Abstract
Amodiaquine is an antimalarial drug used in the prophylaxis and treatment of this disease. However, hepatotoxicity as a life-threatening adverse effect is associated with its clinical use. We evaluated amodiaquine-induced toxicity in isolated rat hepatocytes as an in vitro model for studying drug-induced hepatotoxicity. This study attempts to investigate the protective effects of taurine and N-acetyl cysteine against the cytotoxicity induced by amodiaquine. Hepatocytes were prepared by the method of collagenase enzyme perfusion via portal vein. This technique is based on liver perfusion with collagenase after removal of calcium ion (Ca2+) with a chelator (ethylene glycol tetraacetic acid (EGTA) 0.5 mM). Cells were treated with different concentrations of amodiaquine, taurine and N-acetyl cysteine. Cell death, protein carbonylation, reactive oxygen species formation, lipid peroxidation, and mitochondrial depolarization were assessed as toxicity markers. Amodiaquine cytotoxic mechanism involved protein carbonylation as well as reactive oxygen species formation and lipid peroxidation. In addition, mitochondria seem to be a target for amodiaquine to induce cellular damage. Administration of taurine (200 μM) and/or N-acetyl cysteine (200 μM) reduced oxidative stress, lipid peroxidation and protein carbonylation caused by amodiaquine. Furthermore, amodiaquine-induced mitochondrial injury was significantly mitigated by taurine and/or N-acetyl cysteine. In glutathione-depleted cells, only N-acetyl cysteine protected hepatocytes against amodiaquine, and taurine showed no protective properties in this situation. Taurine and N-acetyl cysteine protect hepatocytes against amodiaquine probably via their antioxidant properties and counteracting oxidative stress.
Keywords: Amodiaquine, N-acetyl cysteine, Oxidative stress, Protein carbonylation, Taurine
INTRODUCTION
Amodiaquine is used in the prophylaxis and treatment of malaria especially against chloroquine-resistant isolates of Plasmodium falciparum (1). However, the clinical use of this drug is associated with hepatotoxicity (2,3). The exact mechanism of amodiaquine-induced hepatotoxicity is not clear yet, but this adverse effect has been attributed to the bioactivation of the drug to a quinoneimine metabolite (4). Oxidative stress has been suggested to be involved in the development of amodiaquine-induced hepatotoxicity due to the ability of redox cycling induction by the quinoneimine metabolite of amodiaquine (5,6). Such reactive metabolites can irreversibly bind to proteins, which might lead to toxicity by disrupting the cell functions. Since reactive intermediates are formed during amodiaquine metabolism, amodiaquine toxicity mechanism could involve protein carbonylation. Lipid peroxidation is a consequence of oxidative stress (7). It has been shown that lipid peroxidation occurred after amodiaquine treatment (8,9). Glutathione reservoirs seems to have critical role in preventing amodiaquine hepatotoxicity (10). Hence, in present study, amodiaquine-induced cytotoxicity was evaluated in intact and glutathione-depleted hepatocytes to elucidate the role of glutathione reservoirs in the toxicity induced by this drug.
Taurine, a conditionally essential amino acid, has several physiological roles (11). There are many reports on taurine protective effects against different chemicals-induced hepatotoxicity (12,13,14). It has been reported that this amino acid could act as an antioxidant in biological systems (15). Hence, the protective effects of taurine could be due to the antioxidant capability of this amino acid. Being an antioxidant, it has also the ability to scavenge the reactive oxygen species; attenuate lipid peroxidation, and consequently stabilize the biological membranes (16,17).
Considering the previously reported hepato-toxicity associated with amodiaquine, it becomes imperative to study on effective protective agents, which could reduce liver injury caused by this drug. Since taurine has shown protective properties such as antioxidative (18), lipid peroxidation attenuating (19), and/or cellular membrane stabilizing (20), this study attempted to evaluate the potential protective effects of this amino acid against amodiaquine-induced cellular injury. N-acetyl cysteine (NAC) was used as a thiol containing protective agent since its hepatoprotective properties has been proven in many investigations (21,22,23). Cell death, reactive oxygen species (ROS) formation, lipid peroxi-dation, protein carbonylation, and mitochondrial depolarization were assessed as toxicity markers after amodiaquine treatment and the protective effects of taurine and/or N-acetyl cysteine were evaluated.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (weighing 250–300 g) obtained from Tabriz University of Medical Sciences, Tabriz, Iran, was used. Animals were fed a standard chow diet and water ad libitum. The animals were handled and used, according to the animal handling protocol that approved by a local ethic committee in Tabriz University of Medical Sciences, Tabriz, Iran.
Isolation and incubation of hepatocytes
Hepatocytes were isolated from male Sprague–Dawley rats by collagenase perfusion method as described previously (24). The cells were suspended (1 × 106 cell/ml) in Krebs-Henseleit buffer containing 12.5 mM HEPES (4-(2-hydroxyethyl)-1-piperazinee-thanesulfonic acid), and incubated under a stream of 95% O2 and 5% CO2. Glutathione- depleted hepatocytes were obtained by preincubating hepatocytes with 0.2 mM 1-bromoheptane for 30 min (25). Taurine (200 μM) was added 30 min before amodiaquine in all experiments.
Cell viability
Hepatocyte viability was assessed by plasma membrane disruption as determined by trypan blue uptake test (26). Cell viability was determined immediately after isolation and at scheduled time intervals following incubation. Approximately 85–90% of hepatocytes were viable at the time of isolation.
Determination of reactive oxygen species
Determinationn of ROS was carried out as explained previously (27). Briefly, 1.6 μM of DCFD-DA (2’,7’-dichlorfluorescein-diacetate) was added to incubation media and at different time points, aliquots of 1 ml were withdrawn. These samples were centrifuged for 1 min at 3000 g. The fluorescence intensity of dichlorofluorescein was measured in supernatant using a Jasco® FP-750 fluorescence spectro-photometer. Excitation and emission wavelengths were 490 nm and 520 nm, respectively (27).
Determination of lipid peroxidation in hepatocytes
Hepatocyte lipid peroxidation was determined by measuring the amount of thiobarbituric acid reactive substances (TBARS) formed during the decomposition of lipid hydroperoxides.
One mL aliquot of hepatocyte suspension containing 106 cells/ml was treated with trichloroacetic acid (70% w/v) and the supernatant was boiled with thiobarbituric acid (0.8% w/v) for 20 min then, the absorbance of developed color was measured at 532 nm in a Ultrospec2000® spectrophotometer (28).
Protein carbonylation assay
Total protein-bound carbonyl content was measured by derivatizing the carbonyl adducts with 2,4-dinitrophenyl hydrazine (DNPH). Briefly, an aliquot of the suspension of cells (0.5 mL, 0.5 × 106 cells) was added to an equivalent volume (0.5 mL) of 0.1% DNPH (w/v) in 2 N HCl and incubated for 1 h at room temperature in dark. This reaction was terminated and total cellular protein precipitated by the addition of an equivalent 1.0 mL volume of 20% trichloroacetic acid (TCA) (w/v). Cellular protein was rapidly pelleted by centrifugation at 10,000 g, and the supernatant was discarded. Excess unin-corporated dinitrophenylhydrazine (DNPH) was extracted three times using an excess volume (0.5 mL) of ethanol: ethyl acetate (1:1) solution.
Following the extraction, the recovered cellular protein was solubilized in 1 mL of Tris-buffered 8.0 M guanidine–HCl, pH 7.2. The resulting solubilized hydrazones were measured at 380 nm by an Ultrospec2000® spectrophotometer (29).
Mitochondrial membrane potential assay
At different time points, 2 mL samples of the cell suspension were taken and centrifuged at 1000 g for 1 min. Then the cell pellet was resuspended in 2 ml of Krebs-Henseleit buffer containing 1.5 μM rhodamine 123 and incubated at 37 °C water bath. Hepatocytes were separated by centrifugation at 3000 g for 1 min and the amount of rhodamine 123 appearing in the incubation medium was measured fluorimeterically using a Jasco® FP-750 fluorescence spectrophotometer at 490 nm excitation and 520 nm emission wavelengths. The capacity of mitochondria to take up the rhodamine 123 was calculated as the difference in fluorescence intensity between control and treated cells (30).
RESULTS
Amodiaquine-induced cytotoxicity
Amodiaquine caused cytotoxicity toward isolated rat hepatocytes in a concentration dependent manner (Fig. 1). It was found that 1 mM amodiaquine caused about 50% cell death in two hours (Lethal concentration 50%, LC50). Taurine and NAC were added to the incubation medium to determine their ability to modulate the toxic response. For this purpose, the LC50 of amodiaquine (1 mM) was selected.
Fig. 1.

Concentration-response curves of amodiaquine-induced cytotoxicity toward isolated rat hepatocytes. Isolated rat hepatocytes (106 cells/mL) were incubated at 37°C in rotating round bottom flasks with 95% O2 and 5% CO2 in Krebs- Henseleit buffer (pH 7.4). Data are given as Mean ± SEM for three independent experiments. *Different from control group (P<0.05).
As shown in Fig. 2, Taurine (200 μM) and NAC (200 μM), effectively reduced amodiaquine-induced cell death. Treatment of hepatocytes with 1-bromoheptane (as a glutathione depletory agent) (25) beforehand, resulted in a dramatic increase in amodiaquine-induced cytotoxicity (Fig. 2). NAC effectively prevented cell death induced by amodiaquine in glutathione-depleted hepatocytes (Fig. 2) but taurine showed no significant effects in this situation (data not given).
Fig. 2.
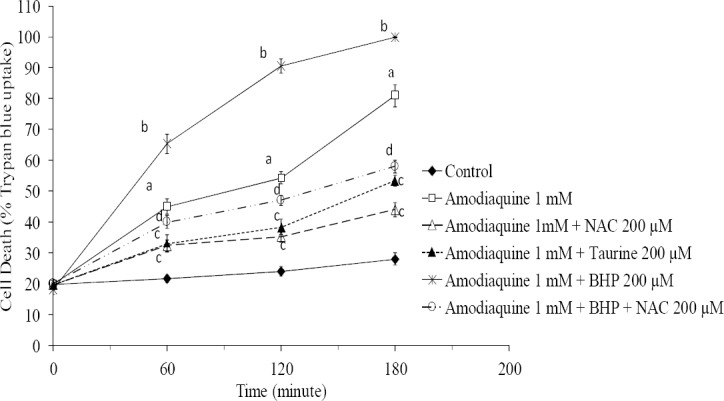
The effect of taurine, N-acetyl cysteine (NAC) and 1-bromoheptane (BHP) on amodiaquine-induced cytotoxicity toward isolated rat hepatocytes. Isolated rat hepatocytes (106 cells/mL) were incubated at 37°C in rotating round bottom flasks with 95% O2 and 5% CO2 in Krebs-Henseleit buffer (pH 7.4). Data are shown as Mean ± SEM for at least three separate experiments. aSignificantly different from control group (n=3, ANOVA, P<0.001). bSignificant difference as compared with amodiaquine-treated hepatocytes (n=3, ANOVA, P<0.05). cSignificantly different from amodiaquine-treated group (n=3, ANOVA, P<0.05). dSignificantly different from glutathione-depleted cells, which were treated with amodiaquine (n=3, ANOVA, P<0.001).
Amodiaquine-induced ROS formation
A significant amount of ROS was formed when hepatocytes were treated with amodiaquine (Fig. 3), which could suggest the occurrence of oxidative stress after amodiaquine administration to hepatocytes. Pre-treatment of hepatocytes with taurine and/or addition of NAC to incubation medium reduced the level of ROS formation caused by amodiaquine (Fig. 3). Glutathione depletion resulted in a sharp increase in ROS formation induced by amodiaquine (Fig. 3). This might prove the vital role of glutathione against amodiaquine-induced oxidative stress and cytotoxicity. NAC effectively reduced the levels of ROS formed after amodiaquine in glutathione-depleted hepatocytes (Fig. 3). Taurine was unable to reduce ROS levels in glutathione-depleted cells (data not given).
Fig. 3.
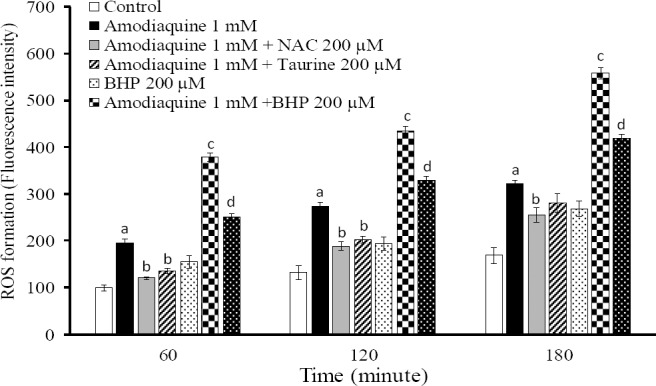
Reactive oxygen species formation induced by amodiaquine and the protective effects of taurine and NAC. BHP: 1-bromoheptane. Isolated rat hepatocytes (106 cells/mL) were incubated at 37°C in rotating round bottom flasks with 95% O2 and 5% CO2 in Krebs-Henseleit buffer (pH 7.4). Data are expressed as Mean ± SEM for three independent experiments. aDifferent from control group (P<0.05). bSignificantly different from amodiaquine-treated hepatocytes (n=3, ANOVA, P<0.05). cSignificantly different from amodiaquine-treated cells (n=3, ANOVA, P<0.001). dSignificantly different from amodiaquine-treated cells which were depleted of glutathione (n=3, ANOVA, P<0.001).
Lipid peroxidation caused by amodiaquine
Lipid peroxidation could be a consequence of ROS or reactive metabolites formation (31). When hepatocytes were incubated with amodiaquine, a significant amount of lipid peroxidation was detectable in normal cells (Fig. 4). The levels of lipid peroxidation were higher when glutathione-depleted cells were treated with amodiaquine (Fig. 4). Addition of taurine (200 μM) and/or NAC (200 μM) decreased the lipid peroxidation induced by amodiaquine in intact hepatocytes (Fig. 4). In glutathione-depleted cells, only NAC coun-teracted the lipid peroxidation caused by amodiaquine (Fig. 4).
Fig. 4.
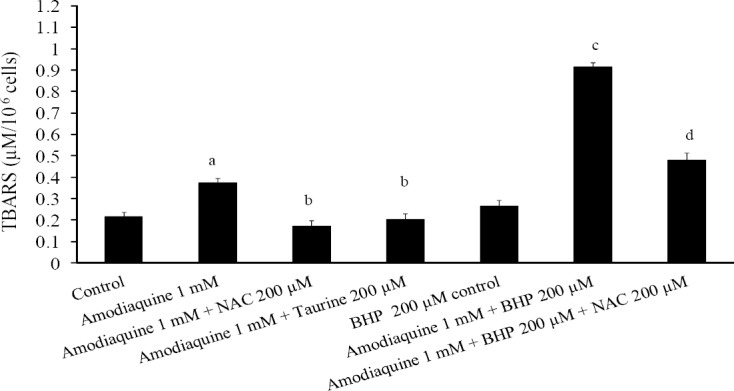
TBARS formation induced by amodiaquine and the preventive role of taurine and/or N-acetyl cysteine. BHP: 1-bromoheptane. Isolated rat hepatocytes (106 cells/mL) were incubated at 37°C in rotating round bottom flasks with 95% O2 and 5% CO2 in Krebs-Henseleit buffer (pH 7.4). Data are shown as Mean ± SEM for three independent experiments as measured after 120 min of incubation. aDifferent from control group (n=3, ANOVA, P<0.05). bDifferent from amodiaquine-treated group (n=3, ANOVA, P<0.05). cDifferent from amodiaquine-treated group (n=3, ANOVA, P<0.05). dDifferent from amodiaquine-treated groups which were depleted of glutathione (BHP-treated) (n=3, ANOVA, P<0.05).
Protein carbonylation
Formation of carbonyl compounds is the most general and widely used marker of protein oxidation both in vitro and in vivo. It was found that protein carbonylation induced by amodiaquine was significantly higher than the control level (Fig. 5). Administration of taurine and/or NAC reduced the level of carbonylated proteins in amodiaquine-treated hepatocytes (Fig. 5). The level of carbonylated proteins raised when cellular glutathione reservoirs were depleted (Fig. 5). NAC effectively reduced protein carbonylation, induced by amodiaquine in glutathione-depleted hepatocytes (Fig. 5).
Fig. 5.
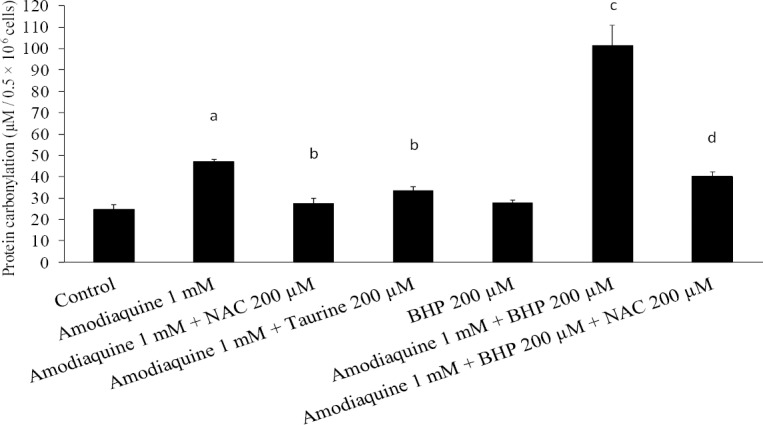
Amodiaquine-induced protein carbonylation. The protective effects of taurine and/or N-acetyl cysteine. BHP: 1-bromoheptane. Data are given as Mean ± SEM for at least three separate experiments as assessed 30 min after incubation. aSignificantly different from control group (n=3, ANOVA, P<0.05). bLower than amodiaquine-treated group (n=3, ANOVA, P<0.05). cHigher than amodiaquine-treated cells (n=3, ANOVA, P<0.05). dLower than amodiaquine-treated cells which were glutathione-depleted (n=3, ANOVA, P<0.05).
Amodiaquine-induced mitochondrial depolarization
The possibility that subcellular components such as mitochondria are the target of amodiaquine to cause hepatocytes injury was investigated by evaluating the changes in mitochondrial membrane potential (ΔΨ) as a key parameter of mitochondrial function. The LC50 of amodiaquine (1 mM) caused reduction in mitochondrial membrane potential as measured with rhodamine 123 test (Fig. 6). This shows that mitochondria could be a target for amodiaquine or its reactive metabolite(s) to cause hepatocyte damage and consequently cell death.
Fig. 6.
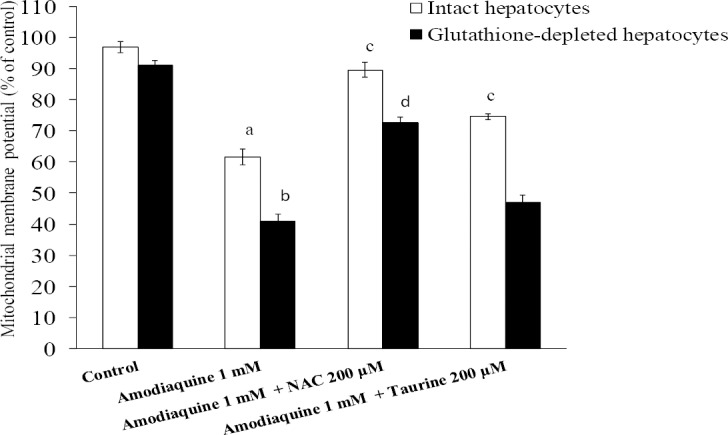
Mitochondrial depolarization induced by amodiaquine and the role of NAC and/or taurine. Data are given as Mean ± SEM for at least three separate experiments as assessed 60 min after incubation. Isolated rat hepatocytes (106 cells/mL) were incubated at 37°C in rotating round bottom flasks with 95% O2 and 5% CO2 in Krebs-Henseleit buffer (pH 7.4). a,bDifferent from control groups (n=3, ANOVA, P<0.05). cDifferent from amodiaquine-treated hepatocytes (n=3, ANOVA, P<0.05). dDifferent from amodiaquine-treated cells which were glutathione-depleted (n=3, ANOVA, P<0.05).
It was found that taurine and/NAC effectively prevented mitochondrial depolarization caused by amodiaquine (Fig. 6). Glutathione depletion deteriorated amodia-quine-induced mitochondrial depolarization (Fig. 6). Taurine could not reduce mitochondrial depolarization in glutathione-depleted hepato-cytes (Fig. 6), but NAC effectively diminished mitochondrial depo-larization caused by amodiaquine in these cells (Fig. 6).
DISCUSSION
Different concentrations of amodiaquine caused cytotoxicity toward isolated rat hepatocytes. The toxicity was accompanied with ROS formation, lipid peroxidation, protein carbonylation, and mitochondrial depolarization. Taurine (200 μM) and/or NAC (200 μM) effectively attenuated the toxic effects of amodiaquine in hepatocytes, but only NAC had significant effects on amodiaquine-induced toxicity in glutathione-depleted cells. As revealed in previous investigations, amodia-quine causes oxidative stress (32,33). We found that ROS level was elevated in amodiaquine-treated hepatocytes. This might indicate the occurrence of oxidative stress in isolated hepatocytes after amodiaquine administration. Being an antioxidant, taurine and NAC can scavenge reactive species and attenuate oxidative stress (16,34). Hence, ROS formed during amodiaquine metabolism are scavenged by taurine and NAC which might have a role in their protective effects against amodiaquine cytotoxicity.
Lipid peroxidation usually is one of the consequences of ROS formation and oxidative stress in biological systems (7). Taurine might reduce lipid peroxidation due to its ability in attenuating oxidative stress (35). Furthermore, taurine could chelate metal ions such as Fe2+ which could have a role in its ability in reducing lipid peroxidation (36). NAC is a well= recognized radical scavenger (34), and it is speculated that alleviation of oxidative stress and scavenging reactive species formed during amodiaquine metabolism could be involved in the protective effects of NAC against amodiaquine-induced hepatotoxicity.
Protein carbonylation induced by reactive carbonyl species generated by peroxidation of polyunsaturated fatty acids play a significant role in the etiology of xenobiotics-induced cellular damage (37). Reducing oxidative stress and quenching reactive species (15), might be attributed to the protective effects of taurine against cellular protein damage caused by amodiaquine.
Mitochondrial depolarization can cause energy crisis and releasing of apoptotic signaling molecules, which could finally encounter cell death (38). Decrease in mitochondrial membrane potential could be a consequence of ROS formation and oxidative stress (39), induced by amodiaquine. The protective effects of taurine and/or NAC against mitochondrial injury might be attributed to their effects against oxidative stress.
Taurine administration ameliorated amodiaquine-induced toxicity in intact hepatocytes, but had no significant effect in glutathione-depleted cells. It has been shown that taurine affects some antioxidant enzymes (40). Since, in this study, the duration of treatment with taurine was not sufficient to upregulate antioxidant enzymes, taurine had no significant effect in glutathione-depleted cells. In intact hepatocytes, taurine by regulating cellular ions such as Ca2+, stabilizing cellular membrane and scavenging reactive species (11), restricts the oxidative stress induced by amodiaquine. However, in glutathione-depleted cells, where hepatocytes are in a more susceptible state and amodiaquine causes dramatic cytotoxicity, taurine is unable to protect cells. However, NAC as a more potent radical scavenger than taurine (41), prevented oxidative stress and toxicity induced by amodiaquine in both intact and glutathione-depleted rat hepatocytes. But taurine could not act effectively in this situation where a vast amount of ROS is formed by amodiaquine (Fig. 3), probably due to its weaker antioxidative and radical scavenging properties than NAC (15).
CONCLUSION
Our results indicate that taurine and/or NAC protect against the multiple targets of amodiaquine-induced damage toward hepatocytes. This study provides insight into the search for other implications of taurine against different drug-induced hepato- cytes injuries specially those, which are accompanied with oxidative stress.
ACKNOWLEDGMENTS
This study was funded (Grant number: 5/79/1436) by Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. The authors thanked Drug Applied Research Center for providing facilities and financial supports to carry out this study. This research was a part of Reza Heidari's PhD thesis that was supported by Students’ Research Committee. The authors are also thankful to the Students’ Research Committee of Tabriz University of Medical Sciences, Tabriz-Iran, for providing supports to the study. Authors wish to thank Dr. M.R. Sattari and Dr. H. Hamzeiy for linguistic corrections of the manuscript.
REFERENCES
- 1.White NJ. The treatment of malaria. N Eng J Med. 1996;335:800–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 2.Larrey D, Castot A, Pessayre D, Merigot P, Machayekhy JP, Feldmann G, et al. Amodiaquine- induced hepatitis. A report of seven cases. Ann Int Med. 1986;104:801–3. doi: 10.7326/0003-4819-104-6-801. [DOI] [PubMed] [Google Scholar]
- 3.Taylor WRJ, White NJ. Antimalarial drug toxicity: a review. Drug safety. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Jewell H, Maggs JL, Harrison AC, O’Neill PM, Ruscoe JE, Park BK. Role of hepatic metabolism in the bioactivation and detoxication of amodiaquine. Xenobiotica. 1995;25:199–217. doi: 10.3109/00498259509061845. [DOI] [PubMed] [Google Scholar]
- 5.Park BK, Pirmohamed M, Tingle MD, Madden S, Kitteringham NR. Bioactivation and bioinactivation of drugs and drug metabolites: Relevance to adverse drug reactions. Toxicol in Vitro. 1994;8:613–621. doi: 10.1016/0887-2333(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 6.Tafazoli S, O’Brien PJ. Amodiaquine-induced oxidative stress in a hepatocyte inflammation model. Toxicology. 2009;256:101–109. doi: 10.1016/j.tox.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama H, Horie T, Awazu S. Oxidative stress in isolated rat hepatocytes during naproxen metabolism. Biochem Pharmacol. 1995;49:991–996. doi: 10.1016/0006-2952(94)00542-t. [DOI] [PubMed] [Google Scholar]
- 8.Farombi EO, Olowu BI, Emerole GO. Effect of three structurally related antimalarial drugs on liver microsomal components and lipid peroxidation in rats. Comp Biochem Physiol C: Comp Pharmacol. 2000;126:217–224. doi: 10.1016/s0742-8413(00)00116-x. [DOI] [PubMed] [Google Scholar]
- 9.Farombi EO, Adoro S, Uhunmwangho S. Antimalarial drugs exacerbate rat liver microsomal lipid peroxidation in the presence of oxidants. Biosc Rep. 2001;21:353–359. doi: 10.1023/a:1013242401009. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu S, Atsumi R, Itokawa K, Iwasaki M, Aoki T, Ono C, et al. Metabolism-dependent hepato-toxicity of amodiaquine in glutathione-depleted mice. Arch Toxicol. 2009;83:701–707. doi: 10.1007/s00204-009-0436-9. [DOI] [PubMed] [Google Scholar]
- 11.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Waters E, Wang JH, Redmond HP, Wu QD, Kay E, Bouchier-Hayes D. Role of taurine in preventing acetaminophen-induced hepatic injury in the rat. Am J Physiol Gastro Liver Physiol. 2001;280:1274–1279. doi: 10.1152/ajpgi.2001.280.6.G1274. [DOI] [PubMed] [Google Scholar]
- 13.Sinha M, Manna P, Sil PC. Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol in Vitro. 2007;21:1419–1428. doi: 10.1016/j.tiv.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Manna P, Sinha M, Sil P. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids. 2009;36:417–428. doi: 10.1007/s00726-008-0094-x. [DOI] [PubMed] [Google Scholar]
- 15.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J. 1988;256:251–255. doi: 10.1042/bj2560251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu QD, Wang JH, Fennessy F, Redmond HP, Bouchier-Hayes D. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am J Physiol. 1999;277:1229–1238. doi: 10.1152/ajpcell.1999.277.6.C1229. [DOI] [PubMed] [Google Scholar]
- 17.You JS, Chang KJ. Taurine protects the liver against lipid peroxidation and membrane disintegration during rat hepatocarcinogenesis. Adv Exp Med Biol. 1998;442:105–112. doi: 10.1007/978-1-4899-0117-0_14. [DOI] [PubMed] [Google Scholar]
- 18.Rashid K, Das J, Sil PC. Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol. 2013;51:317–329. doi: 10.1016/j.fct.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Tabassum H, Rehman H, Banerjee BD, Raisuddin S, Parvez S. Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin Chim Acta. 2006;370:129–136. doi: 10.1016/j.cca.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Timbrell JA, Seabra V, Waterfield CJ. The in vivo and in vitro protective properties of taurine. Gen Pharmacol. 1995;26:453–462. doi: 10.1016/0306-3623(94)00203-y. [DOI] [PubMed] [Google Scholar]
- 21.Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, et al. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2010;22:1235–1238. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]
- 22.Masubuchi Y, Nakayama J, Sadakata Y. Protective effects of exogenous glutathione and related thiol compounds against drug-induced liver injury. Biol Pharm Bull. 2011;34:366–370. doi: 10.1248/bpb.34.366. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad I, Shukla S, Kumar A, Singh BK, Kumar V, Chauhan AK, et al. Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats. Chemico-Biological Interactions. 2013;201:9–18. doi: 10.1016/j.cbi.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Moldeus P, Hogberg J, Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- 25.Khan S, O’Brien PJ. 1-bromoalkanes as new potent nontoxic glutathione depletors in isolated rat hepatocytes. Biochem Biophys Res Comm. 1991;179:436–41. doi: 10.1016/0006-291x(91)91389-t. [DOI] [PubMed] [Google Scholar]
- 26.Niknahad H, O’Brien PJ. Mechanism of sulfite cytotoxicity in isolated rat hepatocytes. Chemico-Biological Interactions. 2008;174:147–154. doi: 10.1016/j.cbi.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Pourahmad J, Mortada Y, Eskandari MR, Shahraki J. Involvement of lysosomal labilisation and lysosomal/mitochondrial cross-talk in diclofenac induced hepatotoxicity. Iran J Pharm Res. 2011;10:877–87. [PMC free article] [PubMed] [Google Scholar]
- 28.Afsharirad B, Eghbal MA, Delazar A, Heidari R. Res Pharm Sci. Vol. 7. in 13th Iranian Pharmaceutical Sciences Congress-Isfahan, Iran; 2012. Effects of Scrophularia striata extracts on isolated rat hepatocytes; p. S137. [Google Scholar]
- 29.Mehta R, Wong L, O’Brien PJ. Cytoprotective mechanisms of carbonyl scavenging drugs in isolated rat hepatocytes. Chemico-Biological Interactions. 2009;178:317–323. doi: 10.1016/j.cbi.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Heidari R, Babaei H, Eghbal M. Mechanisms of methimazole cytotoxicity in isolated rat hepatocytes. Drug Chem Toxicol. 2012 doi: 10.3109/01480545.2012.749272. In press. [DOI] [PubMed] [Google Scholar]
- 31.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Mishra SK, Singh P, Rath SK. A study of toxicity and differential gene expression in murine liver following exposure to anti-malarial drugs: amodiaquine and sulphadoxine-pyrimethamine. Malaria Journal. 2011;10:109–109. doi: 10.1186/1475-2875-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tafazoli S, Spehar DD, O’Brien PJ. Oxidative stress mediated idiosyncratic drug toxicity. Drug Metab Rev. 2005;37:311–325. doi: 10.1081/dmr-55227. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-Acetyl-L-Cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol Sci. 2011;120:87–97. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oudit GY, Trivieri MG, Khaper N, Husain T, Wilson GJ, Liu P, et al. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation. 2004;109:1877–1885. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 36.Flora SJS. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidat Med Cell Long. 2009;2:191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burcham PC, Fontaine F. Extensive protein carbonylation precedes acrolein-mediated cell death in mouse hepatocytes. J Biochem Mol Toxicol. 2001;15:309–316. doi: 10.1002/jbt.10007. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 39.Satoh T, Enokido Y, Aoshima H, Uchiyama Y, Hatanaka H. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res. 1997;50:413–420. doi: 10.1002/(SICI)1097-4547(19971101)50:3<413::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Ozden S, Catalgol B, Gezginci-Oktayoglu S, Arda-Pirincci P, Bolkent S, Alpertunga B. Methiocarb-induced oxidative damage following subacute exposure and the protective effects of vitamin E and taurine in rats. Food Chem Toxicol. 2009;47:1676–1684. doi: 10.1016/j.fct.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Sevgiler Y, Karaytug S, Karayakar F. Antioxidative effects of N-acetylcysteine, lipoic acid, taurine, and curcumin in the muscle of Cyprinus carpio L. exposed to cadmium. Arch Ind Hyg Toxicol. 2011;62:1–9. doi: 10.2478/10004-1254-62-2011-2082. [DOI] [PubMed] [Google Scholar]


