is the biogenesis and assembly of small nuclear ribonucleoproteins (snRNPs), the major component of the spliceosome, it has been hypothesized that deleterious splicing changes initiate the disease process. However, early widespread splicing changes in motor neurons are not a feature of SMA, suggesting that splicing changes in a small number of genes critical for motor unit health induce key pathological processes [122,123]. It was previously shown that SMN reduction alters the snRNP profile in a non-uniform manner, with a preferential reduction of minor snRNPs [124]. Intriguingly, Lotti et al. [125] demonstrated that SMN reduction induces defective splicing and reduces the expression of a discrete set of U12 intron-containing genes in Drosophila and mammalian cells. One of these SMN target genes, stasimon, is required for normal neurotransmitter release in Drosophila and axon outgrowth in zebrafish [66,125]. Restoration of stasimon in SMN-deficient Drosophila corrects some of the neuromuscular junction defects, but not all. Defective splicing and reduced levels of stasimon were also observed in motor neurons in SMA mice. This is the first demonstration of a direct link between SMN reduction, a splicing defect, and specific aspects of the SMA phenotype, supporting the hypothesis that SMA pathogenesis may result from splicing defects in a small number of genes. It will be intriguing to determine whether restoration of normal stasimon levels can rescue the phenotype of the SMNΔ7 mouse and, in the future perhaps, human patients.
Neuroprotection
Another potential therapeutic approach is to prevent or delay motor neuron death and degeneration. However, as discussed above, this aspect of the human pathology is underrepresented in the SMNΔ7 model, and it has been argued that such neuroprotective strategies might intervene too late in the disease pathway to be clinically relevant and/or may be ineffective if restricted to motor neurons. A recent preliminary report of a successful Phase 2/3 trial of olesoxime (TRO19622) in SMA patients is potentially exciting in this context. Olesoxime delayed the loss of motor function for 2 years in Type II and non-ambulatory Type III patients in a double-blind, placebo-controlled trial involving 165 patients at 22 sites in seven European countries [126]. Olesoxime was identified by high-throughput screening as a neuroprotective agent for motor neurons in vitro [127]. It binds two components of the mitochondrial permeability transition pore—voltage-gated anion channel (VDAC) and translocator protein (TSPO)—and thereby prevents cytochrome c efflux in conditions that would otherwise promote apoptosis [127]. While it is possible that olesoxime may also act through other mechanisms in patients, the most parsimonious conclusion is that neuroprotective strategies have real potential to block progression—though not to provide a complete cure—in patients with SMA.
Regenerative medicine approaches to neurodegenerative disease
Patients treated with olesoxime—and even potentially those who undergo SMN-restorative treatments—may experience slowed functional loss but not a complete restoration of muscle strength. In this context, it seems important to consider regenerative therapies that augment the function of the remaining motor units. Therapeutic strategies include enhancing axonal regeneration or sprouting, inducing the hypertrophy of remaining muscle fibers [128–131], or replacing myofibers that have degenerated by the grafting of muscle satellite cells or stem cell-derived skeletal myocytes [70,81–85].
Motor units have substantial capacity for collateral sprouting to re-innervate denervated myofibers, which is an important compensatory mechanism in chronic neuromuscular disease [132–134]. The difficulty of measuring changes in motor unit size due to collateral sprouting in preclinical models of SMA has precluded the assessment of this therapeutic strategy. However, progress has been made in adapting electrophysiological measurements, such as the compound muscle action potential (CMAP), the summated electrical activity of all motor units supplying an individual muscle, and motor unit number estimation (MUNE), a measurement of both motor unit number and size based on CMAP, to mouse models of SMA [135]. CMAP and MUNE have been examined in SMA patients, and are known to correlate with age, SMN2 copy number, and motor function [136,137]. Similar to human patients, CMAP and MUNE measurements of the sciatic nerve in SMNΔ7 mice exhibit preserved neuromuscular function in the early postnatal period, followed by motor unit loss that correlates with progression of the gross motor phenotype [135]. Regenerative therapies that enhance collateral sprouting would be expected to increase CMAP, due to enlarged motor unit territories, but not increase MUNE. Thus, CMAP in combination with MUNE represents a potentially promising method to assess motor unit loss, as well as preservation or collateral sprouting, in response to therapeutic interventions in SMA model mice.
Regeneration of myofibers, in parallel with collateral sprouting, is one critical determinant of the adaptive capacity of the neuromuscular system. Given the growing body of evidence that implicates impaired satellite cell regenerative capacity in SMA pathology [82], enhancing muscle regeneration through satellite cell transplantation could provide therapeutic benefit. However, there are not sufficient data to quantitatively evaluate such an approach in SMA mice, given that local muscle transplantation may not lead to functional improvement. Moreover, systematic delivery of satellite cells or stem cell-derived skeletal myocytes to the neuromuscular system of SMA patients presents a considerable therapeutic hurdle.
In addition to increasing myofiber number, enhancing the strength of functional myofibers through hypertrophy represents another potential therapeutic strategy, which has been evaluated by the modulation of myostatin and other pathways that regulate myofiber growth. However, these treatments may not alone be sufficient to prevent progression and may need to be tested together with a disease-stabilizing agent. Administration or transgenic overexpression of the myostatin inhibitor follistatin in SMNΔ7 mice, for example, modestly increased muscle mass, but had modest [130] or no [131] effect on motor function and survival.
Many challenges remain in validating and developing these regenerative medicine approaches to SMA. However, the applicability of these strategies goes well beyond SMA and they would seem an important strand of any long-term strategy for neurodegenerative disease.
Conclusion
This is an exciting time for SMA patients, families and researchers. Not only are multiple clinical trials based on sound preclinical data completed, underway or planned, we also are rapidly gaining a better understanding of the disease process in both molecular and cellular terms. Encouragingly, key phenotypes of the mouse models—which have a genotype close to that of all patients—mimic the selective neuromuscular degeneration that characterizes SMA. Although many hurdles remain to be cleared, the decision by NINDS (National Institute of Neurological Disorders and Stroke) in 2003 to identify SMA as the neurological disease most promising for rational therapeutic approaches through the establishment of the SMA Project is looking more and more justified.
Acknowledgments
We thank our many colleagues in the Motor Neuron Center and the Henderson lab for wide-ranging discussions over the years. Work in the authors’ laboratory on SMA was funded by the SMA Foundation, Association Française contre les Myopathies, and NINDS. Justin C. Lee is supported by an NRSA fellowship and the MD-PhD program and Daniel M. Lascone by the Neurobiology and Behavior graduate program. Christopher E. Henderson is a Gurewitsch/Vidda Professor of Rehabilitation and Regenerative Medicine.
Abbreviations
- AAV
adeno-associated virus
- ALS
amyotrophic lateral sclerosis
- ASO
antisense oligonucleotide
- ChAT
choline acetyltransferase
- CMAP
compound muscle action potential
- CNS
central nervous system
- ICV
intracerebroventricular
- IGF-1
insulin-like growth factor 1
- iPSC
induced pluripotent stem cell
- MOE
2’-O-methoxyethyl
- MUNE
motor unit number estimation
- scAAV
self-complementary AAV
- scAAV9-SMN
serotype 9 expressing SMN
- snRNP
small nuclear ribonucleoprotein
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- TSPO
translocator protein
- VDAC
voltage-gated anion channel
Disclosures
Christopher E. Henderson is co-founder and shareholder of the drug discovery biotech Trophos, whose data are reported in references 126 and 127. Trophos is a privately owned French company.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/7/4
References
- 1.Hoffmann J. Ueber chronische spinale Muskelatrophie im Kindesalter, auf familiärer Basis. Deutsche Zeitschrift f Nervenheilkunde. 1893;3:427–70. doi: 10.1007/BF01668496. [DOI] [Google Scholar]
- 2.Hoffmann J. Dritter Beitrag zur Lehre von der hereditären progressiven spinalen Muskelatrophie im Kindesalter. Deutsche Zeitschrift f Nervenheilkunde. 1900;18:217–24. doi: 10.1007/BF01635796. [DOI] [Google Scholar]
- 3.Hoffmann J. Weiterer Beitrag zur Lehre von der hereditären progressiven spinalen Muskelatrophie im Kindesalter. Deutsche Zeitschrift f Nervenheilkunde. 1897;10:292–320. doi: 10.1007/BF01668174. [DOI] [Google Scholar]
- 4.Werdnig G. Zwei frühinfantile hereditäre Fälle von progressiver Muskelatrophie unter dem Bilde der Dystrophie, aber anf neurotischer Grundlage. Archiv f Psychiatrie. 1891;22:437–80. [Google Scholar]
- 5.Beevor CE. A case of congenital spinal muscular atrophy (family type) and a case of hemorrhage into the spinal cord at birth, giving similar symptoms. Brain. 1902;25:85–108. doi: 10.1093/brain/25.1.85. [DOI] [Google Scholar]
- 6.Sylvestre M. Paralysie flasque de quatre membres et des muscles du tronc (sauf le diaphragme) chez un nouveau-ne. Bull Soc Pediatr Paris. 1899;1:3–10. [Google Scholar]
- 7.Dubowitz V. Ramblings in the history of spinal muscular atrophy. Neuromuscular disorders: NMD. 2009;19:69–73. doi: 10.1097/01202412-199201020-00020. [DOI] [PubMed] [Google Scholar]
- 8.Kugelberg E, Welander L. Heredofamilial juvenile muscular atrophy simulating muscular dystrophy. AMA archives of neurology and psychiatry. 1956;75:500–9. doi: 10.1001/archneurpsyc.1956.02330230050005. [DOI] [PubMed] [Google Scholar]
- 9.Wohlfart G, Fex J, Eliasson S. Hereditary proximal spinal muscular atrophy, a clinical entity simulating progressive muscular dystrophy. Acta psychiatrica et neurologica Scandinavica. 1955;30:395–406. [PubMed] [Google Scholar]
- 10.Munsat T. Workshop report: international SMA collaboration. Neuromuscul Disord. 1991;19:69–73. [Google Scholar]
- 11.Thomas NH, Dubowitz V. The natural history of type I (severe) spinal muscular atrophy. Neuromuscular disorders: NMD. 1994;4:497–502. doi: 10.1016/0960-8966(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Zerres K, Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Archives of neurology. 1995;52:518–23. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 13.Kolb SJ, Kissel JT. Spinal muscular atrophy: a timely review. Archives of neurology. 2011;68:979–84. doi: 10.1001/archneurol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrar MA, Vucic S, Johnston HM, Du Sart D, Kiernan MC. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. The Journal of pediatrics. 2013;162:155–9. doi: 10.1016/j.jpeds.2012.05.067. [DOI] [PubMed] [Google Scholar]
- 15.Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, Kang PB, Foley AR, Yang ML, Martens WB, Oskoui M, Glanzman AM, Flickinger J, Montes J, Dunaway S, O'Hagen J, Quigley J, Riley S, Benton M, Ryan PA, Montgomery M, Marra J, Gooch C, De Vivo Darryl C. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–7. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–65. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/12798957
- 17.Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AH, McPherson JD. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Human molecular genetics. 1999;8:1177–83. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 18.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6307–11. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burghes Arthur HM, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nature reviews. Neuroscience. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prior TW, Krainer AR, Hua Y, Swoboda KJ, Snyder PC, Bridgeman SJ, Burghes Arthur HM, Kissel JT. A positive modifier of spinal muscular atrophy in the SMN2 gene. American journal of human genetics. 2009;85:408–13. doi: 10.1016/j.ajhg.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prior TW, Snyder PJ, Rink BD, Pearl DK, Pyatt RE, Mihal DC, Conlan T, Schmalz B, Montgomery L, Ziegler K, Noonan C, Hashimoto S, Garner S. Newborn and carrier screening for spinal muscular atrophy. American journal of medical genetics. Part A. 2010;152A:1608–16. doi: 10.1002/ajmg.a.33474. [DOI] [PubMed] [Google Scholar]
- 22.Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, Flynn K, Hendrickson BC, Scholl T, Sirko-Osadsa DA, Allitto BA. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of 72,400 specimens. European journal of human genetics: EJHG. 2012;20:27–32. doi: 10.1038/ejhg.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awano T, Kim J, Monani UR. Spinal muscular atrophy: journeying from bench to bedside. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2014;11:786–95. doi: 10.1007/s13311-014-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, Feng Z, Ling Karen KY, Karp GM, Qi H, Woll MG, Chen G, Zhang N, Gabbeta V, Vazirani P, Bhattacharyya A, Furia B, Risher N, Sheedy J, Kong R, Ma J, Turpoff A, Lee C, Zhang X, Moon Y, Trifillis P, Welch EM, Colacino JM, Babiak J, Almstead NG, Peltz SW, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science (New York, N.Y.) 2014;345:688–93. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718522863
- 25.Arnold WD, Burghes Arthur HM. Spinal muscular atrophy: development and implementation of potential treatments. Annals of neurology. 2013;74:348–62. doi: 10.1016/B978-032303506-4.10308-6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li DK, Tisdale S, Lotti F, Pellizzoni L. SMN control of RNP assembly: from post-transcriptional gene regulation to motor neuron disease. Seminars in cell & developmental biology. 2014;32:22–9. doi: 10.1016/j.semcdb.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrank B, Götz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9920–5. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossoll W, Rossol W, Prior TW, Morris GE, Burghes AH. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(-/-) mice and results in a mouse with spinal muscular atrophy. Human molecular genetics. 2000;9:333–9. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718868343
- 29.Hsieh-Li HM, Chang JG, Jong YJ, Wu MH, Wang NM, Tsai CH, Li H. A mouse model for spinal muscular atrophy. Nature genetics. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718837234
- 30.Le TT, Pham LT, Butchbach Matthew ER, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes Arthur HM. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Human molecular genetics. 2005;14:845–57. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 31.Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marcé M, Griffin JW, Rich MM, Sumner CJ. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:842–51. doi: 10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling Karen KY, Gibbs RM, Feng Z, Ko C. Severe neuromuscular denervation of clinically relevant muscles in a mouse model of spinal muscular atrophy. Human molecular genetics. 2012;21:185–95. doi: 10.1093/hmg/ddr453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monani UR. Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron. 2005;48:885–96. doi: 10.1016/j.neuron.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Dubowitz V. 2. London: W.B. Saunders; 1995. Muscle disorders in childhood. [Google Scholar]
- 35.Byers RK, Banker BQ. Infantile muscular atrophy. Archives of neurology. 1961;5:140–64. doi: 10.1001/archneur.1961.00450140022003. [DOI] [PubMed] [Google Scholar]
- 36.Iannaccone ST, Browne RH, Samaha FJ, Buncher CR. Prospective study of spinal muscular atrophy before age 6 years. DCN/SMA Group. Pediatric neurology. 1993;9:187–93. doi: 10.1016/0887-8994(93)90082-N. [DOI] [PubMed] [Google Scholar]
- 37.Kuzuhara S, Chou SM. Localization of the phrenic nucleus in the rat: a HRP study. Neuroscience letters. 1980;16:119–24. doi: 10.1016/0304-3940(80)90330-4. [DOI] [PubMed] [Google Scholar]
- 38.Iwata M, Hirano A. “Glial bundles” in the spinal cord late after paralytic anterior poliomyelitis. Annals of neurology. 1978;4:562–3. doi: 10.1002/ana.410040617. [DOI] [PubMed] [Google Scholar]
- 39.Shishikura K, Hara M, Sasaki Y, Misugi K. A neuropathologic study of Werdnig-Hoffmann disease with special reference to the thalamus and posterior roots. Acta neuropathologica. 1983;60:99–106. doi: 10.1007/BF00685353. [DOI] [PubMed] [Google Scholar]
- 40.Lee JC, Papandrea D, Chung WK, Pisapia DJ, Goldman JE, CE H. Identifying potential therapeutic targets in SMA through profiling of motor pools that show selective resistance in human patients and mouse models. Families of SMA Research Group Meeting: 2013; 2013. [Google Scholar]
- 41.Lee JC, Papandrea D, Pisapia DJ, Chung WK, Goldman JE, CE H. Early and selective motor neuron loss as a pathological endpoint for assessing potential therapeutic targets in the SMNΔ7 mouse. Families of SMA Research Group Meeting: 2014; 2014. [Google Scholar]
- 42.Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Human molecular genetics. 2010;19:4059–71. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718864639
- 43.Heier CR, Satta R, Lutz C, DiDonato CJ. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Human molecular genetics. 2010;19:3906–18. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718864641
- 44.Narver HL, Kong L, Burnett BG, Choe DW, Bosch-Marcé M, Taye AA, Eckhaus MA, Sumner CJ. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Annals of neurology. 2008;64:465–70. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes, Arthur HM, Kaspar BK. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nature biotechnology. 2010;28:271–4. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ, Azzouz M. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Science translational medicine. 2010;2:35ra42. doi: 10.1126/scitranslmed.3000830. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends in molecular medicine. 2013;19:40–50. doi: 10.1016/j.molmed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Shababi M, Lorson CL, Rudnik-Schöneborn SS. Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? Journal of anatomy. 2014;224:15–28. doi: 10.1111/joa.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudnik-Schöneborn S, Heller R, Berg C, Betzler C, Grimm T, Eggermann T, Eggermann K, Wirth R, Wirth B, Zerres K. Congenital heart disease is a feature of severe infantile spinal muscular atrophy. Journal of medical genetics. 2008;45:635–8. doi: 10.1136/jmg.2008.057950. [DOI] [PubMed] [Google Scholar]
- 50.Distefano G, Sciacca P, Parisi MG, Parano E, Smilari P, Marletta M, Fiumara A. Il coinvolgimento cardiaco nell'atrofia muscolare spinale progressiva. Revisione della letteratura e contributo casistico in età pediatrica. La Pediatria medica e chirurgica: Medical and surgical pediatrics. 1994;16:125–8. [PubMed] [Google Scholar]
- 51.Palladino A, Passamano L, Taglia A, D'Ambrosio P, Scutifero M, Cecio MR, Picillo E, Viggiano E, Torre V, de Luca F, Nigro G, Politano L. Cardiac involvement in patients with spinal muscular atrophies. Acta myologica: myopathies and cardiomyopathies: official journal of the Mediterranean Society of Myology / edited by the Gaetano Conte Academy for the study of striated muscle diseases. 2011;30:175–8. doi: 10.1016/j.nmd.2011.06.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Araujo Alexandra prufer de Queiroz Campos, Araujo M, Swoboda KJ. Vascular perfusion abnormalities in infants with spinal muscular atrophy. The Journal of pediatrics. 2009;155:292–4. doi: 10.1016/j.jpeds.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gogliotti RG, Quinlan KA, Barlow CB, Heier CR, Heckman CJ, DiDonato CJ. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3818–29. doi: 10.1523/JNEUROSCI.5775-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/714597896
- 54.Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, Alvarez FJ, Sumner CJ, O'Donovan MJ. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69:453–67. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/9192959
- 55.Passini MA, Bu J, Richards AM, Kinnecom C, Sardi SP, Stanek LM, Hua Y, Rigo F, Matson J, Hung G, Kaye EM, Shihabuddin LS, Krainer AR, Bennett CF, Cheng SH. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Science translational medicine. 2011;3:72ra18. doi: 10.1126/scitranslmed.3001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.d'Errico P, Boido M, Piras A, Valsecchi V, Amicis E de, Locatelli D, Capra S, Vagni F, Vercelli A, Battaglia G. Selective vulnerability of spinal and cortical motor neuron subpopulations in delta7 SMA mice. PloS one. 2013;8:e82654. doi: 10.1371/journal.pone.0082654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1147093
- 58.Corti S, Nizzardo M, Simone C, Falcone M, Nardini M, Ronchi D, Donadoni C, Salani S, Riboldi G, Magri F, Menozzi G, Bonaglia C, Rizzo F, Bresolin N, Comi GP. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Science translational medicine. 2012;4:165ra162. doi: 10.1126/scitranslmed.3004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desclaux D, Kim Y, Garcia Diaz A, Mekhoubad S, Dimos J, Eggan K, Wichterle H, CE H. An SMN-dependent cell-autonomous survival deficit in human Type 1 SMA ES-derived motor meurons. Families of SMA Research Group Meeting: 2013; 2013. [Google Scholar]
- 60.Ruggiu M, McGovern VL, Lotti F, Saieva L, Li DK, Kariya S, Monani UR, Burghes Arthur HM, Pellizzoni L. A role for SMN exon 7 splicing in the selective vulnerability of motor neurons in spinal muscular atrophy. Molecular and cellular biology. 2012;32:126–38. doi: 10.1128/MCB.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jodelka FM, Ebert AD, Duelli DM, Hastings ML. A feedback loop regulates splicing of the spinal muscular atrophy-modifying gene, SMN2. Human molecular genetics. 2010;19:4906–17. doi: 10.1093/hmg/ddq425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park GH, Maeno-Hikichi Y, Awano T, Landmesser LT, Monani UR. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:12005–19. doi: 10.1523/JNEUROSCI.2208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/719872203
- 63.Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke James P, Xu X, Davis C, Wooley J, Goldhamer DJ, Lutz CM, Rich MM, Sumner CJ. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:8703–15. doi: 10.1523/JNEUROSCI.0204-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/719869525
- 64.Ling KK, Lin M, Zingg B, Feng Z, Ko C. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PloS one. 2010;5:e15457. doi: 10.1371/journal.pone.0015457. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/724181875
- 65.Tarabal O, Caraballo-Miralles V, Cardona-Rossinyol A, Correa FJ, Olmos G, Lladó J, Esquerda JE, Calderó J. Mechanisms involved in spinal cord central synapse loss in a mouse model of spinal muscular atrophy. Journal of neuropathology and experimental neurology. 2014;73:519–35. doi: 10.1097/NEN.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 66.Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD. SMN is required for sensory-motor circuit function in Drosophila. Cell. 2012;151:427–39. doi: 10.1016/j.cell.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718030604
- 67.Renault F, Raimbault J, Praud JP, Laget P. Etude électromyographique de 50 cas de maladie de Werdnig-Hoffmann. Revue d’électroencéphalographie et de neurophysiologie clinique. 1983;13:301–5. doi: 10.1016/S0370-4475(83)80042-2. [DOI] [PubMed] [Google Scholar]
- 68.Wishart TM, Huang JP, Murray LM, Lamont DJ, Mutsaers CA, Ross J, Geldsetzer P, Ansorge O, Talbot K, Parson SH, Gillingwater TH. SMN deficiency disrupts brain development in a mouse model of severe spinal muscular atrophy. Human molecular genetics. 2010;19:4216–28. doi: 10.1093/hmg/ddq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henderson CE, Hauser SL, Huchet M, Dessi F, Hentati F, Taguchi T, Changeux JP, Fardeau M. Extracts of muscle biopsies from patients with spinal muscular atrophies inhibit neurite outgrowth from spinal neurons. Neurology. 1987;37:1361–4. doi: 10.1212/WNL.37.8.1361. [DOI] [PubMed] [Google Scholar]
- 70.Braun S, Croizat B, Lagrange MC, Warter JM, Poindron P. Constitutive muscular abnormalities in culture in spinal muscular atrophy. Lancet. 1995;345:694–5. doi: 10.1016/S0140-6736(95)90869-2. [DOI] [PubMed] [Google Scholar]
- 71.Hayhurst M, Wagner AK, Cerletti M, Wagers AJ, Rubin LL. A cell-autonomous defect in skeletal muscle satellite cells expressing low levels of survival of motor neuron protein. Developmental biology. 2012;368:323–34. doi: 10.1016/j.ydbio.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725275416
- 72.Bricceno KV, Martinez T, Leikina E, Duguez S, Partridge TA, Chernomordik LV, Fischbeck KH, Sumner CJ, Burnett BG. Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics. Human molecular genetics. 2014;23:4745–57. doi: 10.1093/hmg/ddu189. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718361402
- 73.Guettier-Sigrist S, Hugel B, Coupin G, Freyssinet J, Poindron P, Warter J. Possible pathogenic role of muscle cell dysfunction in motor neuron death in spinal muscular atrophy. Muscle & nerve. 2002;25:700–8. doi: 10.1002/mus.10081. [DOI] [PubMed] [Google Scholar]
- 74.Boyer JG, Deguise M, Murray LM, Yazdani A, Repentigny Y de, Boudreau-Larivière C, Kothary R. Myogenic program dysregulation is contributory to disease pathogenesis in spinal muscular atrophy. Human molecular genetics. 2014;23:4249–59. doi: 10.1093/hmg/ddu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee YI, Mikesh M, Smith I, Rimer M, Thompson W. Muscles in a mouse model of spinal muscular atrophy show profound defects in neuromuscular development even in the absence of failure in neuromuscular transmission or loss of motor neurons. Developmental biology. 2011;356:432–44. doi: 10.1016/j.ydbio.2011.05.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruiz R, Casañas JJ, Torres-Benito L, Cano R, Tabares L. Altered intracellular Ca2+ homeostasis in nerve terminals of severe spinal muscular atrophy mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:849–57. doi: 10.1523/JNEUROSCI.4496-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kariya S, Park G, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, Landmesser LT, Monani UR. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Human molecular genetics. 2008;17:2552–69. doi: 10.1093/hmg/ddn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, Monani UR, Morris GE, Burghes Arthur HM. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Human molecular genetics. 2008;17:1063–75. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang J, Krauss RS. Muscle stem cells in developmental and regenerative myogenesis. Current opinion in clinical nutrition and metabolic care. 2010;13:243–8. doi: 10.1097/MCO.0b013e328336ea98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerletti M, Shadrach JL, Jurga S, Sherwood R, Wagers AJ. Regulation and function of skeletal muscle stem cells. Cold Spring Harbor symposia on quantitative biology. 2008;73:317–22. doi: 10.1101/sqb.2008.73.054. [DOI] [PubMed] [Google Scholar]
- 81.Fidziańska A, Goebel HH, Warlo I. Acute infantile spinal muscular atrophy. Muscle apoptosis as a proposed pathogenetic mechanism. Brain: a journal of neurology 1990. 113 (Pt 2):433–45. doi: 10.1093/brain/113.2.433. [DOI] [PubMed] [Google Scholar]
- 82.Nicole S, Desforges B, Millet G, Lesbordes J, Cifuentes-Diaz C, Vertes D, Cao ML, Backer F de, Languille L, Roblot N, Joshi V, Gillis J, Melki J. Intact satellite cells lead to remarkable protection against Smn gene defect in differentiated skeletal muscle. The Journal of cell biology. 2003;161:571–82. doi: 10.1083/jcb.200210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shafey D, Côté PD, Kothary R. Hypomorphic Smn knockdown C2C12 myoblasts reveal intrinsic defects in myoblast fusion and myotube morphology. Experimental cell research. 2005;311:49–61. doi: 10.1016/j.yexcr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Dachs E, Hereu M, Piedrafita L, Casanovas A, Calderó J, Esquerda JE. Defective neuromuscular junction organization and postnatal myogenesis in mice with severe spinal muscular atrophy. Journal of neuropathology and experimental neurology. 2011;70:444–61. doi: 10.1097/NEN.0b013e31821cbd8b. [DOI] [PubMed] [Google Scholar]
- 85.Mutsaers CA, Wishart TM, Lamont DJ, Riessland M, Schreml J, Comley LH, Murray LM, Parson SH, Lochmüller H, Wirth B, Talbot K, Gillingwater TH. Reversible molecular pathology of skeletal muscle in spinal muscular atrophy. Human molecular genetics. 2011;20:4334–44. doi: 10.1093/hmg/ddr360. [DOI] [PubMed] [Google Scholar]
- 86.Wang DD, Bordey A. The astrocyte odyssey. Progress in neurobiology. 2008;86:342–67. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nature reviews Neuroscience. 2013;14:311–21. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB. Inflammation in ALS and SMA: sorting out the good from the evil. Neurobiology of disease. 2010;37:493–502. doi: 10.1016/j.nbd.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature neuroscience. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di Giorgio Francesco Paolo, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nature neuroscience. 2007;10:608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1087760
- 91.Re DB, Le Verche V, Yu C, Amoroso MW, Politi KA, Phani S, Ikiz B, Hoffmann L, Koolen M, Nagata T, Papadimitriou D, Nagy P, Mitsumoto H, Kariya S, Wichterle H, Henderson CE, Przedborski S. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–8. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGivern JV, Patitucci TN, Nord JA, Barabas MA, Stucky CL, Ebert AD. Spinal muscular atrophy astrocytes exhibit abnormal calcium regulation and reduced growth factor production. Glia. 2013;61:1418–28. doi: 10.1002/glia.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718036856
- 93.Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Current opinion in neurobiology. 2007;17:533–40. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Chien YY, Nonaka I. Peripheral nerve involvement in Werdnig-Hoffmann disease. Brain & development. 1989;11:221–9. doi: 10.1016/S0387-7604(89)80040-3. [DOI] [PubMed] [Google Scholar]
- 95.Farrar MA, Vucic S, Lin CS, Park SB, Johnston HM, Du Sart D, Bostock H, Kiernan MC. Dysfunction of axonal membrane conductances in adolescents and young adults with spinal muscular atrophy. Brain: a journal of neurology. 2011;134:3185–97. doi: 10.1093/brain/awr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moosa A, Dubowitz V. Motor nerve conduction velocity in spinal muscular atrophy of childhood. Archives of disease in childhood. 1976;51:974–7. doi: 10.1136/adc.51.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yonekawa T, Komaki H, Saito Y, Sugai K, Sasaki M. Peripheral nerve abnormalities in pediatric patients with spinal muscular atrophy. Brain & development. 2013;35:165–71. doi: 10.1016/j.braindev.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 98.Hunter G, Aghamaleky Sarvestany A, Roche SL, Symes RC, Gillingwater TH. SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Human molecular genetics. 2014;23:2235–50. doi: 10.1093/hmg/ddt612. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718198259
- 99.Baughan TD, Dickson A, Osman EY, Lorson CL. Delivery of bifunctional RNAs that target an intronic repressor and increase SMN levels in an animal model of spinal muscular atrophy. Human molecular genetics. 2009;18:1600–11. doi: 10.1093/hmg/ddp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, Krainer AR. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–6. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13356968
- 101.Mitrpant C, Porensky P, Zhou H, Price L, Muntoni F, Fletcher S, Wilton SD, Burghes Arthur HM. Improved antisense oligonucleotide design to suppress aberrant SMN2 gene transcript processing: towards a treatment for spinal muscular atrophy. PloS one. 2013;8:e62114. doi: 10.1371/journal.pone.0062114. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718399703
- 102.Porensky PN, Mitrpant C, McGovern VL, Bevan AK, Foust KD, Kaspar BK, Wilton SD, Burghes Arthur HM. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Human molecular genetics. 2012;21:1625–38. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718864022
- 103.Williams JH, Schray RC, Patterson CA, Ayitey SO, Tallent MK, Lutz GJ. Oligonucleotide-mediated survival of motor neuron protein expression in CNS improves phenotype in a mouse model of spinal muscular atrophy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:7633–8. doi: 10.1523/JNEUROSCI.0950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/719873895
- 104.Zhou H, Janghra N, Mitrpant C, Dickinson RL, Anthony K, Price L, Eperon IC, Wilton SD, Morgan J, Muntoni F. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Human gene therapy. 2013;24:331–42. doi: 10.1089/hum.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720897100
- 105.Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clinical endocrinology. 2008;69:347–58. doi: 10.1111/j.1365-2265.2008.03292.x. [DOI] [PubMed] [Google Scholar]
- 106.Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science (New York, N.Y.) 2003;301:839–42. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1015280
- 107.Tsai L, Chen C, Ting C, Lin-Chao S, Hwu W, Dodge JC, Passini MA, Cheng SH. Systemic administration of a recombinant AAV1 vector encoding IGF-1 improves disease manifestations in SMA mice. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:1450–9. doi: 10.1038/mt.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718383237
- 108.Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P, Carcenac R, Astord S, Pereira de Moura Aurélie, Voit T, Barkats M. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Human molecular genetics. 2011;20:681–93. doi: 10.1093/hmg/ddq514. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718864507
- 109.Benkhelifa-Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R, Marais T, Barkats M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:282–90. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717976143
- 110.Glascock JJ, Shababi M, Wetz MJ, Krogman MM, Lorson CL. Direct central nervous system delivery provides enhanced protection following vector mediated gene replacement in a severe model of spinal muscular atrophy. Biochemical and biophysical research communications. 2012;417:376–81. doi: 10.1016/j.bbrc.2011.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/719679696
- 111.Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O'Riordan CR, Klinger KW, Shihabuddin LS, Cheng SH. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. The Journal of clinical investigation. 2010;120:1253–64. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725275418
- 112.Bowerman M, Beauvais A, Anderson CL, Kothary R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Human molecular genetics. 2010;19:1468–78. doi: 10.1093/hmg/ddq021. [DOI] [PubMed] [Google Scholar]
- 113.Bowerman M, Murray LM, Boyer JG, Anderson CL, Kothary R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC medicine. 2012;10:24. doi: 10.1186/1741-7015-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Butchbach Matthew ER, Singh J, Thorsteinsdóttir M, Saieva L, Slominski E, Thurmond J, Andrésson T, Zhang J, Edwards JD, Simard LR, Pellizzoni L, Jarecki J, Burghes Arthur HM, Gurney ME. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Human molecular genetics. 2010;19:454–67. doi: 10.1093/hmg/ddp510. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1346973
- 115.Farooq F, Abadía-Molina F, MacKenzie D, Hadwen J, Shamim F, O'Reilly S, Holcik M, MacKenzie A. Celecoxib increases SMN and survival in a severe spinal muscular atrophy mouse model via p38 pathway activation. Human molecular genetics. 2013;22:3415–24. doi: 10.1093/hmg/ddt191. [DOI] [PubMed] [Google Scholar]
- 116.Gogliotti RG, Cardona H, Singh J, Bail S, Emery C, Kuntz N, Jorgensen M, Durens M, Xia B, Barlow C, Heier CR, Plasterer HL, Jacques V, Kiledjian M, Jarecki J, Rusche J, DiDonato CJ. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Human molecular genetics. 2013;22:4084–101. doi: 10.1093/hmg/ddt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwon DY, Motley WW, Fischbeck KH, Burnett BG. Increasing expression and decreasing degradation of SMN ameliorate the spinal muscular atrophy phenotype in mice. Human molecular genetics. 2011;20:3667–77. doi: 10.1093/hmg/ddr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Van Meerbeke James P, Gibbs RM, Plasterer HL, Miao W, Feng Z, Lin M, Rucki AA, Wee CD, Xia B, Sharma S, Jacques V, Li DK, Pellizzoni L, Rusche JR, Ko C, Sumner CJ. The DcpS inhibitor RG3039 improves motor function in SMA mice. Human molecular genetics. 2013;22:4074–83. doi: 10.1093/hmg/ddt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cherry JJ, Osman EY, Evans MC, Choi S, Xing X, Cuny GD, Glicksman MA, Lorson CL, Androphy EJ. Enhancement of SMN protein levels in a mouse model of spinal muscular atrophy using novel drug-like compounds. EMBO molecular medicine. 2013;5:1035–50. doi: 10.1002/emmm.201202305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farooq F, Abadía-Molina F, MacKenzie D, Hadwen J, Shamim F, O'Reilly S, Holcik M, MacKenzie A. Celecoxib increases SMN and survival in a severe spinal muscular atrophy mouse model via p38 pathway activation. Human molecular genetics. 2013;22:3415–24. doi: 10.1093/hmg/ddt191. [DOI] [PubMed] [Google Scholar]
- 121.Wishart TM, Mutsaers CA, Riessland M, Reimer MM, Hunter G, Hannam ML, Eaton SL, Fuller HR, Roche SL, Somers E, Morse R, Young PJ, Lamont DJ, Hammerschmidt M, Joshi A, Hohenstein P, Morris GE, Parson SH, Skehel PA, Becker T, Robinson IM, Becker CG, Wirth B, Gillingwater TH. Dysregulation of ubiquitin homeostasis and β-catenin signaling promote spinal muscular atrophy. The Journal of clinical investigation. 2014;124:1821–34. doi: 10.1172/JCI71318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bäumer D, Lee S, Nicholson G, Davies JL, Parkinson NJ, Murray LM, Gillingwater TH, Ansorge O, Davies KE, Talbot K. Alternative splicing events are a late feature of pathology in a mouse model of spinal muscular atrophy. PLoS genetics. 2009;5:e1000773. doi: 10.1371/journal.pgen.1000773. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/720225751
- 123.Zhang Z, Pinto AM, Wan L, Wang W, Berg MG, Oliva I, Singh LN, Dengler C, Wei Z, Dreyfuss G. Dysregulation of synaptogenesis genes antecedes motor neuron pathology in spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19348–53. doi: 10.1073/pnas.1319280110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718166436
- 124.Gabanella F, Butchbach Matthew ER, Saieva L, Carissimi C, Burghes Arthur HM, Pellizzoni L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PloS one. 2007;2:e921. doi: 10.1371/journal.pone.0000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lotti F, Imlach WL, Saieva L, Beck ES, Le Hao T, Li DK, Jiao W, Mentis GZ, Beattie CE, McCabe BD, Pellizzoni L. An SMN-dependent U12 splicing event essential for motor circuit function. Cell. 2012;151:440–54. doi: 10.1016/j.cell.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717960449
- 126.Dessaud E, Andre C, Scherrer B, Berna P, Pruss R, Cuvier V, Hauke W, SMA Study Investigators, E B Results of a Phase II study to assess safety and efficacy of olesoxime (TRO19622) in 3-25 year old spinal muscular atrophy patients. Families of SMA Research Group Meeting: 2014; 2014. [Google Scholar]
- 127.Bordet T, Patrick Berna P, Abitbol JL, RM P. Olesoxime (TRO19622): A Novel Mitochondrial-Targeted Neuroprotective Compound. Pharmaceuticals. 2010;3(2):345–368. doi: 10.3390/ph3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bosch-Marcé M, Wee CD, Martinez TL, Lipkes CE, Choe DW, Kong L, Van Meerbeke James P, Musarò A, Sumner CJ. Increased IGF-1 in muscle modulates the phenotype of severe SMA mice. Human molecular genetics. 2011;20:1844–53. doi: 10.1093/hmg/ddr067. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/10854956
- 129.Rindt H, Buckley DM, Vale SM, Krogman M, Rose FF, Garcia ML, Lorson CL. Transgenic inactivation of murine myostatin does not decrease the severity of disease in a model of Spinal Muscular Atrophy. Neuromuscular disorders: NMD. 2012;22:277–85. doi: 10.1016/j.nmd.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 130.Rose FF, Mattis VB, Rindt H, Lorson CL. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Human molecular genetics. 2009;18:997–1005. doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.1Sumner CJ, Wee CD, Warsing LC, Choe DW, Ng AS, Lutz C, Wagner KR. Inhibition of myostatin does not ameliorate disease features of severe spinal muscular atrophy mice. Human molecular genetics. 2009;18:3145–52. doi: 10.1093/hmg/ddp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wohlfart G. Collateral regeneration in partially denervated muscles. Neurology. 1958;8:175–80. doi: 10.1212/WNL.8.3.175. [DOI] [PubMed] [Google Scholar]
- 133.Brown MC. Sprouting of motor nerves in adult muscles: a recapitulation of ontogeny. Trends in Neurosciences. 1984;7(1):10–14. doi: 10.1016/S0166-2236(84)80180-0. [DOI] [Google Scholar]
- 134.McComas AJ, Sica RE, Campbell MJ, Upton AR. Functional compensation in partially denervated muscles. Journal of neurology, neurosurgery, and psychiatry. 1971;34:453–60. doi: 10.1136/jnnp.34.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arnold WD, Porensky PN, McGovern VL, Iyer CC, Duque S, Li X, Meyer K, Schmelzer L, Kaspar BK, Kolb SJ, Kissel JT, Burghes Arthur HM. Electrophysiological Biomarkers in Spinal Muscular Atrophy: Preclinical Proof of Concept. Annals of clinical and translational neurology. 2014;1:34–44. doi: 10.1002/acn3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704–12. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/722952449
- 137.Lewelt A, Krosschell KJ, Scott C, Sakonju A, Kissel JT, Crawford TO, Acsadi G, D'anjou G, Elsheikh B, Reyna SP, Schroth MK, Maczulski JA, Stoddard GJ, Elovic E, Swoboda KJ. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle & nerve. 2010;42:703–8. doi: 10.1002/mus.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725275417