Abstract
Isovanillin and isoacetovanillon are two components found in P. spinosa Decne.exBoiss extract with no previously reported effect on ileum contractions. Spasmolytic effect of isovanillin and isoacetovanillon were examined on response to electrical field stimulation (EFS), acetylcholine (ACh) and 5-HT in strips of rat ileum. Longitudinal ileum strips were set up in an organ bath containing oxygenated Tyrode's solution. All strips that was contracted in response to EFS, acetylcholine or 5-HT showed relaxation in the presence of isovanillin (5-320 μg/ml), or isoacetovanillon (5-320 μg/ml). Isovanillin and isoacetovanillon inhibited the response to 5-HT with IC50 values of 356±50μM and 622±110μM respectively. They reduced the response to EFS without significantly affecting the acetylcholine response. P. spinosa extract (5-160 μg/ml) in a concentration dependent manner reduced the response to 5-HT, acetylcholine and EFS. This study demonstrated that isovanillin and isoacetovanillon are relaxant of ileum contractions induced by 5-HT and EFS and they have contribution to the relaxant effect of P. spinosa extract but other components are responsible for the inhibition of acetylcholine by the extract.
Keywords: Isovanillin, Isoacetovanillon, Pycnocycla spinosa extract, Propantheline, Acetylcholine, 5-HT
INTRODUCTION
Pycnocycla spinosa Decne.exBoiss is a wild plant which grows in Middle East including Iran (1). Hydroalcoholic extract of P. spinosa has both antispasmodic and anti-diarrhoeal effect (2,3,4,5). The extract of P. spinosa contains many active substances including flavonoids, glycosides, tannins and alkaloids–like substances (3,4). Fractionations of extracts also indicated presence of a number of active substances in the extract (6).
Further separation and isolation lead to identification of active substances in the extract including isovanillin and isoacetovanillon (Fig. 1) which may be responsible for antispasmodic effect of P. spinosa extract (7).
Fig. 1.
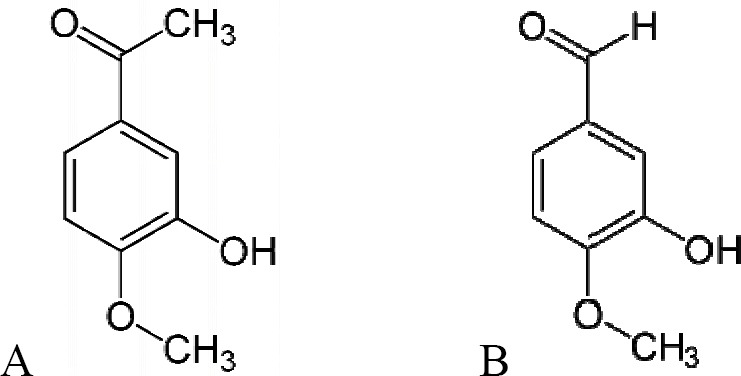
Chemical structure of isoacetovanillon (A) and isovanillin (B).
Although, these two substances are already known as plant material constituents, however, their pharmacological effect on ileum motility has not been reported, so far. Therefore, the aim of this research was to investigate the antispasmodic of isovanillin and isoacetovanillon in comparison with P. spinosa extract and a reference drug; propantheline.
MATERIALS AND METHODS
Drugs and solutions
Drugs used were as follows: Acetylcholine, 5-hydroxytriptamine (5-HT), and propantheline (Sigma, Germany), lidocaine (Pasteur, Tehran) isovanillin and isoacetovanillon (Sigma, China).
Acetylcholine (250 μM), 5-HT (100 μM), lidocaine (7.4 mM) and propantheline (1 mM) stock solutions were made up in distilled water. Isovanillin and isoacetovanillon were made up as 20 mg/ml stock solution in dimethyl sulphoxide (DMSO). Further serial dilutions were prepared in distilled water. Tyrode's solution with the following composition: NaCl; 136.9, KCl; 2.68, CaCl2;1.8, MgCl2; 1.05, NaHCO3; 11.9, NaH2PO4; 0.42 and glucose 5.55 mM were made up in distilled water. All the chemicals were from Merck (Germany) unless stated otherwise.
Plant extract
The aerial parts of Pycnocycla spinosa Decne.exBoiss. var. spinosa (Fam. Umbelli-ferae) were collected from base of Sofah mountain in Isfahan, Iran. The plant was identified by Dr Mehregan, plant taxonomist (Tehran Azad University). A voucher specimen (A24) of the plant was deposited in the herbarium of the School of Pharmacy and Pharmaceutical sciences at the Isfahan University of Medical Sciences, Iran.
Studies on ileum
Male Wistar rats (200-220 g) were killed and longitudinal strips of ileum were taken and placed in oxygenated Tyrode's solution. Each strip was suspended between parallel platinum electrodes in 50 ml organ bath. The bathing fluid was Tyrode's solution at 37°C and gassed with oxygen. The ileum tension was monitored with an isotonic transducer and displayed on a pen recorder (Harvard) device. Each preparation was subjected to a constant 1g weight tension.
Electrical field stimulation (EFS) was performed using rectangular pulses produced by a stimulator (Designed in Isfahan). Tissues were stimulated with maximum of 6 volts using 1s trains of stimuli at 50 Hz. Acetylcholine (0.5 μM) and 5-HT (2 μM) were added into bath with contact time of 20 s. Stimulation was performed once every 10 or 15 min as appropriate.
After the tissue baseline and responses were stabilized, pure compounds or extract were directly added into the bath using two fold increments in concentration unless stated. Value in the text shows the final bath concentration. Adequate time was permitted after adding each agent for equilibration of the response before further testing.
Analysis of data
Contraction to EFS or to added spasmogens were measured relative to the tissue baseline and expressed as percentage of initial response prior to addition of testing agent. The IC50 value (drug concentration causing 50% of maximum response) was determined by plotting a full concentration response curve for each tissue. Potency of the compounds was expressed as pD2 value (the negative log10 of the molar IC50). Values are presented as mean ± standard error of mean (SEM). Statistical analysis was performed using Student's t-test and/or one way analysis of variance (ANOVA) as appropriate. Sigma Plot (version 11) computer program was used for statistical analysis and plotting of graphs.
RESULTS
The response of ileum strip to electrical field stimulation was a rapid contraction (EFS-1), followed by relaxation and 30-90 s later a second contraction (EFS-2) which peaks up and returned to baseline level relatively slowly. Acetylcholine and 5-HT caused a rapid contraction the amplitude of which slightly falls off before it was washout. The response to EFS was relatively smaller than the response to acetylcholine or 5-HT. The responses to EFS were reduced by 5 and 50 μM lidocaine while the acetylcholine and 5-HT responses were not significantly affected. Larger responses to electrical stimulation could be obtained by using a larger pulse, but such response may not be abolished by lidocaine. At 500 μM bath concentration lidocaine however inhibited the response to acetylcholine and 5-HT showing a direct effect of lidocaine on smooth muscle.
All the tested tissues have shown repeatable contractile response to EFS, acetylcholine and/or 5-HT. Tissues with no consistent response were not further pursued.
Propantheline at concentration which totally blocked the response to acetylcholine (0.5 nM-32 nM) partially reduced the contraction in response to EFS without affecting the response to 5-HT (Fig. 2). The remaining response to EFS shows the presence of non-adrenergic non-cholinergic (NANC) responses. There was no significant changes in the contractile response in vehicle treated time matched control tissues.
Fig. 2.

Inhibitory effect of propantheline on tension development in the isolated ileum of rat treated with 5-HT (2 μM), acetylcholine (ACh, 500 nM) and electrical field stimulation (EFS: 6 V, 50 Hz, 1 s duration). Each data point is mean ± SEM (n=6). Ordinant scales: spasm remaining as a % of the contraction prior to propantheline addition. Abscissa scales: log10 concentration of propantheline. Each point is mean and the vertical lines show the SEM. EFS-1=initial contractile response. EFS-2=secondary contractile response. Stars show significant differences at corresponding propantheline concentration with 5-HT. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test).
P. spinosa extract (10-160 μg/ml) concentration dependently inhibited the response to 5-HT (IC50=28 ± 2 μg/ml, n=6), acetylcholine (IC50=55 ± 8 μg/ml, n=6) and EFS (EFS-1, IC50=27 ± 4 μg/ml, n=6), at its highest used bath concentration (320 μg/ml), the extract abolished the response to above stimuli (Fig. 3).
Fig. 3.
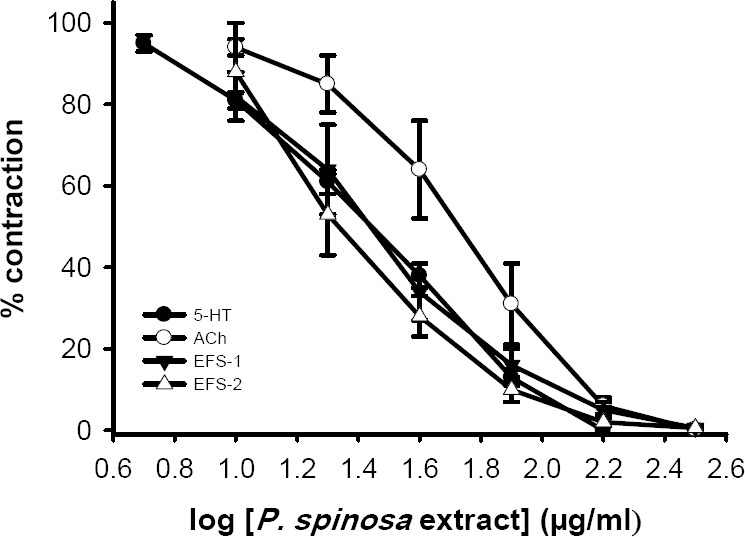
Log concentration inhibitory response curve of P. spinosa extract on tension development in the isolated ileum of rat treated with 5-HT (2 μM), acetylcholine (ACh, 500 nM) and electrical field stimulation (EFS: 6 V, 50 Hz, 1 s duration). Each data point is mean ± SEM (n=6). Ordinant scales: spasm remaining as a % of the contraction prior to extract addition. Abscissa scales: log10 concentration of P. spinosa extract. Each point is mean and the vertical lines show the SEM. EFS-1=initial contractile response. EFS-2=secondary contractile response. There was no statistically significant changes in vehicle treated time matched controls (curves not shown) but the reduction of responses by extract is significant (P<0.001, ANOVA).
Isovanillin (5-320 μg/ml) and isoaceto-vanillon (5-320 μg/ml) concentration depen-dently inhibited the contractile responses to 5-HT with IC50 values of 54 ± 8 μg/ml (pD2=3.5 ± 0.07, n=6) and 103 ± 19 μg/ml (pD2=3.3 ± 0.1, n=6), respectively (see Figs. 4, 5). Both isovanillin and isoacetovanillon reduced the responses to EFS but the inhibitory effect was not complete (Figs. 4, 5). Isovanillin and isoacetovanillon at concentra-tions which inhibited the response to 5-HT did not affected the contractile response to acetylcholine (Figs. 4, 5). In the vehicle treated time matched control tissues, DMSO (0.03%-1.6%) only caused a slight increase in contractile responses to acetylcholine, 5-HT and EFS.
Fig. 4.
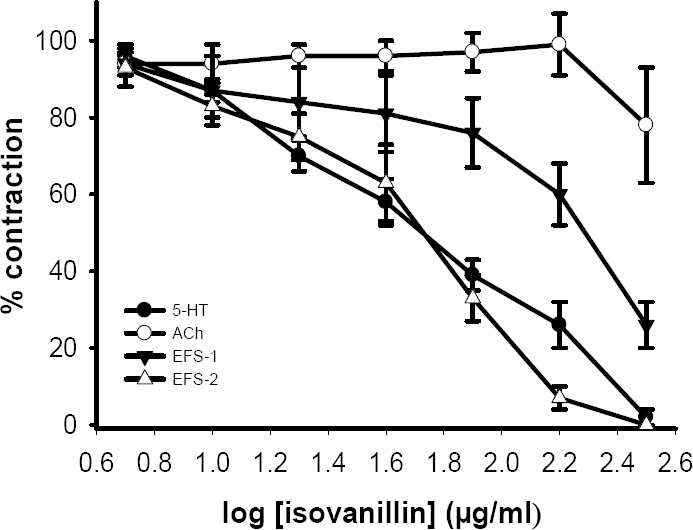
Inhibitory effect of isovanillin on tension development in the isolated ileum of rat treated with 5- HT (2 μM), acetylcholine (ACh, 500 nM) and electrical field stimulation (EFS: 6 V, 50 Hz, 1 s duration). Each data point is mean ± SEM (n=6). Ordinant scales: spasm remaining as a % of the contraction prior to isovanillin addition. Abscissa scales: log10 concentration of isovanillin. Each point is mean and the vertical lines show the SEM. EFS-1= initial contractile response. EFS-2= secondary contractile response. The variation in ACh response is not statistically significant but inhibition of 5-HT and EFS responses are significant (P<0.001, ANOVA).
Fig. 5.
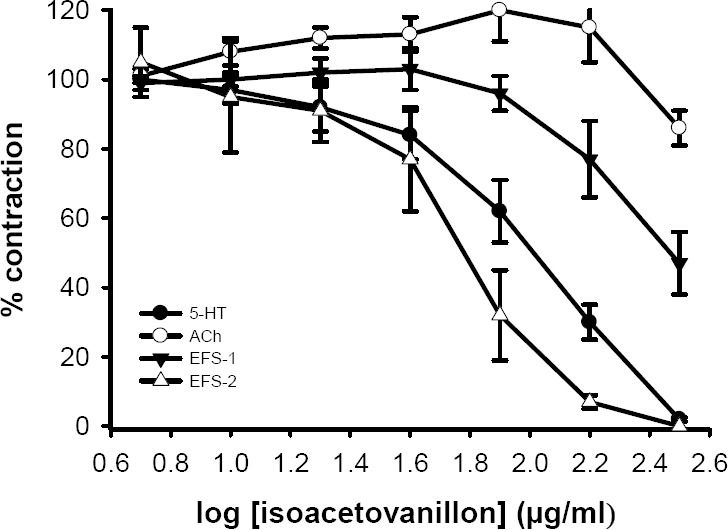
Inhibitory effect of isoacetovanillon on tension development in the isolated ileum of rat treated with 5- HT (2 μM), acetylcholine (ACh, 500 nM) and electrical field stimulation (EFS: 6 V, 50 Hz, 1 s duration). Each data point is mean ± SEM (n=6). Ordinant scales: spasm remaining as a % of the contraction prior to isoacetovanillon addition. Abscissa scales: log10 concentration of isoacetovanillon. Each point is mean and the vertical lines show the SEM. EFS-1= initial contractile response. EFS-2= secondary contractile response. The variation in ACh response is not statistically significant but inhibition of 5-HT and EFS responses are significant (P<0.001, ANOVA).
DISCUSSION
Studies have shown that, a seris of phenols that are structurally related to 3-t-butyl-4-hydroxy-anisole (BHA), have antispasmodic and spasmolytic activity in rat ileum longitudinal muscle (8). Preliminary data indicate that BHA is an active substance which also has antispasmodic effects on vascular smooth muscle (9,10). Isovanillin is a phenolic aldehyde (Fig. 1), an organic compound and isomer of vanillin and it is found in a number of plants including Pimpinella anisum (apiaceae), Evodia rutaecarpa (apiaceae) and Coriandrum sativum (apiaceae) (11,12,13). Isovanillin is a selective inhibitor of aldehyde oxidase (14). It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid (14). Despite presence of isovanillin in number of plant material, however so far there isn’t any official report on its pharmacological action on gastrointestinal function. Isoacetovanillon (Fig. 1) is also found in plant materials (15) but its pharmacological activity is not documented except a short report about analgesic effect and inhibitory action on the gastrointestinal motility as well as low degree of antibacterial activity against Escherichia coli and Shigella flexneri (15).
An isolated organ bath assay was used as pharmacological screening tool to assess concentration-response relationships of isovanillin and isoacetovanillon in rat ileum. These two compounds have been identified in extract of P. spinosa (7). The complex responses of the organ bath assay can be studied while controlling several physiological parameters. Therefore, effects of compounds with unknown molecular targets can be studied because all important tissue-specific expression and modification of molecular targets remain intact in organ bath preparation. Isovanillin, isoacetovanillon and the hydroalcoholic extract of P. spinosa concentration dependently inhibited the contraction induced by acetylcholine, 5-HT and EFS. Two former induces contractions via activating muscarinic M3 receptors and serotonergic 5-HT2 receptors repectively on ileum smooth muscle cells, while EFS stimulate the mesenteric neurons within the tissue including NANC nerves which have been described in all regions of the gastrointestinal tract (16,17,18,19,20).
Propantheline which is a muscarinic receptor antagonist at concentrations which totally removed the response to acetylcholine, only partially reduced the contractile response to EFS. As it would be expected, propantheline had no effect on 5-HT response. On the other hand, isovanillin and isoacetovanillon totally removed the ileum contractile responses to EFS, indicating that it also can remove contraction induced by neurotransmitters released from NANC nerves. In addition, the contractile response to 5-HT was also removed. Furthermore, isovanillin and isoacetovanillon are relaxant of rat ileum contraction induced by KCl (7) while propantheline and atropine have no effect on contraction induced by KCl (21,22). When these results are compared, it could be concluded that the mechanism of action of isovanillin and isoacetovanillon is somehow different from that of simple muscarinic antagonism.
Since isovanillin and isoacetovanillon both inhibited the response to different spasmogens, a more general intracellular mechanism might be involved because these compounds inhibited contraction due to release of Ca2+ ions from intracellular store (acetylcholine and 5-HT) or due to activation of voltage gated calcium channels (KCl) (7). Isoacetovanillon is a structural isomer of acetovanillon which has been widely used as an NADPH oxidase inhibitor in many experimental models (23,24,25,26). Isovanillin is also an inhibitor of aldehyde oxidase in the liver slice (14). Since isovanillin and isoacetovanillon have many structural similarities, it is possible that these two compounds act on similar intracellular target or membrane receptors, probably inhibiting specific enzyme involved in smooth muscle contraction. However, the exact mechanism of action needs to be investigated.
The total hydroalcoholic extract of P. spinosa was as potent as the isovanillin and isoacetovanillon in inhibiting rat ileum contraction. As isovanillin and isoaceto-vanillon only compose a small fraction of P. spinosa extract, it is clear that other active components exist in the extract with significant contribution in relaxant effect of P. spinosa extract. This can be supported by the reports that on gram weight bases some fractions of P. spinosa extract are about 10 times more active than isovanillin and isoacetovanillon (7).
CONCLUSION
In this study we have shown that isovanillin and isoacetovanillon are potent relaxant of rat isolated ileum. As these two components are found in P. spinosa extract it can be concluded that isovanillin and isoacetovanillon have significant contribution in antispasmodic action of P. spinosa extract and they could be used as an alternative remedy for treatment of spasmodic gastrointestinal disorders.
ACKNOWLEDGMENT
We would like to thanks the research department of Isfahan University of Medical Sciences, Isfahan, I.R.Iran, for providing student's grant (NO: 190104) for this research project.
REFERENCES
- 1.Jalili A, Jamzad Z. Tehran: Research Institute of Forests and Rangelands; 1999. Red data book of Iran, A preliminary survey of endemic, rare and endangered plant species in Iran; pp. 689–690. [Google Scholar]
- 2.Sadraei H, Asghari G, Naddafi A. Relaxant effect of essential oil and hydro-alcoholic extract of Pycnocycla spinosa Decne. exBoiss. on ileum contraction. Phytother Res. 2003;17:645–649. doi: 10.1002/ptr.1217. [DOI] [PubMed] [Google Scholar]
- 3.Sadraei H, Asghari G, Hekmatti AA. Antispasmodic effect of three fraction of hydroalcoholic extract of Pycnocycla spinosa. J Ethnopharmacol. 2003;86:187–190. doi: 10.1016/s0378-8741(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 4.Sadraei H, Asghari G, Khazael M. Relaxant effect of four fractions separated from alkaloid extract of Pycnocycla spinosa on rat isolated ileum. Res Pharm Sci. 2008;3:79–86. [Google Scholar]
- 5.Sadraei H, Asghari G, Shams M. Antidiarrhoeal action of hydroalcoholic extract of Pycnocycla spinosa in comparison with loperamide and dicyclomine. Iranian J Pharm Res. 2011;10:835–841. [PMC free article] [PubMed] [Google Scholar]
- 6.Sadraei H, Asghari G, Behzad S. Bioactivity-guided isolation of spasmolytic components of Pycnocycla spinosa. Decne exBoiss. Res Pharm Sci. 2011;6:81–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Jahed M. PharmD [Thesis] Isfahan University of Medical Sciences; 2013. Quantitative comparison of anti-spasmodic action of fractions separated from Pycnocycla spinosa extract on rat ileum using a bioassay technique. [Google Scholar]
- 8.Sgaragli GP, Valoti M, Gorelli B. Calcium antagonist and antiperoxidant properties of some hindered phenoles. Br J Pharmacol. 1993;110:369–377. doi: 10.1111/j.1476-5381.1993.tb13819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorelli B, Pessina F, Fusi F. Calcium antagonist property of some hindered phenols on rat aorta rings. Pharmacol Res. 1995;31:206. [Google Scholar]
- 10.Fusi F, Valoti M, Frosini F. 2,5-di-t-butyl-1,4-benzohydroquinone BHQ induces endothelium-dependent relaxation of rat thoracic aorta. Eur J Pharmacol. 1999;366:181–187. doi: 10.1016/s0014-2999(98)00932-7. [DOI] [PubMed] [Google Scholar]
- 11.Birgitt K, Jurgen R. S-adenosyl-l-methionine: Anol-O-methyltransferase activity in organ cultures of Pimpinella anisum. Phyto Chem. 1996;42:397–403. [Google Scholar]
- 12.Wang Q, Liang J, Chen J. Chemical constituents of Evodia rutaecarpa. J China Pharm Uni. 2005;36:520. [Google Scholar]
- 13.Singh G, Maurya S, Lampasona M. Chemical composition, antifungal, antioxidant and sprout suppressant activities of coriander (Coriandrum sativum) essential oil and its oleoresin. Flavour Fragr J. 2006;21:472–479. [Google Scholar]
- 14.Panoutsopoulos GI, Beedham C. Metabolism of isovanillin by aldehyde oxidase, xanthine oxidase, aldehyde dehydrogenase and liver slices. Pharmacol. 2005;73:199–208. doi: 10.1159/000082860. [DOI] [PubMed] [Google Scholar]
- 15.Sun FZ, Cai M, Lou FC. Analegesic effect and gastro-intestinal motility inhibitory action of 3-hydroxy-4-methoxy-acetophenone from Cynanchum paniculatum (Bunge) Kitagawa. Zhongguo Zhong Yao Za Zhi. 1993;18:362–383. [PubMed] [Google Scholar]
- 16.Levey AI. Immunological localization of M1-M5 muscarinic acetylcholine receptors in peripheral tissue and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 17.Elgen RM, Hege SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 18.Cohen M, Schenck K, Colbert W. Role of 5-HT-2 receptors in serotonin-induced contraction of nonvascular smooth muscle. J Pharmacol Exp Ther. 1985;232:770–774. [PubMed] [Google Scholar]
- 19.Smith GJ, Lefebvre RA. Non adrenergic non cholinergic responses in the rat ileum. Eur J Pharmacol. 1996;303:79–87. doi: 10.1016/0014-2999(96)00089-1. [DOI] [PubMed] [Google Scholar]
- 20.Ekblad E, Sandler F. Motor response in rat ileum evoked by nitric oxide donorsvs. Field stimulation: Modulation by pituitary adenylate cyclase inhibitors. J Pharmacol Exp Ther. 1997;283:23–28. [PubMed] [Google Scholar]
- 21.Sadraei H, Shokoohinia Y, Sajjadi SE, Mozafari M. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res Pharm Sci. 2013;8:137–144. [PMC free article] [PubMed] [Google Scholar]
- 22.Sadraei H, Asghari G, Emami S. Inhibitory effect of Rosa damascena Mill flower essential oil, geraniol and citronellol on rat ileum contraction. Res Pharm Sci. 2013;8:17–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanska J, Sarniak A, Wlodarczyk A. Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res. 2012;38:90–99. doi: 10.3109/01902148.2011.649823. [DOI] [PubMed] [Google Scholar]
- 24.Impellizzeri D, Esposto E, Mazzon E. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol. 2011;81:636–648. doi: 10.1016/j.bcp.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Impellizzeri D, Mazzon E, Esposito E. Effect of apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radic Res. 2011;45:221–236. doi: 10.3109/10715762.2010.526604. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad A, Mondello S, Di Paola R. Protective effect of apocynin, a NADPH-oxidase inhibitor, against contrast-induced nephropathy in the diabetic rats: a comparison with n-acetylcycteine. Eur J Pharmacol. 2012;674:397–406. doi: 10.1016/j.ejphar.2011.10.041. [DOI] [PubMed] [Google Scholar]


