Abstract
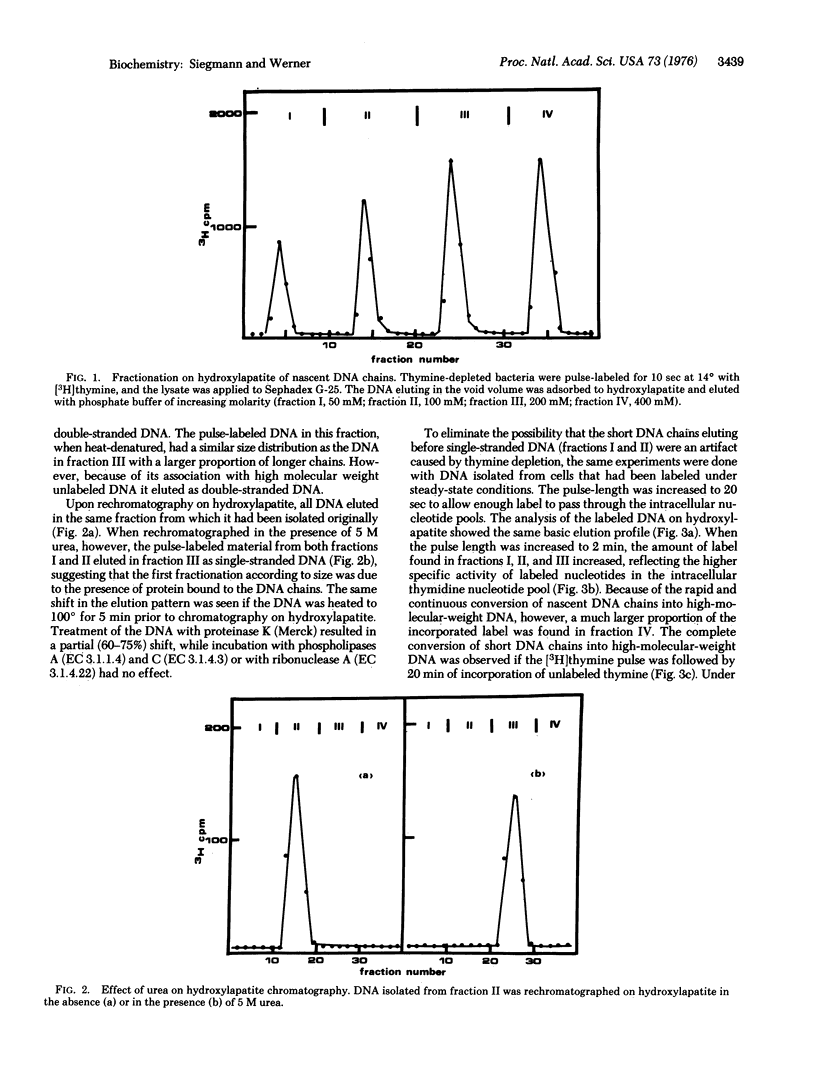
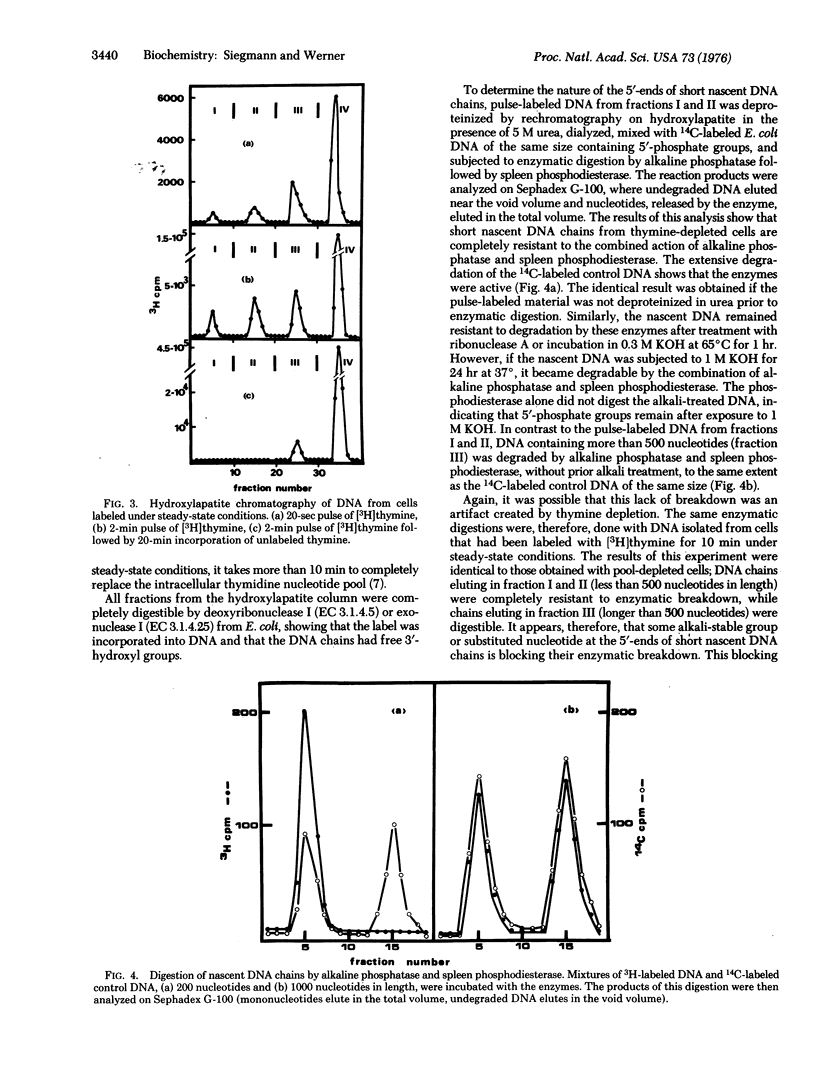
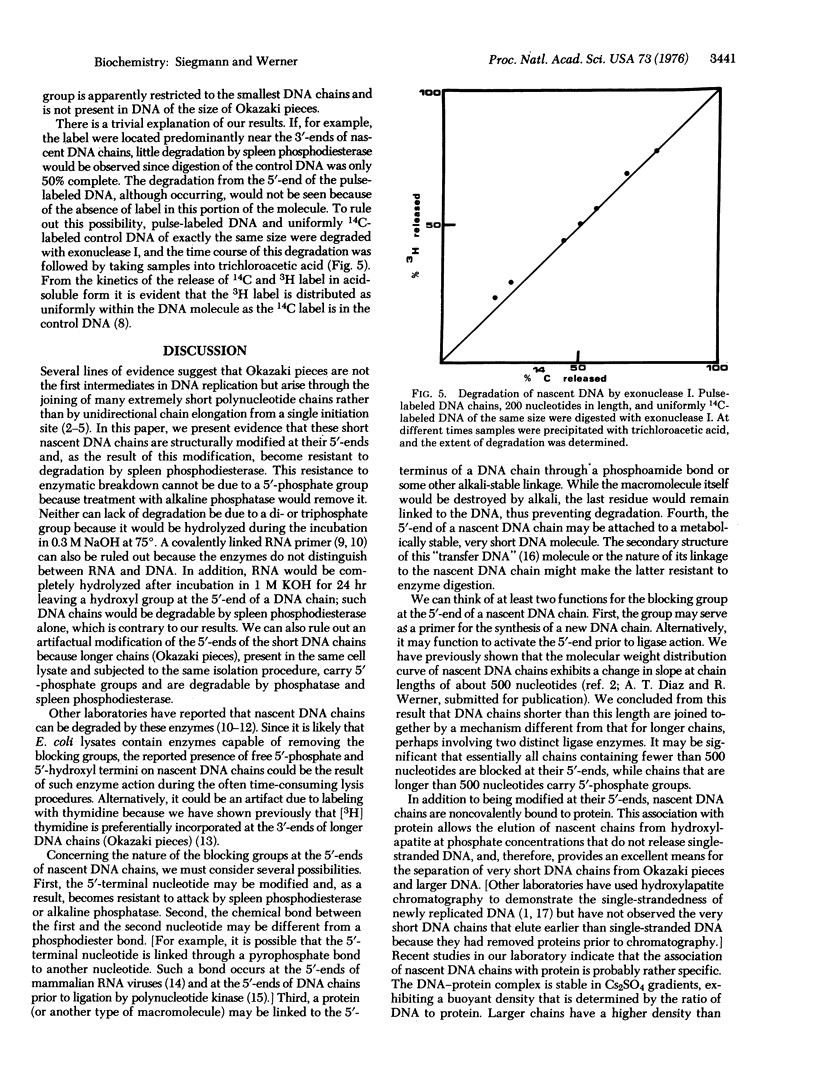
Because of their association with protein short nascent DNA chains in Escherichia coli can be separated from other cellular DNA by chromatography on hydroxylapatite. Protein-free DNA chains of less than 500 nucleotides in length are resistant to degradation from the 5'-end by alkaline phosphatase [orthophosphoric-monoester phosphohydrolase (alkaline optimum); EC 3.1.3.1] and spleen phosphodiesterase (oligonucleate 3'-nucleotidohydrolase; EC 3.1.4.18). In contrast, DNA chains containing more than 500 nucleotides are degradable. From these results we conclude that short nascent DNA chains are structurally modified at their 5'-ends. The nature of this structure and its possible functions are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewin N. [Catalytic role for RNA in DNA replication]. Nat New Biol. 1972 Mar 29;236(65):101–101. doi: 10.1038/newbio236101a0. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Diaz A. T., Werner R. Mechanism of DNA chain growth. J Mol Biol. 1975 Jun 15;95(1):63–70. doi: 10.1016/0022-2836(75)90335-6. [DOI] [PubMed] [Google Scholar]
- Diaz A. T., Wiener D., Werner R. Synthesis of small polynucleotide chains in thymine-depleted bacteria. J Mol Biol. 1975 Jun 15;95(1):45–61. doi: 10.1016/0022-2836(75)90334-4. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Okazaki R., Tamanoi F. Mechanism of DNA chain growth. XI. Structure of RNA-linked DNA fragments of Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):501–517. doi: 10.1016/0022-2836(73)90219-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. K., Lark K. G. DNA replication in Escherichia coli: evidence for two classes of small deoxyribonucleotide chains. J Mol Biol. 1973 Feb 5;73(4):371–396. doi: 10.1016/0022-2836(73)90088-0. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. Novel mutants of Escherichia coli that accumulate very small DNA replicative intermediates. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2150–2154. doi: 10.1073/pnas.72.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth. XV. RNA-linked nascent DNA pieces in Escherichia coli strains assayed with spleen exonuclease. J Mol Biol. 1975 Aug 25;96(4):653–664. doi: 10.1016/0022-2836(75)90144-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Wechsler J. A. DNA replication in dnaB mutants of Escherichia coli: gene product interaction and synthesis of 4 S pieces. J Mol Biol. 1975 Feb 15;92(1):145–163. doi: 10.1016/0022-2836(75)90095-9. [DOI] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Okazaki R. Mechanism of DNA chain growth. IV. Direction of synthesis of T4 short DNA chains as revealed by exonucleolytic degradation. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1242–1248. doi: 10.1073/pnas.64.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Hall Z. W., Anraku Y., Chien J. R., Lehman I. R. On the mechanism of the polynucleotide joining reaction. Cold Spring Harb Symp Quant Biol. 1968;33:27–34. doi: 10.1101/sqb.1968.033.01.008. [DOI] [PubMed] [Google Scholar]
- Wang H. F., Sternglanz R. Thymine-labelled deoxyoligonucleotide involved in DNA chain growth in Bacillus subtilis. Nature. 1974 Mar 8;248(5444):147–150. doi: 10.1038/248147a0. [DOI] [PubMed] [Google Scholar]
- Werner R. Mechanism of DNA replication. Nature. 1971 Apr 30;230(5296):570–572. doi: 10.1038/230570a0. [DOI] [PubMed] [Google Scholar]
- Werner R. Nature of DNA precursors. Nat New Biol. 1971 Sep 22;233(38):99–103. doi: 10.1038/newbio233099a0. [DOI] [PubMed] [Google Scholar]


