Abstract
Melissa officinalis L. (Labiatae) traditionally used in treating neurological disorders has also been identified as a memory-enhancing herb. The extract of M. officinalis has a cholinergic property. The role of basal forebrain cholinergic neurons, the neurons that are destroyed in Alzheimer's disease (AD), in learning and memory, is also well known. The aim of this study is to investigate the role of cholinergic system on the memory improving activity of M. officinalis extract. The leaves of M. officinalis were extracted with ethanol 80% using the maceration method. Rats received intra-peritoneal injections of M. officinalis extract in different doses (50–400 mg/kg) alone or in combination with scopolamine (1 mg/kg) before being trained in a Morris water maze (MWM) in a single-day training protocol. After training, the acetylcholinesterase enzyme (AChE) activity was measured in the hippocampus. Administration of M. officinalis extract (200 mg/kg) could significantly enhance learning and memory of naïve rats (p<0.001) and significantly ameliorate scopolamine-induced learning deficit, but the effect of the extract was not dose dependent, and doses above 200 mg/kg could neither enhance memory in naïve rats nor reverse scopolamine-induced memory impairment. Also, inhibition of AChE activity was observed in both naïve and scopolamine-induced memory-impaired rats. These results suggest that M. officinalis can improve memory and that the cholinergic property of the extract may contribute to the memory-improving effects observed in this study. Then M. officinalis extract has potential therapeutic value in alleviating certain memory impairment observed in AD.
Keywords: Melissa officinalis, Scopolamine, Alzheimer's disease, Water maze, Memory, Acetylcholinesterase activity
INTRODUCTION
Alzheimer's disease (AD) is a neuro-degenerative disease that is characterized by loss of learning ability with ageing (1). This impairment of memory is correlated with loss of basal forebrain cholinergic neurons (2,3). The role of these neurons in learning and memory is well known, and pharmacological blockage of them leads to impairment of learning and memory (4). Scopolamine-induced amnesic animal models are used to screen for agents that are claimed to have cognition-enhancing activity through stimulation of the cholinergic system, thus making them candidates for treatment of AD (5). The drugs that are currently available for treating AD patients are tacrine, donepezil, galantamine, and rivastigmine, which act through inhibiting acetylcholinesterase (AChE) and increasing the level of acetylcholine (ACh) in the brain. These drugs have improved the cognitive functions of AD patients symptomatically, but they cannot inhibit the progression of the disease (6). Due to the complex etiology of AD, new attempts are focusing on agents that target the progression of the disease from different pathways and on developing multi-functional compounds to combat the causes and symptoms (7). Herbal extracts include several materials with heterogeneous pharmacological effects were attended for complex situation like AD (8).
Melissa officinalis L. (Labiatae) has been frequently used in Iranian traditional medicine to treat neurological disorders such as depression and anxiety, and it is also mentioned as a memory enhancing herb (9). The plant also has anti-inflammatory and antioxidant capabilities (10). The extract of M. officinalis has a cholinergic property (11), as well as neurotropic action (12). Our previous study showed that M. officinalis extract can protect PC12 cells (pheochromocytoma cell line) against beta-amyloid induced toxicity (13). The aim of the present study was to evaluate the role of the cholinergic system in the effects of M. officinalis extract on learning and memory.
MATERIALS AND METHODS
Animals
Male albino Wistar rats weighing 180 to 220 g were purchased from the Pasteur Institute of Iran, housed in groups of five in stainless-steel cages, and given food and water ad libitum under a standard 12 h light/12 h dark cycle. All training and test sessions were performed in a room where only the water maze was placed in standard conditions and carried out in the constant hour in the morning between 9:00-12:00 (AM). The animal experiments were carried out in accordance with recommendations from the Declaration of Helsinki and the internationally accepted principles for the use of experimental animals.
Materials
Scopolamine hydrobromide, 5-dithiobis-(2-nitrobenzoic acid) (DTNB), and acetylthiocholine iodide (ATCh) were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, U.S.A).
Plant material and extraction procedure
The leaves of M. officinalis were collected from Gorgan (Golestan province, Iran) in June 2009 and identified by M. Kamalinejad, botanist from Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences. A voucher specimen (no. 545). was kept in Herbarium of Faculty of Pharmacy at this universityin Tehran, Iran.
The total plant extract was obtained by extraction of dried and milled plant leaves with ethanol 80% (1:10) using the maceration method for 4 days. After every 24 h, the mixture was filtered, and fresh solvent was added to the plant powder. The combined extracts were concentrated to dryness.
Treatments
The animals were randomly divided into the following groups, with 8 rats in each group: the normal saline -treated group which received normal saline intra-peritoneally (ip) as extract vehicle one h before training; the extract groups which received four different doses of M. officinalis extract (50, 100, 200, and 400 mg/kg, ip, dissolved in normal saline) 1 h before training., the scopolamine group which received scopolamine (1 mg/kg, ip, dissolved in normal saline) 30 min before training and the scopolamine + extract groups which were administered four different doses of M. officinalis extract (50, 100, 200, and 400 mg/kg) 1 h before training and scopolamine (1 mg/kg) 30 min later, and they were trained in a Morris water maze (MWM) 30 min after scopolamine administration (14).
Morris water-maze task
The water maze was a black, circular tank 136 cm in diameter and 60 cm tall filled with water (20 °C ± 2 °C) to a depth of 25 cm. The Plexiglass escape platform used for the spatial task was submerged to a depth of 1 cm from the water surface. The single training session consisted of two blocks, and each block included four trials that were performed on the training day (15,16). A trial was started by releasing a rat into the pool. The rats were allowed to swim to the hidden platform, but if an animal did not escape within 90 s it was manually guided to the escape platform by the experimenter. The rats were allowed to rest on the platform for 20 s between trials, and then they were placed in a holding cage for 5 min between the two blocks. This procedure was repeated with each rat starting in each of the four quadrants, with their order being randomized. The submerged platform was in the same quadrant on every trial. The probe test was performed on the second day (24 h later). In the probe test, the hidden platform was removed, and the animal was released from the north location and allowed to swim freely for 60 s. The positions of rats were recorded by a video camera mounted above the center of the pool and connected to a computer. The recordings were analyzed using an automated tracking system (Ethovision, Nodulus, Wageningen, The Netherlands) for escape latency (time to find the hidden platform), swimming speed on the training day, time spent in the target quadrant, and the number of platform-area crossings in the probe test.
Measurement of acetylcholinesterase enzyme activity
After training, the animals were sacrificed by decapitation under ether anesthesia, and the hippocampuses were dissected out (17) and homogenized in ice cold 0.1 M phosphate buffer saline (PBS), (pH 8.0). The homogenates were then centrifuged at 1000 × g for 10 min at 4 °C, and the supernatant was used as source of AChE. The AChE activity was measured as described earlier by Ellman (18). Briefly, 0.1 M PBS (pH 8.0), ATCh 75 mM as a substrate, and DTNB 10 mM in a ratio of 150:2:5 were mixed. Absorbance was then measured at 412 nm using a microplate reader (Bio Tech, USA) immediately after the enzyme source (10 μl) was added to the reaction mixture during 6 min. Protein concentration in the supernatant was measured using the Bradford method (19).
Statistical analysis
The statistical analysis was performed using Graph-Pad Prism software, with each data value being presented as the mean ± SEM. The MWM test training trial measures were analyzed by two-way ANOVA followed by Bonferroni post-hoc analysis using the block as one variable and treatment as a second. The MWM probe test results and enzyme activity results were analyzed by one-way ANOVA followed by Newman-Keuls post-hoc, multiple-comparison testing. The results were considered to be statistically significant if the p value was less than 0.05.
RESULTS
The effects of M. Officinalis extract on learning and memory of naïve rats
The effect of M. officinalis extract on spatial memory was assessed using the MWM test. Fig. 1A represents the mean escape latency during four trials in block1 and block 2 for each group. Statistical analysis revealed a significant block effect (p<0.05) and a treatment effect (p<0.05). There were significant differences between block 1 and block 2 in all groups (p<0.05), which imply that the rats in all groups learned water-maze performance during the training trials, but there were significant differences between the treated groups and the normal saline -treated group.
Fig. 1.
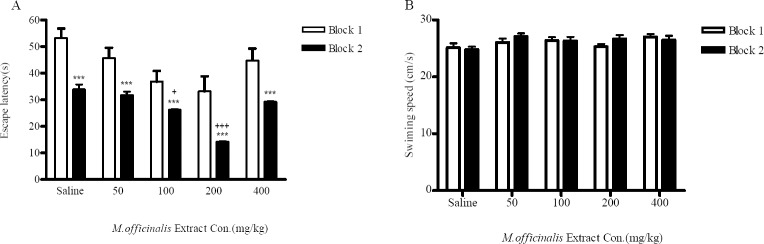
The effect of M. officinalis extract on escape latency (A) and swimming speed (B) of naïve rats in Morris water maze task during training sessions. Training performed in one day and consisted of two blocks, and each block included four trials. Each animal received extract or normal saline intra-peritoneally 1 h before training. Each column represents mean ± SEM for 8 animals. *Represent significant differences between block 1 and block 2 within each group (ANOVA, ***p<0.001). +Represent significant differences versus normal saline-treated group in block 2 (ANOVA, +;p<0.05, ++;p<0.01).
A comparison of these measures in block 2 revealed that 100 and 200 mg/kg M. officinalis extracts administration significantly decreased the escape latency in these groups compared with the normal saline-treated group (p<0.05, p<0.001 respectively). Also, animals in the100 and 200-mg/kg-treated groups spent significantly more time in the target quadrant compared with the normal saline-treated group in the probe test (p<0.05, p<0.001 respectively) (Fig. 2), which represents learning improvement in the treated group. There were no significant differences in speed between the groups (Fig. 1B).
Fig. 2.
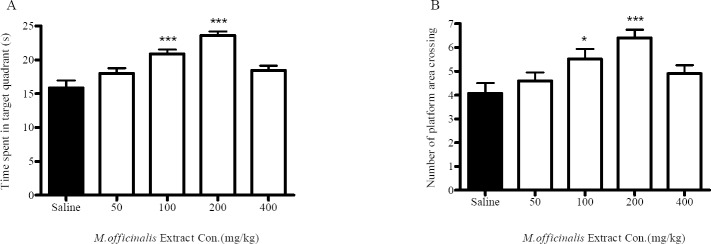
The effect of M. officinalis extract on time spent in target quadrant (A) and number of platform area crossing (B) of naïve rat in Morris water maze task during probe trail. Each animal received extract or normal saline intraperitoneally 1 h before training in training day and the probe trail was performed 24 h after training session. Each column represents mean ± SEM for 8 animals. *Represent significant differences versus normal saline-treated group (ANOVA*p<0.05, ***p<0.001).
The effects of M. Officinalis extract on scopolamine-induced learning and memory impairment in the Morris water-maze task
As shown in Fig. 3A, in the scopolamine-treated group there were not significant differences between bock 1 and block 2 in escape latency, and these animals spent less time in the target quadrant compared with the normal saline -treated group in the probe test (Fig. 4), which implies that the scopolamine treatment impaired the learning ability of the animals.
Fig. 3.
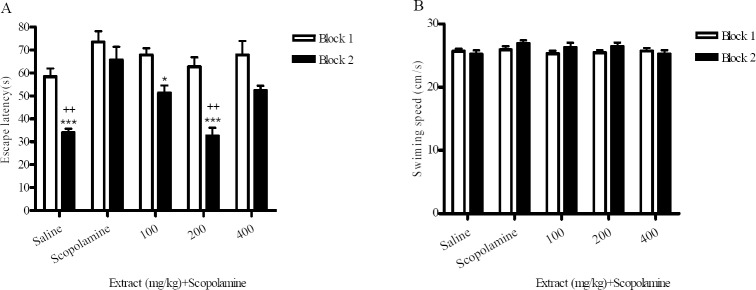
The effect of M. officinalis extract on scopolamine-induced memory impairment in Morris water maze task during training sessions. Training performed in one day and consisted of two blocks, and each block included four trials. Each animal administrated intra-peritoneally extract 1 h and scopolamine 1 mg/kg, 30 min before training. Each column represents mean ± SEM for escape latency (A) and swimming speed (B) for 8 animals. Sco:Scopolamine. *Significant differences between block 1 and block 2 within each group (ANOVA, *p<0.05, ***p<0.001). +Significant differences versus scopolamine-treated group in block 2 (ANOVA, ++p<0.01).
Fig. 4.
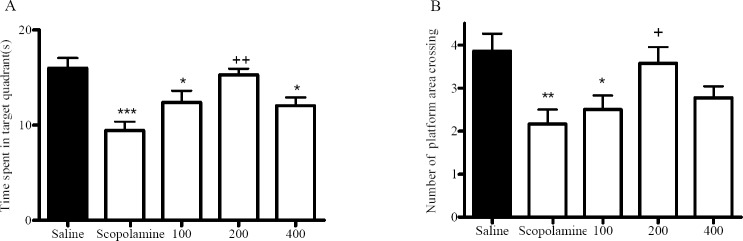
The effect of M. officinalis extract on scopolamine-induced memory impairment in Morris water maze task during probe trail. Each animal administrated intra-peritoneally extract 1 h and scopolamine 30 min before training in training day and the probe trail was performed 24 h later. Each column represents Mean ± SEM for time spent in target quadrant (A) and number of platform area crossing (B) for 8 animals. Sco:Scopolamine. *Versus normal saline-treated group (ANOVA, *p<0.05, **p<0.01, ***p<0.001). +Versus scopolamine-treated group (ANOVA, +p<0.05, ++p<0.01).
From these Figs (p<0.05), a significant treatment effect can be observed and the administration of M. officinalis extract (200 mg/kg) before scopolamine injection could significantly ameliorate scopolamine-induced memory impairment. In addition, it can be observed that M. officinalis extract (400 mg/kg) could not reverse scopolamine-induced memory impairment (Figs. 3A). In the probe test, animals pretreated with extract (200 mg/kg) spent significantly more time in the target quadrant compared with the scopolamine-treated group (Fig. 4). There were no significant differences in speed between the groups (Fig. 3B).
Acetylcholinesterase enzyme activity
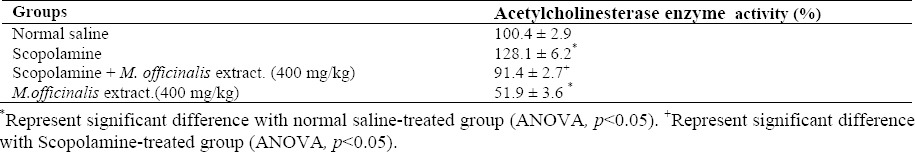
The results of AChE activity measurement in the hippocampus are presented in Table 1. AChE activity in the hippocampus of the scopolamine-treated group was significantly increased compared with the control group (p<0.05). Treatment of animals with M. officinalis extract (400 mg/kg) prior to scopolamine injection could ameliorate scopolamine-induced enhancement in AChE activity. Also, inhibition of AChE was observed in the M. officinalis extract-treated group (400 mg/kg).
Table 1.
Effect of M.officinalis extract on hippocampus acetylcholinesterase enzyme activity in naïve and scopolamine-treated rats.
DISCUSSION
Cholinergic neurons in the basal forebrain and hippocampus, and acetylcholine as a major neurotransmitter in these neurons, have important roles in learning and memory processes (18). Age-related dementia and memory deficit observed in AD, are correlated with the loss of cholinergic neurons in the basal forebrain and hippocampus (1). In addition, pharmacological blockage of cholinergic neurons in these areas causes impairment of memory and learning in experimental animals (19). Several studies have employed scopolamine; a nonselective muscarinic receptor antagonist, treated animals as a test model of cognitive function (5), and this model has been widely used to investigate the role of the cholinergic system in learning and memory and test new substances, designed to treat cognitive dysfunction (20,21,22). The results of present study showed that M. officinalis (200 mg/kg) can improve the learning ability of normal, healthy rats compared with a control group, but this effect was not dose dependent, and animals that received 400 mg/kg of extract did not show improvement in maze performance. This pattern was also observed when M. officinalis extract was administered to scopolamine-treated animals and only M. officinalis extract (200 mg/kg) administration before scopolamine could attenuate scopolamine-induced impairment in maze performance.
On the other hand, AChE activity in the hippocampus of rats treated with M. officinalis extract (400 mg/kg) was inhibited by about 50%, although this group did not show memory improvement. In the scopolamine-treated group, scopolamine caused an enhancement in AChE activity, this finding agrees with previous reports. In several studies wherescopolamine-induced amnesic animals have been used as a model for evaluation of antiamnesic effect of new drugs, following injection of scopolamine an increase in AChE activity in the brain tissue have been observed (14,23,24). In the present study despite attenuation of scopolamine induced AChE activity enhancement by M. officinalis extract (400 mg/kg), amelioration of memory impairment was not observed. An inverted U-shaped dose-response curve for antagonizing scopolamine-induced memory impairment by cholinesterase inhibitors was reported previously (25). Rivastigmine 0.5–2.5 mg/kg significantly attenuated the effects of scopolamine on reference and working memory and inhibited cholinesterase in the cortex and hippocampus by 20–60%, but a dose of 3.5 mg/kg, which inhibited cholinesterase in all brain areas by more than 70%, failed to prevent the memory impairment induced by scopolamine in healthy rats (26). The same results were reported for tacrine (27), physostigmine (25), donepezil, and heptyl physostigmine (28). It seems that over inhibition of AChE activity has deleterious effect on learning and memory processes. It is suggested that the reason for memory impairment of higher doses of cholinesterase inhibitors is the accumulation of acetylcholine in the synaptic cleft, then excessive stimulation of nicotinic receptors, followed by the blocking of nicotinic receptors.
Nicotinic receptors have an important role in learning and memory processes (29,30). Nicotine can improve memory when it is injected into the hippocampus (31), and blockage of nicotinic receptor by antagonist drugs causes memory impairment (32). Also, it has been reported that the ameliorating effect of cholinesterase inhibitors on scopolamine-induced memory impairment is modulated through nicotinic receptor and that mecamylamine, a nicotinic receptor antagonist, diminishes the protective effect of cholinestrese inhibitors on scopolamine-induced memory impairment (33)
The known major components of M. officinalis are hydroxycinnamic acid derivatives, particularly rosmarinic acid, caffeic acids and chlorogenic acid, flavonoids; including luteolin and isoquercitrin, and triterpene acid, including ursolic acid (34,35). The citronellal, citronellol, linalool and geranial are the major chemical compositions of the essential oil of the lemon balm (36). The anti-cholinestrase activity of M. officinalis extract and its main constituent rosmarinic acid was reported previously (37), also it is reported that chlorogenic acid can ameliorate scopolamine -induced amnesia and inhibit cholinestrase activity (14).
Our results showed that the effect of M. officinalis extract on scopolamine-induced memory impairment was similar to that of other cholinesterase inhibitors. Thus, it is suggested that inhibition of AChE activity by M. officinalis extract is the at least one of the mechanisms contributed in memory improving activity of this plant. Also, it has been reported that M. officinalis extract has nicotinic receptor activity and that it can displace [3H]-(N)-nicotine from nicotinic receptors in homogenates of human cerebral cortical cell membranes (11). Thus, another mechanism that can substitute for M. officinalis is stimulation of the nicotinic receptors, which results in memory improvement in healthy rats and reduced memory impairment in scopolamine-treated rats. It has also been suggested that higher doses over-stimulate the nicotinic receptors and result in nicotinic receptor blockage and memory impairment.
The memory improving property for the other constituent of extract was reported. luteolin can improve scopolamine-induced impairment of passive avoidance response in rats (38) and attenuate beta-amyloid-induced impairment of water maze performance (39). Ursolic acid can improve age related cognition deficit via activation of antioxidant enzymes and reduction of lipid peroxidation (40). Then the involvement of other mechanisms in the memory-enhancing activity of this extract was not rule out.
CONCLUSION
The present study showed that M. officinalis extract can improve memory in healthy rats and scopolamine-induced memory impairment. These effects of M. officinalis extract may be attributed to the AChE-inhibitory activity that was observed in this study or to nicotinic receptor activity, which was reported earlier, although other mechanisms of actions could be involved too. The memory-enhancing activity of this extract via the cholinergic system and the potent antioxidant activity of this extract make it a candidate for treatment of AD.
REFERENCES
- 1.Terry AV, Callahan PM, Hall B, Webster SJ. Alzheimer's disease and age-related memory decline (preclinical) Pharmacol Biochem Behav. 2011;99:190–210. doi: 10.1016/j.pbb.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer's disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 4.Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 5.Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database. Syst Rev. 2006;1:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmaco-therapies for Alzheimer's disease: Beyond cholinesterase inhibitors. Pharmacol Ther. 2012;134:8–25. doi: 10.1016/j.pharmthera.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Anekonda TS, Reddy PH. Can herbs provide a new generation of drugs for treating Alzheimer's disease? Brain Res Rev. 2005;50:361–376. doi: 10.1016/j.brainresrev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Naghibi F, Mosaddegh M, Mohammadi Motamed S, Ghorbani A. Labiatae Family in folk medicine in Iran: from ethnobotany to pharmacology. Iran J Pharm Res. 2005;2:63–79. [Google Scholar]
- 10.Dastmalchi K, Damien Dorman HJ, Oinonena PP, Darwis Y, Laakso I, Hiltunen R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT. 2008;41:391–400. [Google Scholar]
- 11.Wake G, Court J, Pickering A, Lewis R, Wilkins R, Perry E. CNS acetylcholine receptor activity in european medicinal plants traditionally used to improve failing memory. J Ethnopharmacol. 2000;69:105–114. doi: 10.1016/s0378-8741(99)00113-0. [DOI] [PubMed] [Google Scholar]
- 12.Soulimani R, Fleurentin J, Mortier F, Misslin R, Derrieu G, Pelt JM. Neurotropic action of the hydroalcoholic extract of Melissa officinalis in the mouse. Planta Med. 1991;57:105–109. doi: 10.1055/s-2006-960042. [DOI] [PubMed] [Google Scholar]
- 13.Sepand M, Soodi M, Soleimani M, Hajimehdipoor H. Protective effects of Melissa officinalis extract against beta-amyloid-induced oxidative stress in PC12 cells. J Med Plants. 2012;11:74–85. [Google Scholar]
- 14.Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010;649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 15.De Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 16.Akbari E, Naghdi N, Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav Brain Res. 2006;173:47–52. doi: 10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Spijker S. Dissection of rodent brain regions. In: Wan Li KW, editor. Neuromethods, Spiringer publisher; 2011. pp. 13–26. [Google Scholar]
- 18.Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 19.Bradford M.M. rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ennaceur A, Meliani K. Effects of physostigmine and scopolamine on rats’performances in object-recognition and radial-maze tests. Psychopharmacol. 1992;109:321–330. doi: 10.1007/BF02245880. [DOI] [PubMed] [Google Scholar]
- 22.Hodges DB, Jr, Lindner MD, Hogan JB, Jones KM, Markus EJ. Scopolamine induced deficits in a battery of rat cognitive tests: comparisons of sensitivity and specificity. Behav Pharmacol. 2009;20:237–251. doi: 10.1097/FBP.0b013e32832c70f5. [DOI] [PubMed] [Google Scholar]
- 23.Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, et al. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 24.Jeong EJ, Lee KY, Kim SH, Sung SH, Kim YC. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur J Pharmacol. 2008;588:78–84. doi: 10.1016/j.ejphar.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Suzuki N. Antiammnestic and cholinomimetic side effects of the cholinesterase inhibitors, physostigmine, tacrine and NIK-247 in rats. Eur J Pharmacol. 1993;150:117–124. doi: 10.1016/0014-2999(93)90628-u. [DOI] [PubMed] [Google Scholar]
- 26.Bejar C, Wang RH, Marta Weinstock M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur J Pharmacol. 1999;383:231–240. doi: 10.1016/s0014-2999(99)00643-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Tang XC. Reversal of scopolamine-induced deficits in radial maze performance by (-)-huperzine A: comparison with E2020 and tacrine. Eur J Pharmacol. 1998;349:137–142. doi: 10.1016/s0014-2999(98)00199-x. [DOI] [PubMed] [Google Scholar]
- 28.Braida D, Paladini E, Griffini P, Lamperti M, Maggi A, Sala M. An inverted U-shaped curve for heptylphysostigmine on radial maze performance in rats: comparison with other cholinesterase inhibitors. Eur J Pharmacol. 1996;302:13–20. doi: 10.1016/0014-2999(96)00072-6. [DOI] [PubMed] [Google Scholar]
- 29.Tian S, Huang F, Li P, Li Z, Zhou S, Deng H, et al. Nicotine enhances contextual fear memory reconsolidation in rats. Neurosci Lett. 2011;487:368–371. doi: 10.1016/j.neulet.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 30.Placzek AN, Zhang TA, Dani JA. Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta Pharmacol Sin. 2009;30:752–760. doi: 10.1038/aps.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M, Roghani A. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem. 2005;95:1078–1090. doi: 10.1111/j.1471-4159.2005.03454.x. [DOI] [PubMed] [Google Scholar]
- 32.Decker MW, Majchrzak MJ. Effects of central nicotinic cholinergic receptor blockade produced by chlorisondamine on learning and memory performance in rats. Behav Neural Biol. 1993;60:163–171. doi: 10.1016/0163-1047(93)90271-i. [DOI] [PubMed] [Google Scholar]
- 33.Masuoka T, Kamei C. The role of nicotinic receptors in the amelioration of cholinesterase inhibitors in scopolamine-induced memory deficits. Psychopharmacol. 2009;206:259–265. doi: 10.1007/s00213-009-1603-7. [DOI] [PubMed] [Google Scholar]
- 34.Gruenwald J, Brendler T, Jaenicke C. 2nd ed. Montvale: 2000 Medical Economics Company, Inc; 2000. PDR for Herbal Medicines; pp. 461–463. [Google Scholar]
- 35.Patora J, Klimek B. Flavonoids from lemon balm (Melissa officinalis L., Lamiaceae) Acta Pol Pharm. 2002;59:139–143. [PubMed] [Google Scholar]
- 36.Norouzi M, Soleimani T, Pasha Zanousi M. Essential oil component in leaf and flower of Lemon balm (Melissa officinalis L.) Res Pharm Sci. 2012;7:S749. [Google Scholar]
- 37.Dastmalchi K, Ollilainen V, Lackman P, Boije af Gennas G, Dorman HJ, Jarvinen PP, et al. Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg Med Chem. 2009;17:867–871. doi: 10.1016/j.bmc.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 38.Tsai FS, Peng WH, Wang WH, Wu CR, Hsieh CC, Lin YT, et al. Effects of luteolin on learning acquisition in rats: involvement of the central cholinergic system. Life Sci. 2007;80:1692–1698. doi: 10.1016/j.lfs.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 39.Tsai FS, Cheng HY, Hsieh MT, Wu CR, Lin YC, Peng WH. The ameliorating effects of luteolin on beta-amyloid-induced impairment of water maze performance and passive avoidance in rats. Am J Chin Med. 2010;38:279–291. doi: 10.1142/S0192415X10007841. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol. 2007;74:1078–1090. doi: 10.1016/j.bcp.2007.07.007. [DOI] [PubMed] [Google Scholar]


