Abstract
A 70-year-old male was referred with hyperthyroidism and multinodular goiter (MNG). Thyroid ultrasonography showed 2 nodules, one in the isthmus and the other in the left lobe, 51 and 38 mm in diameter, respectively. Neck CT showed a large MNG, thyroid scintigraphy showed increased uptake in the nodule in the left lobe, and fine-needle aspiration biopsy showed a benign cytology of the nodule in the isthmus. The patient declined surgery and was treated with methimazole. After being lost to follow-up for 3 years, the patient returned with complaints of dyspnea, dysphagia, and hoarseness; he was still hyperthyroid. Cervical CT showed a large mass in the isthmus and left lobe with invasion of surrounding tissues, the trachea, the esophagus, and the recurrent laryngeal nerve. Bronchoscopy showed extensive infiltration and compression of the trachea to 20% of its caliber. A tracheal biopsy revealed an anaplastic thyroid carcinoma. The tumor was considered unresectable, and radiotherapy was given. One month later, the patient died. The association between a toxic thyroid nodule and anaplastic thyroid carcinoma has apparently not been reported so far.
Key Words: Anaplastic thyroid carcinoma, Toxic nodule, Hyperthyroidism
What Is Known about This Topic?
• Hot thyroid nodules have been rarely associated with thyroid malignancy. Recent data suggests a higher prevalence of cancer in toxic multinodular goiter than reported previously.
What Does This Case Report Add?
• A rare association between toxic thyroid nodules and anaplastic thyroid carcinoma. We are not aware of any similar case published in the literature.
Introduction
Thyroid nodules larger than 1 cm should be evaluated with regard to their dimensions and characteristics, functionality, and malignancy. High-resolution ultrasonography (US), sensitive thyrotropin (TSH) assay, and fine-needle aspiration biopsy (FNAB) represent the standard for the management of thyroid nodules. Exceptions to this rule are hot (hyperfunctioning) nodules, which rarely represent clinically significant malignant lesions and therefore do not justify the systematic use of FNAB [1]. The real prevalence of malignancy in hot nodules is very difficult to assess because the published data regarding this association are few and mostly limited to case reports or series with small numbers of patients [2,3,4]. Pazaitou-Panayiotou et al. [5] recently reviewed data regarding this association and reported that the probability of a hot nodule being associated with malignancy ranges between 1 and 10.3%. Traditionally, hyperthyroidism has been seen as a protective condition for thyroid cancer, and a relationship between low serum TSH levels and a lower incidence of papillary carcinoma has been suggested [6,7]. However, a recent meta-analysis could not confirm this association [8]. Larger and prospective studies are required to evaluate this issue. Moreover, there are some data reporting unusual toxic thyroid carcinomas [9], and recent studies suggest that the frequency of malignancy in toxic multinodular goiter (TMNG) is significantly higher than usually assumed (3% [10] vs. 18% [11]). Cerci et al. [12] found no significant difference in the incidence of thyroid cancer between TMNG and nontoxic multinodular goiter (MNG). The risk of malignancy in Graves' disease might be higher than in MNG or toxic nodules (TN) [13]. Recent guidelines still recommend that patients with TMNG should only have an FNAB of cold nodules with clinical or US suspicious features [14]. Although TMNG is less common than Graves' disease, its prevalence increases with age and in iodine-deficient areas [15].
Anaplastic thyroid carcinoma (ATC) is a rare disease, responsible for 1.7% of all thyroid malignancies; it most often originates in an abnormal thyroid gland. A history of goiter is reported in more than 80% of cases, and differentiated thyroid carcinomas are well described as preexistent lesions [16]. We present a case with an unusual association of a TN with an ATC.
Case Report
A 70-year-old Caucasian male was referred to our department due to the recent growth of a TMNG. TSH was suppressed (<0.001 mU/l) and thyroid antibodies (TPO-Ab, Tg-Ab, and TSHR-Ab) were absent. Thyroid US showed 2 nodules, one in the isthmus and the other in the left lobe, 51 and 38 mm in diameter, respectively. A neck CT scan showed a large MNG. Thyroid scintigraphy demonstrated increased uptake in the left lobe nodule with suppressed uptake in the surrounding tissue and in the contralateral lobe. FNAB of the isthmus nodule revealed follicular hyperplasia. The patient declined surgery and was treated with methimazole and β-adrenergic blockers.
After 3 years without follow-up, the patient returned with complaints of dyspnea, hoarseness, and dysphagia. Thyroid US showed the 2 nodules; their diameter was 50 and 30 mm, respectively, similar to their previously observed size. The CT scan revealed a large left lobe with new substernal extension and tracheal compression. FNAB of the isthmus nodule was repeated and follicular hyperplasia was confirmed. The patient was still hyperthyroid with negative antibodies and focal uptake in the left lobe (fig. 1). Methimazole was resumed.
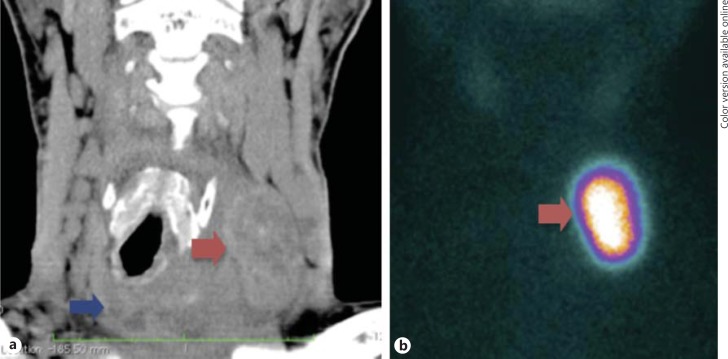
Fig. 1.
a Neck CT scan showing a large mass in the isthmus (blue arrow) and a nodule (red arrow) in the left thyroid lobe, compressing and deviating the trachea to the right. b Iodine-123 thyroid scintigraphy showing uptake in the nodule in the left thyroid lobe [with a similar image on the CT scan (red arrow)].
Three months later, since he had no relief of the compressive symptoms, the patient was referred to an ear-nose-throat specialist who detected vocal cord paralysis and requested a new CT scan. Neck CT was repeated, showing a suspicious mass involving the left lobe and the isthmus, measuring 70 × 60 × 50 mm, with invasion of the surrounding soft tissue, trachea, and recurrent nerve; there were also cervical and mediastinal adenopathies (fig. 2). Thoracoabdominal CT showed no evidence of lung and/or abdominal metastases.
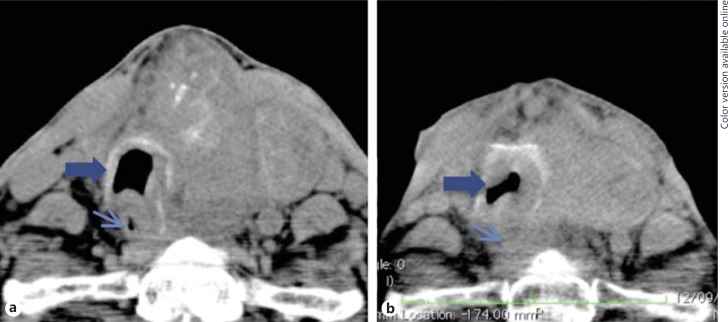
Fig. 2.
Serial neck CT. a CT performed after ENT evaluation (just before the diagnosis of ATC) showing a large mass in the isthmus and left lobe of the thyroid with compression of the trachea (thick arrow) and esophagus (thin arrow). b CT scan after radiotherapy revealing disease progression (mass growth and worsening of the trachea (thick arrow) and esophagus compression (thin arrow)).
The patient was referred to an oncology center where a bronchoscopy was performed, revealing a large tumor infiltration of the trachea (55 mm in extension), with a significant reduction of the lumen. A tracheal stent was placed and a tracheal biopsy revealed squamous cell thyroid cancer (fig. 3). The patient refused an elective tracheostomy, and a percutaneous endoscopic gastrostomy was made because of esophageal compression.
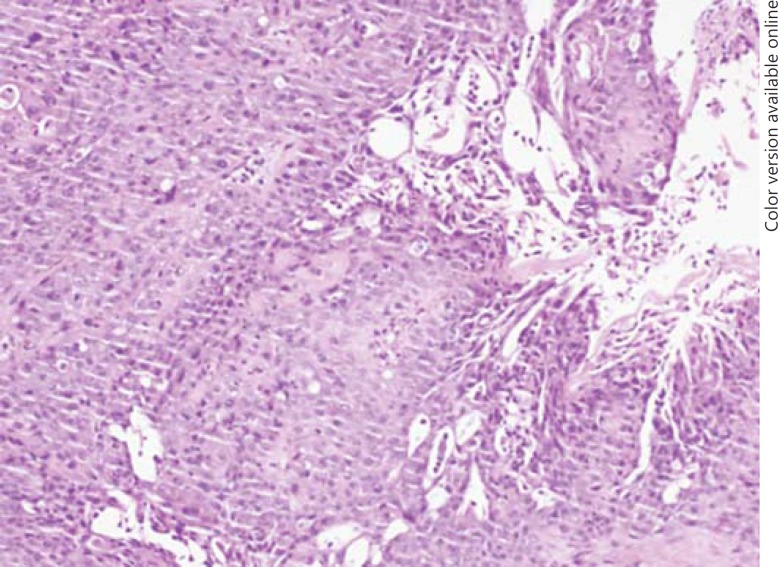
Fig. 3.
Endotracheal biopsy. Histology showing ATC with squamous cell differentiation.
After discussion in a multidisciplinary team, the tumor was considered unresectable, and palliative radiotherapy was started (70 Gy in 30 sessions for 2 months). Until the beginning of radiotherapy, the patient remained hyperthyroid on methimazole. One month after the radiotherapy, the compressive symptoms worsened, and a tracheostomy was done in view of acute airway distress. A neck CT scan did not show any reduction of the tumor or lymph node metastasis (fig. 2). One week after the tracheostomy, the patient died of tracheal compression.
Discussion
The presented case documents an association of thyrotoxicosis with concomitant development of ATC. The patient presented with a TN in an MNG. He had hyperthyroidism, with negative thyroid antibodies and increased uptake in the left lobe nodule on thyroid scintigraphy.
Despite the fact that TN are rarely malignant in patients with MNG, it is important to investigate concomitant cold nodules when they are larger than 1 cm and/or present US suspicious features.
When we first observed this patient, an FNAB of the isthmus ‘cold’ nodule had a benign result. Despite the fact that some cases of carcinomas in TN have been described [9], FNAB of a TN is not routinely recommended. Thyroid cancer causing thyrotoxicosis usually represents differentiated thyroid carcinoma [17], with preserved molecular functions for the production of thyroid hormones.
The patient was managed with methimazole and β-adrenergic blockers. Surgery was suggested given the presence of 2 large nodules (one of them being toxic), but it was refused by the patient. Radioiodine therapy was considered as a definitive alternative treatment approach after controlling the hyperthyroidism; it was not performed because the patient was lost to follow-up. Three years later, he returned with compressive symptoms in the neck. An ATC was diagnosed by endotracheal biopsy. He was still hyperthyroid, with a TN on the scintiscan (fig. 1).
There are few cases in the literature reporting hyperthyroidism in patients with ATC. In most described cases, thyrotoxicosis results from the destruction of normal thyroid follicles by the rapidly infiltrating tumor cells, releasing preformed hormones into the circulation [18,19,20]. Basaria et al. described this condition as anaplastic pseudothyroiditis [21].
Our patient had a TN with a concomitant ATC, an association that to the best of our knowledge has not been described so far. The origin of the ATC in our patient is unclear. It is well described that most (80%) ATC patients have a long history of MNG, and the association between well-differentiated thyroid carcinoma and ATC ranges from 7 to 89% of cases. Many ATC develop as a result of dedifferentiation of preexisting well-differentiated thyroid carcinomas [16], but this does not apply to our patient. Another possibility is ‘de novo’ development of ATC. At the time of the diagnosis of ATC, our patient had an unresectable tumor and the only treatment option considered was radiotherapy. Despite this treatment, the patient had no clinical or radiologic improvement and died from tracheal compression.
Hot thyroid nodules are rarely malignant and therefore FNAB is not routinely recommended. Nevertheless, the presence of concomitant cold nodules (especially if they are clinically suspicious) should prompt further evaluation. ATC are rare and have aggressive behavior. They usually present with sudden symptoms and have a rapid progression and a dismal prognosis, with failure of most treatment options. Although hyperthyroidism is described in some cases of ATC, the concomitant existence of a TN and ATC has not been documented in the literature.
Disclosure Statement
The authors have nothing to declare.
References
- 1.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, Vitti P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16((suppl 1)):1–43. doi: 10.4158/10024.GL. [DOI] [PubMed] [Google Scholar]
- 2.Majima T, Doi K, Komatsu Y, Itoh H, Fukao A, Shigemoto M, Takagi C, Corners J, Mizuta N, Kato R, Nakao K. Papillary thyroid carcinoma without metastases manifesting as an autonomously functioning thyroid nodule. Endocr J. 2005;52:309–316. doi: 10.1507/endocrj.52.309. [DOI] [PubMed] [Google Scholar]
- 3.Uludag M, Yetkin G, Citgez B, Isgor A, Basak T. Autonomously functioning thyroid nodule treated with radioactive iodine and later diagnosed as papillary thyroid cancer. Hormones. 2008;7:175–179. doi: 10.1007/BF03401510. [DOI] [PubMed] [Google Scholar]
- 4.Tfayli HM, Teot LA, Indyk JA, Witchel SF. Papillary thyroid carcinoma in an autonomous hyperfunctioning thyroid nodule: case report and review of the literature. Thyroid. 2010;20:1029–1032. doi: 10.1089/thy.2010.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazaitou-Panayiotou K, Michalakis K, Paschke R. Thyroid cancer in patients with hyperthyroidism. Horm Metab Res. 2012;44:255–262. doi: 10.1055/s-0031-1299741. [DOI] [PubMed] [Google Scholar]
- 6.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A, Vitti P. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251–1260. doi: 10.1677/ERC-09-0036. [DOI] [PubMed] [Google Scholar]
- 7.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 8.Negro R, Valcavi R, Toulis KA. Incidental thyroid cancer in toxic and nontoxic goiter: is TSH associated with malignancy rate? Results of a meta-analysis. Endocr Pract. 2013;19:212–218. doi: 10.4158/EP12234.OR. [DOI] [PubMed] [Google Scholar]
- 9.Als C, Gedeon P, Rosler H, Minder C, Netzer P, Laissue JA. Survival analysis of 19 patients with toxic thyroid carcinoma. J Clin Endocrinol Metab. 2002;87:4122–4127. doi: 10.1210/jc.2001-011147. [DOI] [PubMed] [Google Scholar]
- 10.Kang AS, Grant CS, Thompson GB, van Heerden JA. Current treatment of nodular goiter with hyperthyroidism (Plummer's disease): surgery versus radioiodine. discussion 923, Surgery. 2002;132:916–923. doi: 10.1067/msy.2002.128691. [DOI] [PubMed] [Google Scholar]
- 11.Smith JJ, Chen X, Schneider DF, Nookala R, Broome JT, Sippel RS, Chen H, Solorzano CC. Toxic nodular goiter and cancer: a compelling case for thyroidectomy. Ann Surg Oncol. 2013;20:1336–1340. doi: 10.1245/s10434-012-2725-4. [DOI] [PubMed] [Google Scholar]
- 12.Cerci C, Cerci SS, Eroglu E, Dede M, Kapucuoglu N, Yildiz M, Bulbul M. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53:157–160. doi: 10.4103/0022-3859.33855. [DOI] [PubMed] [Google Scholar]
- 13.Cappelli C, Braga M, De Martino E, Castellano M, Gandossi E, Agosti B, Cumetti D, Pirola I, Mattanza C, Cherubini L, Rosei EA. Outcome of patients surgically treated for various forms of hyperthyroidism with differentiated thyroid cancer: experience at an endocrine center in Italy. Surg Today. 2006;36:125–130. doi: 10.1007/s00595-005-3115-3. [DOI] [PubMed] [Google Scholar]
- 14.Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, Ross DS, Sosa JA, Stan MN. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 15.Laurberg P, Pedersen KM, Vestergaard H, Sigurdsson G. High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area versus high incidence of Graves' disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland. J Intern Med. 1991;229:415–420. doi: 10.1111/j.1365-2796.1991.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 16.Aldinger KA, Samaan NA, Ibanez M, Hill CS., Jr Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41:2267–2275. doi: 10.1002/1097-0142(197806)41:6<2267::aid-cncr2820410627>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Paul SJ, Sisson JC. Thyrotoxicosis caused by thyroid cancer. Endocrinol Metab Clin North Am. 1990;19:593–612. [PubMed] [Google Scholar]
- 18.Alagol F, Tanakol R, Boztepe H, Kapran Y, Terzioglu T, Dizdaroglu F. Anaplastic thyroid cancer with transient thyrotoxicosis: case report and literature review. Thyroid. 1999;9:1029–1032. doi: 10.1089/thy.1999.9.1029. [DOI] [PubMed] [Google Scholar]
- 19.Heymann RS, Brent GA, Hershman JM. Anaplastic thyroid carcinoma with thyrotoxicosis and hypoparathyroidism. Endocr Pract. 2005;11:281–284. doi: 10.4158/EP.11.4.281. [DOI] [PubMed] [Google Scholar]
- 20.Villa ML, Mukherjee JJ, Tran NQ, Cheah WK, Howe HS, Lee KO. Anaplastic thyroid carcinoma with destructive thyrotoxicosis in a patient with preexisting multinodular goiter. Thyroid. 2004;14:227–230. doi: 10.1089/105072504773297902. [DOI] [PubMed] [Google Scholar]
- 21.Basaria S, Udelsman R, Tejedor-Sojo J, Westra WH, Krasner AS. Anaplastic pseudothyroiditis. Clin Endocrinol (Oxf) 2002;56:553–555. doi: 10.1046/j.1365-2265.2002.01495.x. [DOI] [PubMed] [Google Scholar]