Abstract
Stachys lavandulifolia (S. lavandulifolia) is a plant that has been widely used as an herbal medicine in Iran. Unfortunately, despite the prevalent medicinal uses of the plant, there are no reports on the possible toxic effects of S. lavandulifolia. In the present study the potential toxicity of S. lavandulifolia after acute and subchronic administration in rats was evaluated. Rats were orally treated with single doses of S. lavandulifolia aqueous extract and screened for signs of toxicity two weeks after administration. In the sub-chronic toxicity study, S. lavandulifolia was administered for 45 days. Mortality, clinical signs, body weight changes were assayed during the study. After 45 days animals were sacrificed and hematological and biochemical parameters, as well as weight of some organs were measured. All of the rats treated with different concentrations of water extracts of S. lavandulifolia were alive for all 14 days of observation. No hematological changes were observed a part from significant increase in WBC and neutrophils counts. Moreover, serum aspartate aminotransferase (AST) and creatinine significantly increased. Histopathological examinations showed fatty change, degeneration of hepatocytes and renal glomerular atrophy in the male rats. In the female rats, atrophy of hepatocytes and dilatation of sinusoids in liver, hyperemia and degeneration of renal epithelium mostly in cortical region were observed. Based on the results of this study no observed adverse effect level (NOAEL) of the total aqueous extract of S. lavandulifolia considered to be 250 and 100 mg/kg/day for the male and female rats, respectively.
Keywords: Stachys lavandulifolia, Rat, Acute, Subchronic, Toxicity
INTRODUCTION
The Lamiaceae family is one of the largest and most widely recognized families of flowering plants and comprises 258 genera and around 7000 species worldwide (1).
The genus Stachys, one of the largest genera of the Lamiaceae family, contains about 300 species. More than39 species of this genus are grown and distributed in various regions of Iran. (2).
Stachys lavandulifolia Vahl. is a native plant that is widely distributed in different regions of Iran and known as “Chaye-e-Kohi” or “Lolopashmak” (3,4,5,6). This plant is used as herbal tea and a medicinal plant in Iranian folk medicine (4,5). The aqueous extract obtained from the aerial parts of S. lavandulifolia is used as antipyretic, anti-inflammatory, spasmolytic and sedative medicament (5). Also, this plant has antibacterial, antioxidant, anxiolytic, analgesic and wound healing effects (5,6,7,8,9).
Despite its widespread use, little toxicological data is available regarding the safety of repeated exposure to S. lavandulifolia. As part of a safety evaluation of S. lavandulifolia, a toxicological study was thus carried out to investigate its potential toxicity after single and 45-day repeated oral dosing of an aqueous extract of S. lavandulifolia aerial parts in Wistar rats.
MATERIALS AND METHODS
Plant material and aqueous extract preparation
The aerial parts of the plant were collected from Bostan Abad area in East Azerbaijan province (Iran) on May 2012 and dried away from direct sunlight. The plant was authenti-cated by Dr. Nastaran Jalilian (botanist) and a voucher specimen (Registration Number 5594) has been deposited in the Herbarium of Research Center of Agriculture and Natural Resources of Kermanshah Province (Kermanshah, Iran).
Aqueous extract was prepared by adding 500 ml boiled distillated water to 50 g of coarsely powdered plant material in an Erlenmeyer and heating on water bath (90 °C) with occasional stirring for 1 h. The aqueous extract was filtered and evaporated under vaccum at 40 °C.
Acute toxicity study
Four-week-old Wistar rats of both sexes were purchased from University of Medical Sciences (Kermanshah, Iran). The animals were maintained according to standard guidelines. The animals were randomly assigned to control and treatment groups (five rats per sex per group) and housed in clear plastic cages containing wood shavings for bedding. Each cage contained five rats of the same sex.
Environmental conditions were maintained at 23 ± 2 °C and a relative humidity of 40 ± 10% with a 12 h light/dark cycle. At the onset of dosing, the males weighed 178 ± 19 g, and the females weighed 163 ± 15 g. The animals were allowed to acclimatize for two weeks before the initiation of experiments with food and water available ad libitum.
The research was conducted in accordance with internationally accepted principles for laboratory animal use and care (NIH Publication no. 85–23, revised in 1985).
Treatment
In an experiment aimed to determine the oral LD50, the extract was administered to Wistar rats (5/sex/group) as a single oral dose of 5 g/kg body weight via gavage. The animals were fasted for 4 h prior to dosing. The control rats received tap water by gavage in the same volume. Observations were made and recorded systematically 1, 2, 4 and 6 h after administration of the extract, and daily thereafter for a total of 14 days.
They were also monitored daily for mortality, any changes in food and water consumption and any additional clinical or behavioral signs of toxicity. The animal body weights were measured prior to dosing and on days 7 and 14. At the end of the study, all animals were killed by ether inhalation. The rats were dissected and the organs (heart, liver, spleen, kidneys and lungs) were assessed. The relative organ weight of each organ was then calculated using the following formula:
Relative organ weight = Weight of organ/Body weight of rat × 100%
All of the animals were sacrificed on day 15.
Subchronic toxicity study
Group assignment and treatment
The animals were randomly caged in clear plastic cages containing wood shavings for bedding. Dosing was initiated when the rats were 8 weeks old, at which point the males and females weighed 110 ± 19 g and 114 ± 10 g, respectively. The animals were divided into four dose groups (five rats per sex per group).
The first group was given 1 ml distilled water and used as a control. The second, third, and fourth groups were given single doses of 100, 250, and 500 mg/kg of S. lavandulifolia by gavage daily. All of the solutions were prepared just prior to dosing and were kept chilled and tightly capped. Animals were observed for signs of abnormalities during the treatment period. Besides, the body weights of animals were recorded on day 0 and at weekly intervals throughout the course of the study.
Biochemical and hematology analysis
After 45 days, 12 h-fasted animals were anesthetized with ether and the blood collected with and without anticoagulant (ethylene diamine tetra acetate) by retro-orbital puncture, using capillary tubes for hematological and biochemical studies, respectively (10). The following hematological parameters were determined with the Sysmex K-1000 fully automated hematology analyzer: Erythrocyte (RBC), total and differential leukocyte (WBC), neutrophil, lymphocyte, eosinophil, basophil, hematocrit (Hct), hemoglobin (Hb), platelet count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean platelet volume (MPV), platelet distribution width (PDW), and red distribution width (RDW).
Blood samples for biochemical analyses were centrifuged at 3,000 × g for 5 min and plasma was collected and analyzed for glucose, creatinine, albumin, cholesterol, triglycerides, aspartate amino-transferase (AST), alanine aminotransferase (ALT), phosphorus, potassium, sodium, calcium, urea and total protein using a COBAS Mira S chemistry analyzer (Roche Diagnostic Systems, West Sussex, England).
Necropsy
Following blood collection, the rats were sacrificed by decapitation, thereafter the organs listed in Table 1 were removed weighed and finally examined macrosco-pically. The organs were preserved in 10% neutral buffered formalin and prepared for microscopic histopathological examinations.
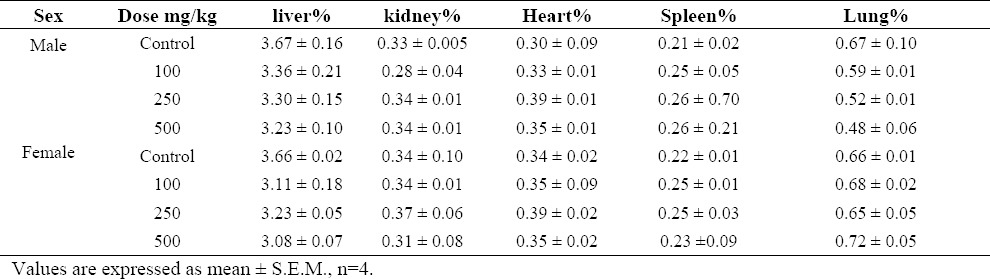
Table 1.
Effect of S. lavandulifolia on group mean organ weights adjusted to overall necropsy body weight in male and female rats.
Statistical analysis
The mean ± SEM was calculated for body weights, organ/body weight ratios, and hematological and biochemistry factors. The difference between dose groups and controls was separately evaluated for males and females with a one-way analysis of variance (ANOVA) followed by Tukey's test. P-values of 0.05 or less were considered to be significant. Because no treatment-related animal deaths were observed, the LD50 values were not measured.
RESULTS
Acute study
The obtained result in the study of acute toxicity indicated that the high dose of S. lavandulifolia (2 g/kg) did not produce any symptoms of toxicity. All of the rats treated with different concentrations of total extracts of S. lavandulifolia were alive for all 14 days of observation.
Normal body weight gains were observed in the males and females of all of the dose groups. No abnormal gross findings were observed in any of the animals. The oral acute toxicity of total extract (LD50) was therefore considered to be unclassified; doses up to 2 g/kg did not induce death or toxic symptoms.
Subchronic study
There was no significant difference in body weights between the control and treatment groups (Fig. 1). No death was found in any of the groups throughout the experimental period, and no abnormal gross findings were observed in any of the animals.
Fig. 1.
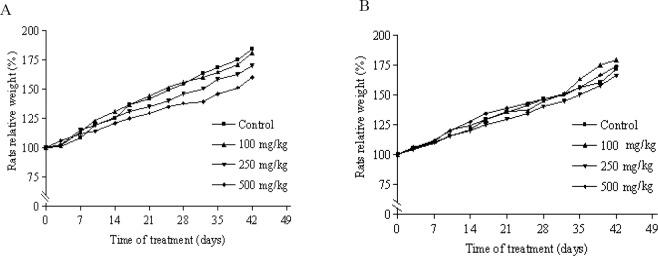
Changes in male (A) and female (B) rats body weight with duration of subchronic treatment. Each point represents mean ± SEM, n = 5.
Moreover, there was no significant difference in relative organ weights measurements (Table 1). In hematological parameters some statistically significant differences were noted when the control and treatment groups were compared (Table 2).
Table 2.
Hematologic parameters in Wistar rats after 45 days treatment with S. lavandulifolia.
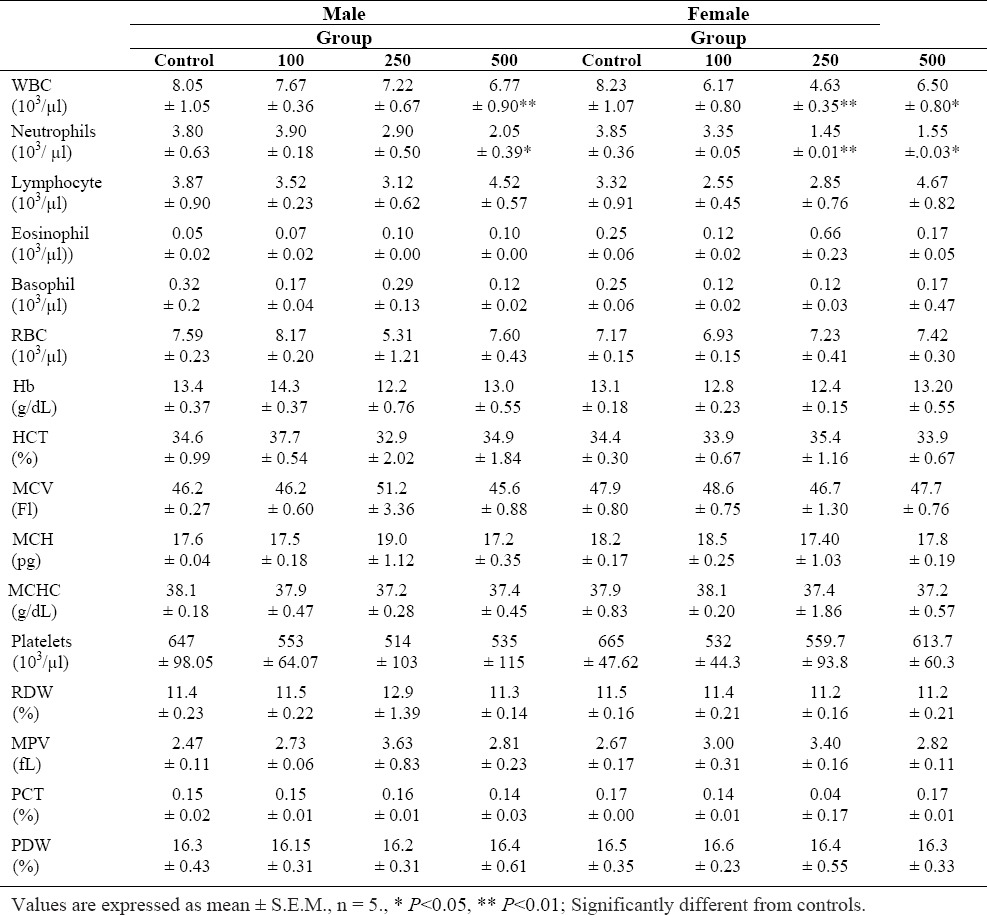
WBC and neutrophils were reduced significantly in the male group that received 500 mg/kg and female rats that received 250 mg/kg and 500 mg/kg of S. lavandulifolia compared to control.
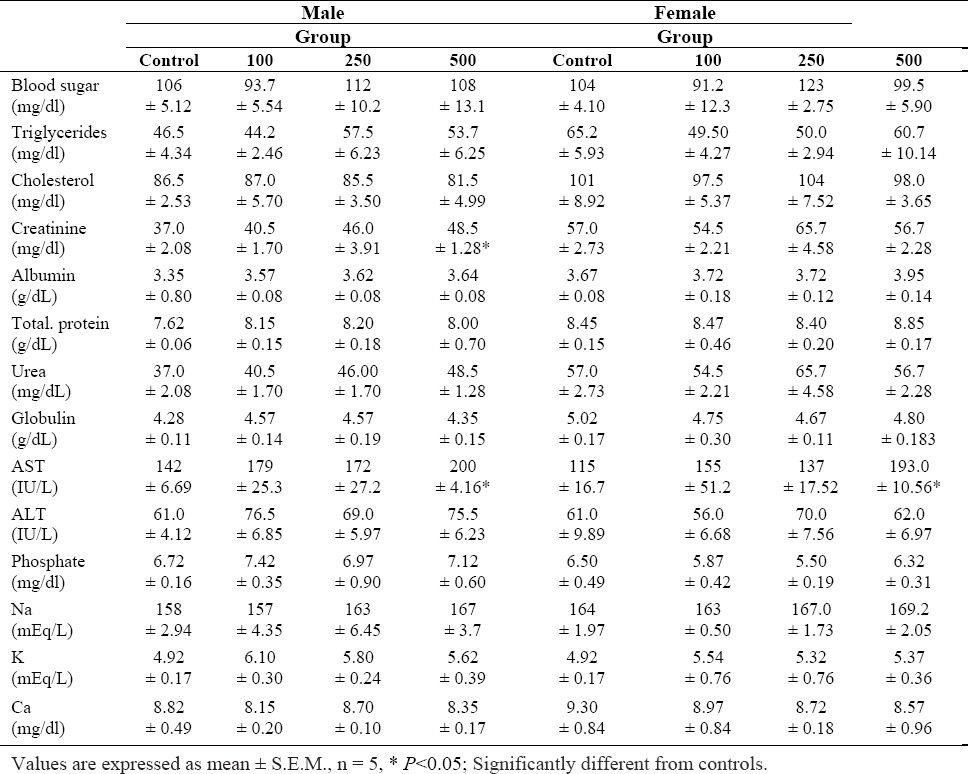
In biochemical analyses, a statistically significant increase in AST was observed at a dose of 500 mg/kg in both sexes. Moreover, creatinin level was significantly increased (P<0.05) in male rats treated with 500 mg/kg of the S. lavandulifolia extract for 45 days, compared with controls.
The other parameters (sodium, potassium, chloride, urea, albumin, total protein and ALT) showed no significant changes during the treatment period (Table 3).
Table 3.
Biochemical parameters of Wistar rats after 45 days treatment with S. lavandulifolia
Histopathological examinations showed fatty change, degeneration of hepatocytes and renal glomerular atrophy in the male rats (500 mg/kg).
In the female rats, atrophy of hepatocytes and dilatation of sinusoids in liver, hyperemia and degeneration of renal epithelium mostly in cortical region were observed (500 mg/kg) (Figs 2 and 3). No histological findings in the spleen or heart could be attributed to the S. lavandulifolia.
Fig. 2.
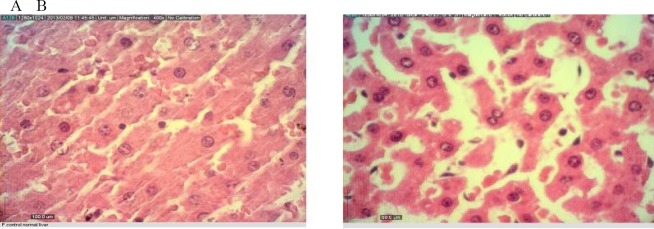
Liver tissue from A) control and B) treatment rats exposed to S. lavandulifolia at subchoronic toxic dose of 500 mg/kg after 45 days
Fig. 3.
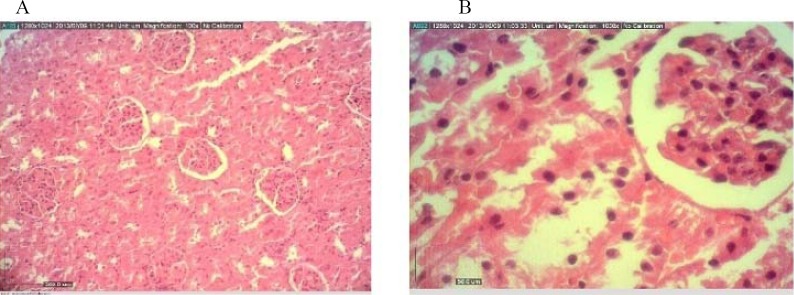
Kidney tissue from A) control and B) treatment rats exposed to S. lavandulifolia at subchoronic toxic dose of 500 mg/kg after 45 days.
DISCUSSION
Many studies have shown that S. lavandulifolia extract has many pharmacology properties such as antipyretic, anti-inflammatory, spasmolytic and sedative effects (11). The present study demonstrated the toxicity of S. lavandulifolia aqueous extract in rats in an acute and subchronic study and presents useful data about oral administration of S. lavandulifolia aqueous extract as no subchronic toxicity study has yet been reported. The results presented in this study did not show any overt toxic reactions of S. lavandulifolia. In the acute toxicity study, no adverse effect was observed up to the dose 5 g/kg of S. lavandulifolia. Therefore, the median acute toxicity value (LD50) of S. lavandulifolia is considered above 5 g/body weight. It is indicated that substances which present LD50 greater than 2.0 g/kg when administrated orally can be categorized practically non-toxic (12).
Therefore, it can be suggested that S. lavandulifolia is devoid of acute oral toxicity. The principle goals of the subchronic study were to further identify and characterize the specific organs affected by the test compound after repeated administration (13).
On the other hand, evaluation of oral toxicity through a repeated dose of 45-day experiment has been applied in many herbal safety assessment studies (14,15). Sub-chronic toxicity study showed minor changes in the blood parameters in some treated groups. Altered neutrophil and WBC count indicated that the plant extract would have some mild effect on the hematological parameter. It seems that S. lavandulifolia has toxic effects on bone marrow which leads to reduced WBC count which may reduce the response to inflammation and infections (16).
This is not, however, an established conclusion and surely needs further investigations. ALT and AST concentrations usually help to detect chronic liver disease. The results of the present study indicate that significant alternations in liver function have occurred. AST elevated in both sex groups that received the highest dose of the extract. Moreover, this study showed some remarkable pathological changes in the liver of the high-dose groups including fatty change, degeneration and atrophy of hepatocytes and dilatation of sinusoids. In an earlier toxicity study on hydroalcoholic extract in female mice, significant alternations in AST were observed in treated animals (17).
Kidney function was evaluated by means of serum urea, creatinine, potassium, sodium and chloride. Serum creatinine, which results from the catabolism of creatine phosphate in skeletal muscle, increases when renal function is poor and decreases with the loss of skeletal muscle (18). Hence, elevated blood ceratinine is a reliable indicator of a negative impact on kidney function or impaired glomerular filtration.
The creatinine level significantly increased in a dose-dependent manner at 500 mg/kg in male rats compared to the control. On the other hand histhopathological findings showed renal glomerular atrophy in male and hyperemia and degeneration of renal epithelium mostly in cortical region in female rats. These results are consistent with a previously described findings that S. lavandulifolia hydroalcoholic extract was able to induce renal toxicity in rat through degeneration of renal tubular epithelial cells (19).
CONCLUSION
In conclusion, a number of significant clinical and pathological changes were associated with the subchronic oral administration of S. lavandulifolia to Wistar rats. Several of these observations support the fact that kidney and liver are plausible target organs for both sexes.
Based on the results of this study no observed adverse effect level (NOAEL) of the total extract of S. lavandulifolia was considered to be 250 and 100 mg/kg/day for the male and female rats, respectively.
ACKNOWLEDGMENTS
The results presented here are extracted from the Pharm.D thesis of Ms. N. Nematy. This study was financially supported by the Research Council of Kermanshah University of Medical Sciences.
REFERENCES
- 1.Morteza-Semnani K, Akbarzadeh M, Changizi S. Essential oils composition of Stachys byzantina, S. inflata, S. lavandulifolia and S. laxa from Iran. Flavour Fragr J. 2006;21:300–303. [Google Scholar]
- 2.Rechinger KH, Hedge IC. Akademiche Druck Verlagsanstalt, Graz Austria; 1982. Flora Iranica; pp. 150,359–361,365. [Google Scholar]
- 3.Andalib S, Vaseghi A, Vaseghi G, Motavallian Naeini A. Sadative and hypnotic effects of Iranian traditional medicinal herbs used for treatment of insomnia. EXCLI J. 2011;10:192–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Mahzooni-kachapi SS, Mahdavi M, Roozbeh-nasira’ei L, Akbarzadeh M, Rezazadeh F, Motavalizadehkakhky A. Antimicrobial activity and chemical composition of essential oils of Stachys lavandulifolia Vahl. From Mazandaran. Iran J Med Plants Res. 2012;6:4149–4158. [Google Scholar]
- 5.Nabavizade F, Alazadeh A.M, Adeli S, Golestan M, Kamalinejad M. Gastroprotective of Stachys lavandulifolia extract on experimental gastric ulcer. Afr. J Pharm Pharmaco. 2011;5:155–159. [Google Scholar]
- 6.Ghasemi Pirbalouti A. Wound healing activity of extracts of Malvasylvestris and Stachys Lavandulifolia. Int J Biol. 2011;3:55–62. [Google Scholar]
- 7.Rabbani M, Sajjadi SE, Zarei HR. Anxiolytic effects of Stachys lavandulifolia Vahl. on the elevated plus-maze model of anxiety in mice. J Ethnopharmacol. 2003;89:271–276. doi: 10.1016/j.jep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Işcan G, Demirci B, Demirci F, Göger F, Kirimer N, Köse YB, et al. Antimicrobial and antioxidant activities of Stachys lavandulifolia subsp. lavandulifolia essential oil and its infusion. Nat Prod Commun. 2012;7:1241–1244. [PubMed] [Google Scholar]
- 9.Hajhashemi V, Ghannadi A, Sedighifar S. Analgesic and anti-inflammatory properties of the hydro-alcoholic, polyphenolic and boiled extracts of Stachys lavandulifolia. Res Pharm Sci. 2007;2:92–98. [Google Scholar]
- 10.De Angelis Pereira MC, Carvalho JCT, Lima LM, Caputo LRG, Ferreira LR, Fiorini JE, et al. Toxicity of a subchronic treatment with hydroalcoholic crude extract from Solanum grandiflorum (Ruiz et Pav) in rats. J Ethnopharmacol. 2003;89:97–99. doi: 10.1016/s0378-8741(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 11.Lobat Jafarzadeh, Mahmoud Rafieian-Kopaei, Roya Ansari Samani, AzamAsgari The effect of hydroalcoholic extract of S. Lavandolifolia V. on pregnant mice. EXCLI J. 2012;11:357–362. [PMC free article] [PubMed] [Google Scholar]
- 12.Mirghazanfari S, Hosseinzadeh L, Shokoohinia Y, Aslany M, Kamali-Nejad M. Acute and subchronic toxicological evaluation of Echinophora platyloba DC. (Apiaceae) total extract in Wistar rats. CLINICS. 2012;67:497–502. doi: 10.6061/clinics/2012(05)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis D. 7th edition. Klaassen, McGraw Hill; 2007. Casarett & Doull's Toxicology; pp. 30–31. [Google Scholar]
- 14.Rasekh HR, Nazari P, Kamali Nejad M, Hosseinzadeh L. Acute and subchronic oral toxicity of Galega officinalis in rats. J Ethnopharmacol. 2008;116:21–26. doi: 10.1016/j.jep.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Rasekh HR, Hosseinzadeh L, Mehri S, Kamli-Nejad M, Aslani M, Tanbakoosazan F. Safety assessment of Ocimum basilicum hydroalcoholic extract in Wistar rats: Acute and subchronic toxicity studies. Iranian J Basic Med Sci. 2012;15:645–653. [PMC free article] [PubMed] [Google Scholar]
- 16.Maurice E, Knuckles, Inyang F, Ramesh A. Acute and subchronic oral toxicity of fluoranthene in F-344 rats. Ecotox Environ Safe. 2004;59:102–108. doi: 10.1016/S0147-6513(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 17.Monji F, Hossein Tehrani H, Halvaei H, Arbabi Bidgoli S. Acute and subchronic toxicity assessment of the hydroalcoholic extract of Stachys lavandulifolia in Mice. Acta Medica Iranica. 2011;49:12–18. [PubMed] [Google Scholar]
- 18.Yee Hor S, Ahmad M, Farsi E, Yam M, Hashim M, Pin Lim Ch, et al. Safety assessment of methanol extract of red dragon fruit (Hylocereuspolyrhizus): Acute and subchronic toxicity studies. Reg Toxicol Pharmacol. 2012;63:106–114. doi: 10.1016/j.yrtph.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Taghikhani M, Nasri H, Asgari A, Afrough H, Namjoo AR, Ansari-Samani R, et al. The renal toxicity of hydroalcoholic extract of Stachys lavandulifolia Vahl in Wistar rats. Life Sci J. 2012;9:3025–3033. [Google Scholar]


