Abstract
Ferula assa-foetida L. is distributed throughout central Asia and Mediterranean area and grows wildly in Iran and Afghanistan. Asafoetida is an oleo-gum-resin that is the exudates of the roots of Ferula assa-foetida and some other Ferula species. In Iranian traditional medicine, asafoetida is considered to be sedative, analgesic, carminative, antispasmodic, diuretic, antihelmintic, emmenagogue and expectorant. The aim of this study was to evaluate the antinociceptive effect of asafoetida in mice. The analgesic activity of asafoetida (25, 50 and 100 mg/kg) was compared with that of sodium diclofenac (30 mg/kg) or morphine sulfate (8 mg/kg) by using hot plate and acetic acid induced writhing tests. In hot plate test, the percentage of maximum possible effect (%MPE) against the thermal stimulus at 15 min post treatment time point for all doses of asafoetida was significantly greater than the control group. The number of writhes in all three doses of asafoetida was significantly less than the control group. GraphPad Prism 5 software was used to analyze the behavioral responses. Data were analyzed using repeated measure one-way ANOVA and P<0.05 was considered as the significant level. According to our findings, asafoetida exhibited a significant antinociceptive effect on chronic and acute pain in mice. These effects probably involve central opioid pathways and peripheral anti-inflammatory action.
Keywords: Asafoetida, Analgesic, Hot plate, Writhing test
INTRODUCTION
Pain is a serious unpleasant sensation that diminishes the quality of life (1). Despite the availability of effective and widely used analgesics, most of them exhibit many undesirable side effects which limit their clinical use (2). Today, the search for new analgesic compounds has been a priority of pharmacologists and pharmaceutical industries. Studying the pharmacological potency of plant species that traditionally have been used as pain killers is considered as a logical research strategy in finding new analgesic drugs (3). The Ferula genus from the Umbelliferae family has been found to be rich sources of bioactive natural products (4). The oleo-gum-resin of Ferula assa-foetida L. (asafoetida) extracted from the incisions on the stem and roots of this plant is considered as an important matter of pharmacological and industrial application (5). In different culture, asafoetida has used for treatment of various disorders. In Iranian folk medicine, asafoetida is used as an antispasmodic, antihelminthic, carminative and analgesic agent (6). Asafoetida is used by Americans as a powerful antihelminthic and antispasmodic but in China, asafoetida is used for treatment of intestinal parasites and Fijian people take water extract of the dried gum for the treatment of upset stomach (5). The oleo-gum-resin extracted from Ferula assa-foetida contains about 40-64% resin, 25% endogeneous gum, 10-17% volatile oil and 1.5-10% ash (5). Its resin fraction consists of ferulic acid esters, free ferulic acid, umbelliferone and coumarin derivatives such as foetidin and kamolonol, farnesiferoles A, B and C (7). The compositions of its gum fraction are known to be glucose, galactose, L-arabinose, rhamnose and glucuronic acid (5). In recent studies, several pharmacological and biological activities of asafetida have also investigated and have shown this oleo gum resin has antioxidant, antiviral, antifungal, cancer chemopreventive, anti-diabetic, antispasmodic, hypotensive and molluscicidal effects (5). A literature survey revealed that analgesic effect of asafetida has not yet been reported. This study was, therefore, conducted to investigate the effect of asafoetida on inflammatory and neurogenic pain in mice.
MATERIALS AND METHODS
Animals
Eighty male albino mice weighing 25 to 30 g and 6-8 weeks old, breeding in animal house of Shahid Sadoughi Medical School were selected and housed at controlled temperature of 22 ± 2 °C with a 12 h light/dark cycle with standard lab chow and tap water ad libitum. Each animal was used only once. Experiments were carried out in accordance with current ethical guidelines for the investigation of experimental pain in conscious animals (8). The number of animals and intensities of noxious stimuli were considered in minimum necessary for demonstrating the consistent effects. Animals were randomly and equally divided into 10 groups.
Plant oleo-gum resin
Asafoetida was collected from Tabas region (Yazd province, Iran) during the summer and the plant species was botanically identified by the botanist in Yazd Agricultural Research Center and voucher number of the specimen was 2365. The dried powdered of asafoetida (10 g) was soaked in distilled water (100 ml) overnight at room temperature. The solution obtained from this extraction was used as the stock for intraperitoneal (i.p.) injections. Concentrations and dosages were expressed as crude amount of the asafoetida used in the preparing of the stock solution.
Drugs and extract administration
Morphine sulfate (Temad, Iran) (8 mg/kg) and sodium diclofenac (Pharma chemie, Iran) (30 mg/kg) were used as positive control drugs, distilled water (as the drug's vehicle) as negative control and asafoetida in 25, 50 and 100 mg/kg as test agent. All administrations were carried out intraperitonealy at volume of 10 ml/kg to control and test groups.
Hot-plate test
Hot-plate test was carried out according to the method previously described (9). Mice were habituated to the cylinder of the apparatus for 5 min before the initiation of the experiment.
The hot-plate apparatus (Borj Sanat, Iran) was set to 54 ± 0.1 °C. Animals were placed on the hot surface inside the Plexiglas cylinder (20 cm in diameter) and the time (in seconds) spent to licking of their hind paws or jumping (whichever occurred first), was recorded as the pain response latency (reaction time). A 30 second cut-off was set to prevent tissue damage.
After recording a baseline reaction time for each animal, mice were immediately received their own administration (morphine sulfate, asafoetida or distilled water) and their reaction time to hot plate was recorded after 15, 30, 45 and 60 min.
The mean reaction time in each time point after drug administration was compared to the baseline reaction time in each group for evaluating the optimum response time. For between-group comparisons the percentage of maximum possible effect (%MPE) against thermal stimulus in each time point for each animal was calculated by the following formula:

Acetic acid-induced writhing test
The abdominal constriction test described by Collier and coworkers (10) was used with slight modification to measure the analgesic activity of asafoetida. Male mice pre-treated either with asafoetida solution (25, 50 and 100 mg/kg), diclofenac sodium (30 mg/kg) or distilled water (10 ml/kg) was treated with i.p. injection of 0.6% acetic acid (10 ml/kg) after 15 min. Acetic acid induces a typical writhing response. Five min after acetic acid injection, mice were kept in individual cages and the number of writhes (hind limb stretching and abdominal muscle contraction) of each mouse was counted for a period of 30 min by an individual who was unaware of the experiment details.
Mean number of writhes was considered as a measure of analgesic effect in each group. The percentage of writhing inhibition was calculated by using the following formula:

Acute toxicity study
At the end of the experiments, the mice were observed for symptoms of short and long term toxicity at first for 4 h and then for 10 consecutive days to rull out the behavioral changes (tremor, paralysis), weight loss and mortality (11).
Data analysis
All data were expressed as mean ± standard error of the means (SEM). GraphPad Prism 5 software was used to analyze the behavioral responses. Data were analyzed using repeated measure one-way ANOVA followed by the Tukey Kramer post-test for multiple comparisons. P<0.05 was considered as the significant level.
RESULTS
Hot-plate test
Latency responses for animals in different groups are shown in Table 1. The latencies for time 0 (base line latency) were statically analyzed by one way ANOVA and there was no significant difference between the groups. Despite the similarity in base line latencies in different groups, for more accuracy the %MPE against thermal stimulus was calculated for each animal in each time point after the treatment. Fifteen min after the treatment, the analgesic effects of different doses of asafoetida was obvious and declined for the next time points except for 50 mg/kg asafoetida which was elevated again at 60 min. The most effective dose of asafoetida was 50 mg/kg and its maximum effect was observed 60 min after drug administration (Fig. 1). As shown in Fig. 1, the %MPE at 15 min post treatment time point for all doses of asafoetida was significantly greater than that of the control group. The %MPE against heat stimulus at 15 min time point in animals receiving 25 and 50 mg/kg asafoetida were statically similar to those who administrated by morphine sulfate (P>0.05).
Table 1.
Hot plate latency responses of animals in different groups (n=8).
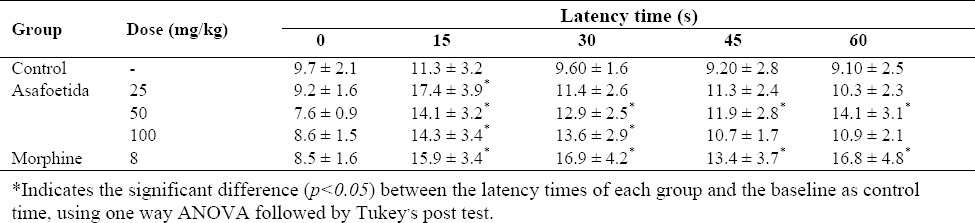
Fig. 1.
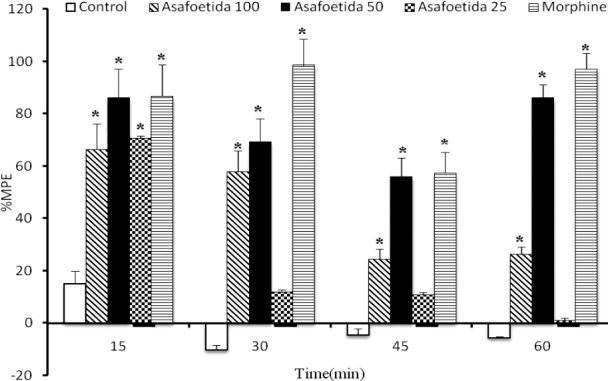
The percentage of maximum possible effect (%MPE) of different treatments on acute pain inhibition at different time points in hot plate test (n=8). *Indicates the significant difference (p<0.05) as compared to the control group.
Acetic acid-induced writhing test
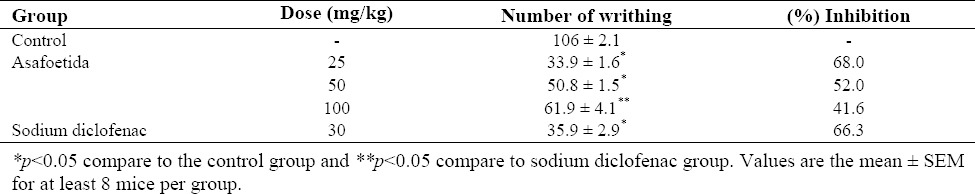
The effect of different treatments on acetic acid induced writhing is presented in Table 2. The percentage of writhing inhibition induced by different doses of asafoetida was inversely dose dependent and the number of writhes in all three doses of asafoetida was significantly less than control group. Maximum inhibition percent (68%) of writhes was observed by 25 mg/kg asafoetida which was statistically similar to that of 30 mg/kg sodium diclofenac (66.3%).
Table 2.
The effect of asafoetida on acetic acid-induced writhing in mice (n=8).
Acute toxicity
Asafoetida at doses used in the present study did not show any short or long term toxic effect. This was evidenced by the absence of tremor, paralysis, weight loss and autonomic behavioral changes as compared to control group. Also there was no mortality in treated animals during 10 days of observations.
DISCUSSION
In this study, we investigated anti-nociceptive effect of asafoetida on neurogenic and chronic pain in mice. Our findings indicated that asafoetida reduces the number of acetic acid induced writhes in an inverse dose-dependent manner, in which the lower (25 mg/kg) and moderate (50 mg/kg) doses produced an analgesic effect comparable to that of the sodium diclofenac.
The analgesic effect of asafoetida may be due either to its action on visceral receptors sensitive to acetic acid or to the inhibition of production/action of prostaglandins (12). On the other hands, hot-plate test is one of the most common tests for monitoring the phasic nociceptive responses to a noxious heat stimulus of high intensity (13).
Pain induced by this thermal noxious stimulus is specific for centrally mediated antinociception (14) and is thought to involve opioid pain inhibitory pathways (15). In the hot plate test, a significant effect of the asafoetida at all doses were obtained 15 min after treatment and the overall analgesic pattern of the most effective dose (50 mg/kg) was very similar to morphine sulfate. Therefore, the analgesic effect of asafoetida in hot plate test may be related to the opioid pain inhibitory pathways. However, the ability of 50 mg/kg asafoetida in prolonging the reaction latency in 60 min time point after a period of attenuated action reveals a biphasic mode of action which we could not find any reason for it. Asafoetida is a good source of active biological components such as flavonoids and terpenoids. The fractionation of asafoetida showed that this oleo-gum-resin consists of three major components including resin, gum and essential oil.
The gum contains monosaccharides, polysaccharides and glyco-proteins, while the major constituents of the resin are phenolic compounds such as ferulic acid and its esters, coumarins, sesquiterpene coumarins and other terpenoids (5). Several studies have shown that terpenoids have anti-inflammatory activities. Mandegari and coworkers attributed the anti inflammatory and antinociceptive activity of Ferula gummosa to its terpenoids and alkaloids (16).
Moreover, the antinociceptive activity of asafoetida may be due to the phenolic compounds such as ferulic acid which are present in a high contents in asafoetida (17). It has been suggested that o-prenylated acetophenone, 2-(3-methylbut-2- enyloxy) acetophenone, extracted from the roots of Ferula communis L, induces the analgesic and anti-inflammatory activity (18). Several recent reports on the therapeutic effects of plant extracts have ascribed to the analgesic activities exerted by presence of phenolic compounds (19,20). The fact that asafoetida produced its analgesic action in both tonic and phasic nociceptive models, is indicative that it may possess both central and peripheral antinociception. One possible mechanism of action for the active principles of this oleo-gum-resin could be related to lipooxygenase and/or cyclooxygenase in the arachidonic acid cascade at the peripheral route (21).
It has been demonstrated that sesquiterpene coumarins are the most bioactive components of asafoetida (5). Umbelliprenin which is one of these sesquiterpene coumarin of asafoetida can inhibit the activity of 5-lipooxygenase and shows the anti-inflammatory action (22).
Free radicals and related reactive species are strongly involved in several pathological and physiological processes, including cancer, cell death, inflammation and pain (23,24). Ferulic acid which is an important asafoetida component has a potent antioxidant activity (5) and may acts as an analgesic and anti inflammatory component of asafoetida.
CONCLUSION
In conclusion, this study demonstrates the analgesic activity of asafoetida which is in accordance with the traditional use of this extract as an analgesic and antinflammatory medicine. However, its active components and their mechanism of actions need to be elucidated by further studies.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Abbas Zarezadeh from Yazd Agricultural Research Center for his assistance in providing the asafoetida used in this research.
REFERENCES
- 1.Milano J, Oliveira SM, Rossato MF, Sauzem PD, Machado P, Beck P, et al. Antinociceptive effect of novel trihalomethyl-substituted pyrazoline methyl esters in formalin and hot-plate tests in mice. Eur J Pharmacol. 2008;581:86–96. doi: 10.1016/j.ejphar.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Jage J. Opioid tolerance and dependence. Do they matter? Eur J Pain. 2005;9:157–162. doi: 10.1016/j.ejpain.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Rang HP, Dale MM, Ritter JM. New York: Churchill Livingston; 1998. Pharmacology; pp. 25–33. [Google Scholar]
- 4.Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 5.Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)- a review. J Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 6.Zargari A. 6th ed. Iran: Tehran: University Publications; 1996. Medicinal Plants; pp. 628–632. [Google Scholar]
- 7.Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. J Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 9.Esmaeili-Mahani S, Rezaeezadeh-Roukerd M, Esmaeilpour K, Abbasnejad M, Rasoulian B, Sheibani V, et al. Olive (Olea europaea L.) leaf extract elicits antinociceptive activity, potentiates morphine analgesia and suppresses morphine hyperalgesia in rats. J Ethnopharmacol. 2010;132:200–205. doi: 10.1016/j.jep.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Collier HOJ, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drug in the mouse. Br J Pharmacol Chemother. 1968;36:313–320. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menezes IA, Marques MS, Santos TC, Dias KS, Silva AB, Mello IC, et al. Antinociceptive effect and acute toxicity of the essential oil of Hyptis fruticosa in mice. Fitoterapia. 2007;78:192–195. doi: 10.1016/j.fitote.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Lawan A, Katsaya UA, Yaro AH. Anti-inflammatory and anti-nociceptive effects of the methanolic extract of the stem bark of Ficus vallischoudae delile (Moraceae) Afr J Pharm Pharacol. 2008;2:200–203. [Google Scholar]
- 13.Mandegary A, Sayyah M, Heidari MR. Antinociceptive and anti inflammatory activity of the seed and root extracts of Ferula gummosa Boiss. in mice and rats. DARU. 2004;12:58–62. [Google Scholar]
- 14.Magaji M, Anuka J, Abdu-Aguye I, Yaro AH, Hussaini IM. Preliminary studies on anti-inflammatory and analgesic activities of Securinega virosa (Euphorbiaceae) in experimental animal models. J Med Plants Res. 2008;2:39–44. [Google Scholar]
- 15.Besra SE, Sharma RM, Gomes A. Anti-inflammatory effect of petroleum ether extract of leaves of Litchi chinensis Gaertn (Sapindaceae) J Ethnopharmacol. 1996;54:1–6. doi: 10.1016/0378-8741(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 16.Mandegary A, Sayyah M, Heidari MR. Antinociceptive and anti- inflammatory activity of the seed and root extracts of Ferula gummosa Boiss in mice and rats. DARU. 2004;12:58–62. [Google Scholar]
- 17.Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activity of methanol extract of Ferula assa-foetida and its essential oil composition. Grasas Aceites. 2009;60:405–412. [Google Scholar]
- 18.Paulino N, Paulino AS, Vautier P, Pisco L, Passarelli C, Costa JMF, et al. Evaluation of antinociceptive and anti-inflammatory effects of synthetic o-prenylated phenolic derivatives. Pharmacology and Pharmacy. 2012;3:348–357. [Google Scholar]
- 19.Küpeli E, Sahin FP, Yesilada E. In vivo anti-inflammatory and antinociceptive activity of phenolic compounds from Sideritis stricta. Z Naturforsch. 2007;62c:519–525. doi: 10.1515/znc-2007-7-810. [DOI] [PubMed] [Google Scholar]
- 20.Backhouse N, Delporte C, Apablaza C, Farías M, Goïty L, Arrau S, et al. Antinociceptive activity of Buddleja globosa (matico) in several models of pain. J Ethnopharmacol. 2008;119:160–165. doi: 10.1016/j.jep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Rajendra W, Armugam A, Jeyaseelan K. Toxins in anti-nociception and anti-inflammation. Toxicon. 2004;44:1–17. doi: 10.1016/j.toxicon.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Iranshahi M, Askari M, Sahebkar A, Hadji pavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU. 2009;17:99–103. [Google Scholar]
- 23.Ferreira PMP, Farias DF, Oliveira JTDA, Carvalho ADFU. Moringa oleifera: Bioactive compounds and nutritional potential. Revista de Nutricao. 2008;21:431–437. [Google Scholar]
- 24.Halliwell B, Gutteridge JMC. 4th ed. UK: Oxford: Oxford University Press; 2007. Free Radicals in Biology and Medicine; pp. 1263–1290. [Google Scholar]


