Abstract
Background & objectives:
Chronic pancreatitis is progressive and irreversible destruction of the pancreas. Matrix metalloproteinase-7 (MMP-7) is a secreted matrilysin, which contributes to angiogenesis and breakdown of basement membranes of pancreatic tissues. The present study was aimed to investigate the association of MMP-7 −181A/G (rs11568818) gene promoter polymorphism in patients with chronic pancreatitis.
Methods:
A total of 100 chronic pancreatitis patients and 150 unrelated healthy individuals were included in this case control study. The genotyping of the MMP-7 gene (− 181 A/G) (rs11568818) was carried out based on PCR-RFLP. The serum levels of MMP-7 were determined by ELISA. Association between genotypes and chronic pancreatitis was examined by odds ratio (OR) with 95% confidence interval (CI).
Results:
The frequencies of the genotypes in promoter of MMP-7 were AA 49 per cent, AG 25 per cent and GG 26 per cent in chronic pancreatitis patients and AA 53 per cent, AG 38 per cent and GG 9 per cent in control subjects. Frequency of MMP-7 −181GG genotype and − 181G allele was significantly associated with chronic pancreatitis compared to healthy subjects [OR = 1.58 (95% CI: 1.06 –2.36), P =0.019]. There was no significant difference in the serum MMP-7 levels in the patients compared to control subjects.
Interpretation &conclusions:
The present study revealed a significant association of MMP-7 -181A/G (rs11568818) GG genotype with chronic pancreatitis patients, indicating its possible association with the disease.
Keywords: Chronic pancreatitis, genetic susceptibility, genotypes, inflammation, matrix metalloproteinase, myofibroblasts
Chronic pancreatitis (CP), an inflammatory disorder of the pancreas like acute pancreatitis, occurs when digestive enzymes attack the pancreas and nearby tissues, and is clinically characterized by recurrent attacks of abdominal or back pain, pancreatic stone formation, exocrine and endocrine pancreatic insufficiency in advanced stages1. Matrix metalloproteinases (MMPs) form an important family of metal-dependent endopeptidases that represent the major class of enzymes responsible for degradation of extracellular matrix components2. MMPs are classified as five main classes: collagenases, gelatinases, stromelysins, membrane-type and others, on the basis of their putative substrate specificity and internal homologies3. Matrix metalloproteinase-7 (MMP-7) (matrilysin, pump-1 protease or PUMP-1), is among the smallest members of the MMP family. It is a protease with broad substrate specificity, being able to degrade elastin, proteoglycans, fibronectin and type IV collagen2,4. In addition, MMP-7 can cleave non-matrix substrates from the cell surface, including E-cadherin, pro-tumour necrosis factor α, and Fas ligand, which is called ‘sheddase’ function in apoptosis5,6,7.
There are at least three regulatory mechanisms that influence activities of MMPs: regulation of transcription, activation of latent MMPs and inhibition of MMP function by tissue inhibitors of metalloproteinases. However, the most important step may be transcriptional regulation, since most MMP genes express only when active physiological or pathological tissue remodelling takes place. Growing evidence indicates that natural sequence variations in promoter regions of the MMP genes may result in variable expression of MMPs8. These polymorphisms have been associated with susceptibility to various diseases including acute myocardial infarction9 rheumatoid arthritis10 multiple sclerosis11 and cancers12,13,14. A single nucleotide polymorphism (SNP) in the promoter region of the MMP-7 gene, especially an A to G transition at the -181 base pair (-181 A/G) (rs11568818) has been proved to be functional in vitro and may influence coronary artery dimensions15. The present study was aimed at understanding the role of the biallelic polymorphism in the -181 promoter region of the MMP-7 gene in the pathophysiology of chronic pancreatitis.
Material & Methods
Subjects: A total of 100 clinically evaluated consecutive chronic pancreatitis patients referred to the Gastroenterology unit of Gandhi Hospital and Osmania General Hospital, Hyderabad, India from January 2010 to February 2012 were included in the study, with the approval of protocol from the Institutional Ethics Committee. The patients were confirmed for chronic pancreatitis based on the clinical diagnosis, biochemical findings and imaging analysis. A total of 150 asymptomatic control subjects were randomly selected among the individuals visiting the Institute of Genetics and Hospital for Genetic Diseases, Hyderabad, for regular health check-up who had no complaints or evidence of pancreatitis or any other gastric problem. Informed written consent was obtained from all the subjects. The demographic characteristics such as sex, age, duration, addictions like smoking, and alcohol consumption were noted based on the standard proforma. Subjects who were consuming 80 g alcohol/day for a period of more than two years were considered as alcoholics16,17.
DNA extraction: Venous blood (5 ml) was drawn from each individual in vacutainers containing EDTA and stored at 4°C. Genomic DNA was extracted from the peripheral blood samples by following the salting out procedure of Lahiri et al18, and stored in TE buffer at -20°C until further use.
MMP-7 SNP genotyping: The MMP-7-181A/G (rs11568818) genotypes were determined as previously described by Zhang et al19 by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) assay19. The primers for amplifying the MMP-7 fragment were 5’-TGG TAC CAT AAT GTC CTG AAT G-3’ (forward) and 5’-TCG TTA TTG GCA GGA AGC ACA CAA TGA ATT-3’ (Reverse) [Bioserve, India]. The fourth nucleotide close to the 3’ end of the reverse primer was mutated from T to A to create an EcoRI recognition site when the -181G allele exists. The PCR was performed in a 25 μl volume containing 100 ng DNA template, 2.0 ml of 10x PCR buffer, 1.5 mM MgCl2, 1 U Taq-DNA polymerase (Bangalore Gene, India), 200 mM dNTPs and 200 nM each primer. The PCR cycling conditions were: 5 min at 94°C followed by 35 cycles of 30 sec at 94°C, 30 sec at 65°C and 30 sec at 72°C, and with a final step at 72°C for 5 min to allow for the complete extension of all PCR fragments. An 8-μl aliquot of PCR product was subjected to digestion at 37°C overnight in a 10 μl reaction containing 10 U EcoRI (Fermentas, USA) and 1x reaction buffer. After digestion, the products were separated on a 3 per cent agarose gel stained with ethidium bromide (Figure). As a result, the -181G alleles were represented by DNA fragments of size at 120 and 30 bp, the -181A alleles size of 150 bp, whereas the heterozygotes displayed a combination of both alleles of size (150, 120 and 30 bp). Ten per cent of the samples were randomly taken, and the assay was repeated and found no bias in the genotyping. The findings were similar on replicative study with the results being 100 per cent concordant.
Fig.
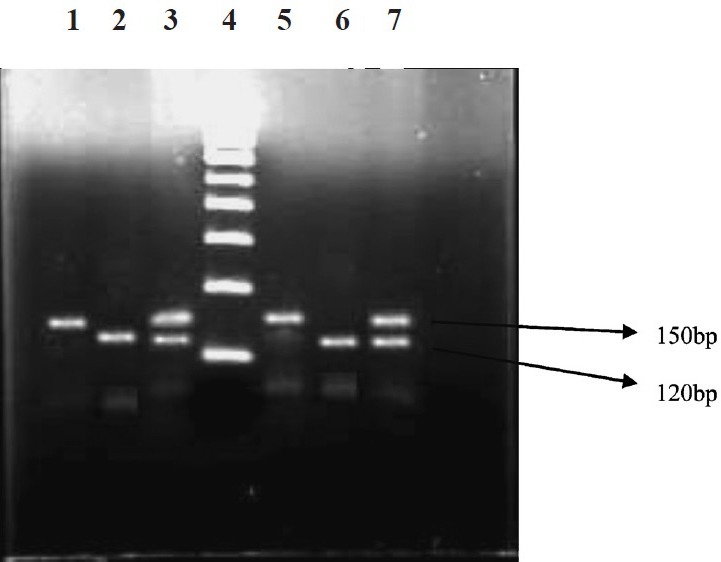
RFLP products of MMP-7 (-181 A/G) genotypes. Lanes 1&5-homozygous A/A (150 bp); lanes 2&6-homozygous G/G (120 bp); lanes 3&7-heterozygous A/G (150 bp/120 bp); lane 4- 100 bp ladder.
MMP-7 ELISA: Quantitative sandwich enzyme immunoassay for human MMP7 was performed as recommended by the manufacturer (Raybiotech®, Inc, Norcross, GA, USA). In brief, 100 μl of each standard and sample were added into the wells and incubated at room temperature for 2 h. After washing four times with washing buffer, the biotinylated antibody was added, and the plate was further incubated for 1 h; after two washings 100 μl of prepared streptavidin solution was added to each well and incubated for 45 min at room temperature with gentle shaking. The solution was discarded and washed properly using wash buffer, and 100 μl of tetra-methylbenzidine (TMB) one step substrate reagent was added to each well, and incubated for 30 min at room temperature in dark with gentle shaking. The absorbance was read at 450 nm immediately. The MMP-7 concentrations for each sample were calculated from the standard curve obtained.
Statistical analysis: Hardy-Weinberg analysis was performed to compare the observed and expected genotype frequencies using χ2 test. Comparison of the MMP-7 genotype distribution in the study groups was performed by means of two-sided contingency tables using χ2 test. MMP-7 levels were compared using student t- test and ANOVA. The odds ratio (OR) and 95% confidence interval (CI) were calculated using an unconditional logistic regression.
Results
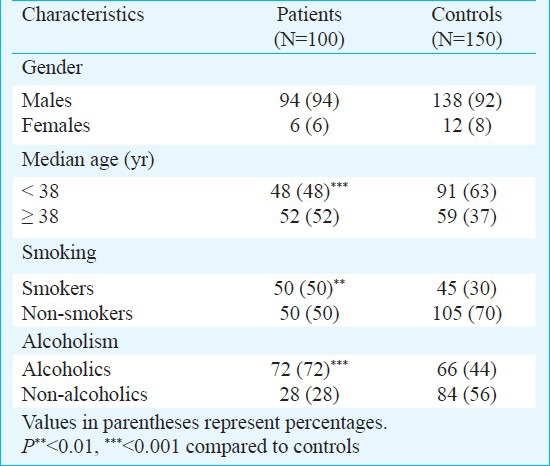
The demographic data of the samples under study are presented in Table I. The gender-wise distribution of patients and control subjects revealed no significant difference. Age (median)-wise comparison showed significant (P<0.001) difference in patients compared to control subjects. The proportion of smokers in CP patients (50%) was significantly higher than the healthy controls (30%) [OR = 2.333 (1.335-4.085), P=0.002]. Similarly, the frequency of alcoholics in chronic pancreatitis patients was significantly different from that in healthy controls [OR = 3.273 (2.839-5.849) P<0.001].
Table I.
Demographic and clinical data of chronic pancreatitis patients and control subjects
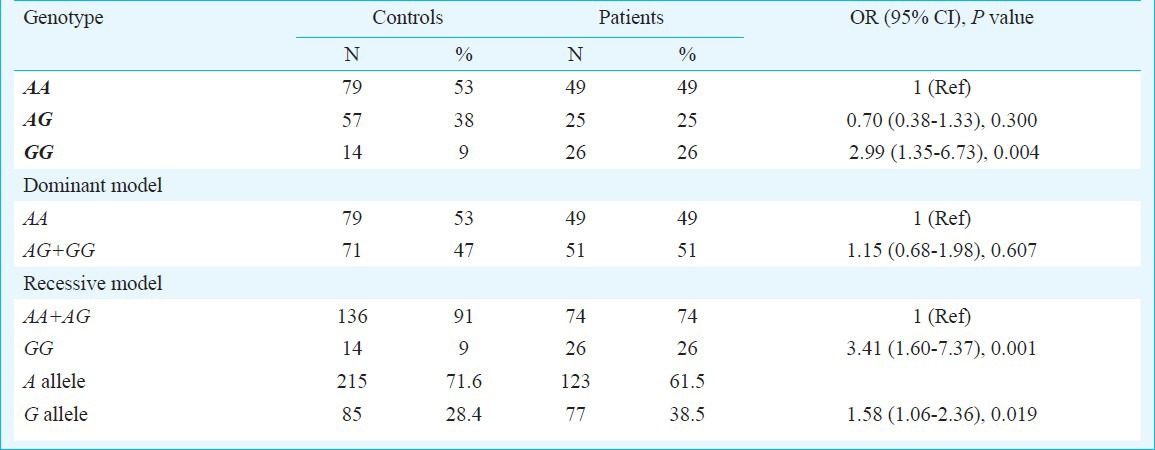
Genotype analysis: The frequencies of A/A, A/G and G/G genotypes in CP were 49, 25 and 26 per cent compared to 53, 38 and 9 per cent in the control subjects, respectively (Table II). The MMP-7 (-181 A/G) GG genotype frequency was higher in patients as compared to control group. When the MMP-7 (181A>G) AA genotype was used as the reference, it was found that CP patients with MMP-7 (181AG) GG genotype were significantly associated with the risk for the disease (OR=2.99, 95% CI= (1.35-6.73), P=0.004). Moreover, in recessive model (OR=3.41, 95% CI=(1.60-7.37), P=0.001), the results showed that MMP-7 (-181AG) GG allele was conferring significant increased risk for CP. Further, the frequency of the -181G allele in chronic pancreatitis patients was significantly higher than that in healthy controls [OR=1.58 (95% CI: 1.06–2.36), P=0.019]. The Hapmap GIH frequency of MMP7 (-181A/G) was found to be 37.5 per cent as per International HAP Map consortium20 and the present study revealed 28.5 per cent, which could be due to the ethnic variation within different regions of Indian population. The MMP-7 gene frequency in chronic pancreatitis patients was not found to be in Hardy Weinberg equilibrium at 5 per cent level of significance. The gene frequency of control subjects was in Hardy Weinberg equilibrium at 5 per cent level of significance.
Table II.
Frequency distribution of genotypes and of MMP-7 (-181 A/G) genotypes with risk of chronic pancreatitis
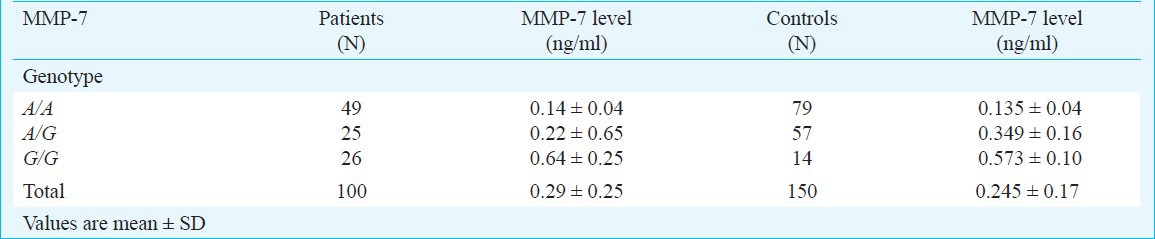
MMP-7 serum levels: Table III gives the MMP-7 levels with respect to MMP-7 genotype in controls and chronic pancreatitis patients. In general MMP-7 levels were found to be increased in individuals with G/G genotype when compared to A/G and A/A genotypes though the difference was not significant. There was no significant difference between the mean levels of MMP-7 between chronic pancreatitis (0.29 ± 0.25 ng/ml) and controls (0.245 ± 0.17 ng/ml) [P=0.344].
Table III.
Mean serum levels (ng/ml) of MMP-7 in chronic pancreatitis patients and control subjects with respect to the genotypes
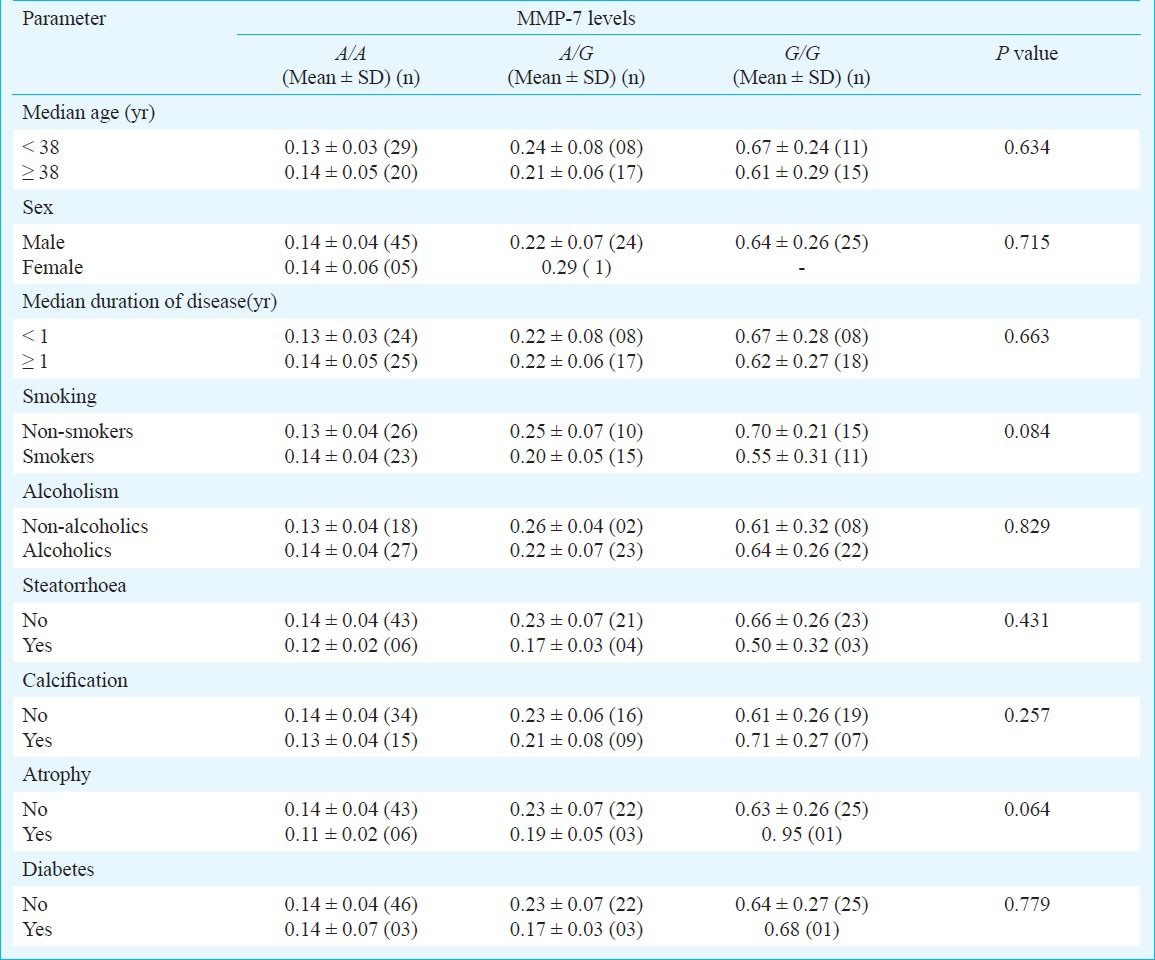
Association of MMP-7 (181A>G) genotypes and serum levels with demographic and clinical characteristics of CP: Potential associations were explored between MMP-7 genotypes and demographic and clinical characteristics such as median age, median duration of the disease, addictions like smoking and alcoholism, steatorrhoea, calcification, and atrophy (Table IV). No significant association of the demographic and clinical features was found in the present study.
Table IV.
Intragroup comparison of MMP-7 genotypes and levels with respect to demographic and clinical parameters of chronic pancreatitis
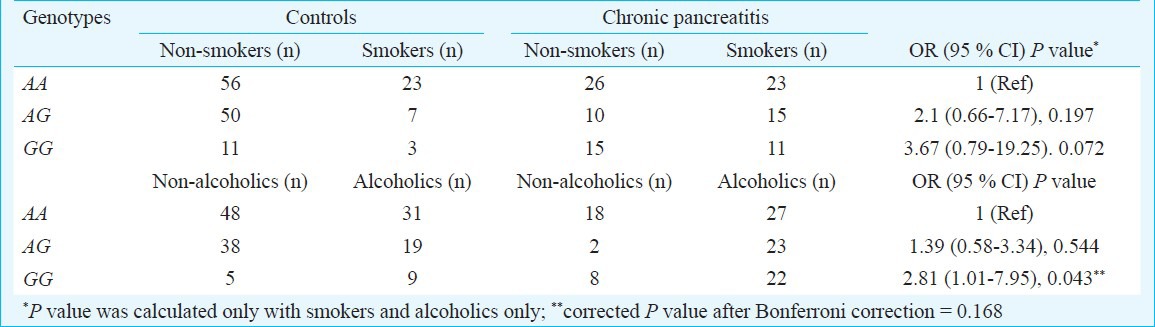
Interaction of MMP-7 (181A>G) genotypes with environmental factors: On analyzing gene environment interaction, a significant modulation of chronic pancreatitis by MMP-7 (181A>G) GG genotype was found with alcoholism, but significance was lost after Bonferroni correction (Pc = 0.168) (Table V).
Table V.
Interaction of MMP-7 (-181A>G) genotypes with smoking and alcoholism
Discussion
Chronic pancreatitis is the progressive and permanent destruction of the pancreas resulting in exocrine and endocrine insufficiency and, often, with chronic disabling pain. Males are about three to four times more likely to be affected than females21. MMP-7 is the smallest known member of MMP family and possesses the highest extracellular matrix (ECM) degrading activity against a variety of ECM components, including elastin, gelatin, type IV collagen, fibronectin, vitronectin, laminin, entactin, aggrecan and proteoglycans21,22. It is also capable of triggering the activation of an MMP cascade23.
Previous studies in chronic pancreatitis highlighted the role of MMP-7 based on expression data and immunohistochemical analyses23. MMP-7 is expressed exclusively in the metaplastic ductal epithelium of chronic pancreatitis patients and has been shown to regulate acinar cell apoptosis through proteolytic release of its pro-apoptotic molecule Fas ligand24. Sires et al25 have reported that MMP-7 efficiently cleaves the basement membrane protein entactin, which bridges laminin and collagen type 4, and suggested a potentially important role for MMP-7 in the disruption of basement membranes by inflammatory cells.
The initial event in chronic pancreatitis is damage to one type or all tissues, compartments or cell types of the pancreas, leading to cell necrosis and/or apoptosis and subsequent release of cytokines/growth factors like tumour growth factor β1 either from migrating inflammatory cells, especially neutrophils, and/or nearby pre-existent epithelial or mesenchymal cells. In a second step, the damaged cells are phagocytosed by neutrophils and macrophages, and the released cytokines cause activation and proliferation of resident fibroblasts/pancreatic stellate cells in the immediate vicinity of the original site of damage and induce them to transform into myofibroblast cells. In the last phase, myofibroblasts produce and deposit ECM, which replaces the inflammatory infiltrate and affects the architecture and function of the surviving pancreatic tissues26,27.
The present study revealed an increased frequency of GG genotype of MMP 7 in chronic pancreatitis patients compared to control subjects, indicating its possible association with the disease. Earlier studies on transient transfection by Jormsjo et al15 revealed that promoter activity of the -181G allele was 2- to 3-fold higher than that of the -181A allele. Similarly, higher risk in alcoholics was further enhanced due to interaction of MMP-7 -181GG genotype. The profibrotic role of MMP7 might be multiple considering its broad substrate specificity that includes basement membrane and extracellular matrix components. MMP-7 is required for efficient repair of damaged epithelium, and controls the transepithelial influx of neutrophils across the colonic mucosa in response to injury28. Re-epithelialization is a desired outcome of any form of injury in any tissue. In contrast, although neutrophils are an essential arm of innate immunity, an overabundance of these granulocytes can cause indiscriminate, severe, and potentially mortal damage. Reports on liver cirrhosis showed that MMP-7 was released from the bile ductular epithelial cells to the lumen during the tissue repair29. Thus, the presence of -181G allele in chronic pancreatitis may lead to an elevated expression of MMP-7, which results in pancreatic tissue damage by increased neutrophil influx as well as increased activation of other members of the MMP family such as MMP-230.
In conclusion, our results suggest a possible association of MMP-7(-181A/G) (rs11568818) gene polymorphism in the pathophysiology of chronic pancreatitis. However, this is a preliminary study and the results need to be confirmed in a large cohort.
References
- 1.Shidhu SS, Tandon RK. The pathogenesis of chronic pancreatitis. Postgrad Med J. 1995;71:67–70. doi: 10.1136/pgmj.71.832.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ke P, Wu ZD, Wen HS, Ying MX, Long HC, Qing LG. Current evidence on associations between the MMP-7 (-181A>G) polymorphism and digestive system cancer risk. Asian Pac J Cancer Prev. 2013;14:2269–72. doi: 10.7314/apjcp.2013.14.4.2269. [DOI] [PubMed] [Google Scholar]
- 3.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–6. [PubMed] [Google Scholar]
- 4.Quantin B, Murphy G, Breathnach R. Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry. 1989;28:5327–34. doi: 10.1021/bi00439a004. [DOI] [PubMed] [Google Scholar]
- 5.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–50. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–8. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9:1441–7. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–7. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nojiri T, Morita H, Imai Y, Maemura K, Ohno M, Ogasawara K, et al. Genetic variations of matrix metalloproteinase-1 and -3 promoter regions and their associations with susceptibility to myocardial infarction in Japanese. Int J Cardiol. 2003;92:181–6. doi: 10.1016/s0167-5273(03)00100-1. [DOI] [PubMed] [Google Scholar]
- 10.Mattey DL, Nixon NB, Dawes PT, Ollier WE, Hajeer AH. Association of matrix metalloproteinase 3 promoter genotype with disease outcome in rheumatoid arthritis. Genes Immun. 2004;5:147–9. doi: 10.1038/sj.gene.6364050. [DOI] [PubMed] [Google Scholar]
- 11.Fiotti N, Zivadinov R, Altamura N, Nasuelli D, Bratina A, Tommasi MA, et al. MMP-9 microsatellite polymorphism and multiple sclerosis. J Neuroimmunol. 2004;152:147–53. doi: 10.1016/j.jneuroim.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Fang S, Jin X, Wang R, Li Y, Guo W, Wang N, et al. Polymorphisms in the MMP1 and MMP3 promoter and non-small cell lung carcinoma in North China. Carcinogenesis. 2004;26:481–6. doi: 10.1093/carcin/bgh327. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Jin X, Fang S, Li Y, Wang R, Guo W, et al. The functional SNP in the matrix metalloproteinase-3 promoter modifies susceptibility and lymphatic metastases in esophageal squamous cell carcinoma but not in gastric cardiac adenocarcinoma. Carcinogenesis. 2004;25:2519–24. doi: 10.1093/carcin/bgh269. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Pan K, Xing D, Liang G, Tan W, Zhang L, et al. Correlation between a single nucleotide polymorphism in the matrix metalloproteinase-2 promoter and risk of lung cancer. Cancer Res. 2002;62:6430–3. [PubMed] [Google Scholar]
- 15.Jormsjö S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P. Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2001;21:1834–9. doi: 10.1161/hq1101.098229. [DOI] [PubMed] [Google Scholar]
- 16.Peterson K. Biomarkers for alcohol use and abuse-a summary. Alcohol Res Health 2004. 2005;28:30–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Chandak GR, Idris MM, Reddy DN, Mani KR, Bhaskar S, Rao GV, et al. Absence of PRSS1 mutations and association of SPINK1 trypsin inhibitor mutations in hereditary and non-hereditary chronic pancreatitis. Gut. 2004;53:723–8. doi: 10.1136/gut.2003.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahiri DK, Nurnberger JI. A rapid non-enzymatic method for the preparation of HMW DNA from blood. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Jin X, Fang S, Wang R, Li Y, Wang N, et al. The functional polymorphism in the matrix metalloproteinase-7 promoter increases susceptibility to esophageal squamous cell carcinoma, gastric cardiac adenocarcinoma and non-small cell lung carcinoma. Carcinogenesis. 2005;26:1748–53. doi: 10.1093/carcin/bgi144. [DOI] [PubMed] [Google Scholar]
- 20.International HAP Map Consortium. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–8. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klöppel G, Mallet B. Chronic pancreatitis: evolution of the disease. Hepatogastroenterology. 1991;38:408–12. [PubMed] [Google Scholar]
- 22.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 23.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase 7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–44. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sires UI, Griffin GL, Broekelmann TJ, Mecham RP, Murphy G, Chung AE, et al. Degradation of entactin by matrix metalloproteinases. Susceptibility to matrilysin and identification of cleavage sites. J Biol Chem. 1993;25:2069–74. [PubMed] [Google Scholar]
- 26.von Bredow DC, Cress AE, Howard EW, Bowden GT, Nagle RB. Activation of gelatinase-tissue-inhibitors-of-metalloproteinase complexes by matrilysin. Biochem J. 1998;331:965–72. doi: 10.1042/bj3310965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klöppel G, Detlefsen S, Feyerabend B. Fibrosis of the pancreas: the initial tissue damage and the resulting pattern. Virchows Arch. 2004;445:1–8. doi: 10.1007/s00428-004-1021-5. [DOI] [PubMed] [Google Scholar]
- 28.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, et al. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–31. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–12. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barillé S, Bataille R, Rapp MJ, Harousseau JL, Amiot M. Production of metalloproteinase-7 (matrilysin) by human myeloma cells and its potential involvement in metalloproteinase-2 activation. J Immunol. 1999;163:5723–8. [PubMed] [Google Scholar]


