Abstract
Background & objectives:
Clinical spectrum of most of the diseases in developing countries is different from the west. Similarly whether renal cell carcinomas (RCC) in a developing country like India is seen in the same spectrum in relation to the age at presentation as in the west is not described in the literature. This study was carried out to investigate the spectrum of RCC in India with regards to age of onset, stage at presentation and survival.
Methods:
Patients with renal tumour, treated between January 2000 to December 2012 in a tertiary care hospital in north India, were analyzed for age at presentation, clinical features and histopathological characteristics. Clinical diagnosis was made by contrast enhanced computerized tomography (CECT) scans and/or magnetic resonance imaging (MRI). Renal masses diagnosed as angiomyolipoma, infective masses and hydatid cysts were excluded from the analysis. Impact of various age groups on gender, tumour size, TNM stage, Fuhrman grade, histopathological subtypes, lymph node, inferior vena cava (IVC) involvement and survival was analyzed. Patients were grouped in five age groups i.e. ≤39, 40-49, 50-59, 60-69 and more than 70 yr of age.
Results:
Of the total 617 patients with 617 renal tumours (2 patients had bilateral tumours but only the larger tumour was considered) clinically suspected as RCC, 586 had epithelial cell tumour and the remaining 31 had non epithelial cell tumour. The mean tumour size was 8.08±3.5 cm (median 7, range 1-25 cm). Tumour of less than 4 cm size was present in only 10.4 per cent patients. The mean age at diagnosis was 55.15±13.34 (median 56, range 14-91 yr) years. A total of 30.03 per cent of renal tumours presented in patients younger than 50 yr of age. Though there was no difference in stage, Fuhrman's grade, IVC involvement and lymph nodal spread among various age groups, younger patients had higher proportion of non clear cell RCC and only 48.59 per cent of them presented with conventional RCC. Mean survival was lower in patients younger than 39 yr with HR of 1.7 (0.8-3.2).
Interpretation & conclusion:
Our results showed that renal cell carcinoma was more frequent in younger people in India. One third of the patients were less than 50 yr of age and only 10.4 per cent patients had tumour of less than 4 cm (T1a). Younger patients of <39 yr of age had relatively lower survival rates.
Keywords: Age at presentation, clear cell, renal cell carcinoma, survival, tumour
Renal cell carcinoma (RCC) accounts for 3 per cent of all adult cancer and 85 per cent of all kidney tumours1. RCC is primarily a disease of elderly and typically presents in the sixth and seventh decades of life2,3. Incidence rates are race dependent and have been reported as 10 to 20 per cent higher in African Americans for unknown reasons3. Data from Indian population on age at presentation and its relation with various histopathological characteristics are lacking. Clinical spectrum of most of the diseases in developing countries is different from the west. This study was aimed at analyzing the clinico-pathological characteristics of RCC and survival of patients in relation to the age at presentation in India.
Material & Methods
From January 2000 to December 2012, patients with renal masses treated at the Sanjay Gandhi Post-graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India, were analyzed. Data were collected from the hospital information system (HIS) and oncology data registry cell in the department of Urology. All the patients had either contrast enhanced computerized tomography (CECT) scans and/or magnetic resonance imaging (MRI) to characterize the renal tumour. Renal masses diagnosed as angiomyolipoma, infective masses and hydatid cysts were excluded from the analysis. To see the association of the disease at presentation and its clinical and pathological characteristics with age, patients were divided into five age groups as ≤39, 40-49, 50-59, 60-69 and more than 70 years. Variables studied were gender, tumour size, TNM stage, Fuhrman grade, histopathological subtypes, lymph node involvement and inferior vena cava (IVC) thrombus.
Pathological tumour staging and grading were done according to an updated staging system by the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC)4,5. T1 was considered as tumours of <7 cm of size, T2 of > 7cm, T3 included tumours involving perinephric fat, renal vein or IVC thrombus, T4 included Gerota's fascia involvement or contiguous involvement of the adrenal gland and metastatic RCC included non contiguous adrenal metastasis, extra regional lymph node or soft tissue disease. Owing to the shift in treatment of RCC towards partial nephrectomy for tumours of < 4 cm, T1 tumours were further classified as T1a for tumours of <4 cm and T1b for tumours of 4-7 cm size. Histological subtypes were divided as per the classification of World Health Organization6.
All the age groups were compared for clinical and histopathological features. Frequencies and proportions were evaluated for categorical variables by chi-square test. A Cox proportional hazard analysis was also performed, including relative risk and confidence intervals (CI). Finally, the Cox proportional risk regression model was fitted to data to estimate the independent prognostic importance of survival. Twenty four patients, who were lost to follow up, were excluded from the analysis. Also 11 patients who died in immediate post-operative period (8) and due to other causes (5) in follow up were excluded from the analysis. Cancer specific survival (CSS) analysis was done only in 551 patients. The end point of interest was survival time, defined as the time from treatment initiation to the death or the last follow up date. The relationship between CSS and each of the variables was analyzed using the log-rank test for categorical variables. Survival analysis was done by using Kaplan-Meier curve.
All P values were two-sided, and P<0.05 was considered significant. The analysis was done using the Statistical Package for the Social Sciences version 17 (SPSS Inc, Chicago, IL, USA).
Results
Of the total 617 patients with 617 renal tumours (2 patients had bilateral tumours but only the larger tumour was considered) which were clinically suspected as RCC, 586 had epithelial cell tumour. The remaining 31 patients had non epithelial cell tumour. Patients and tumour characteristics are described in Tables I and II.
Table I.
Age distribution and frequency of renal tumour at presentation (N=617)
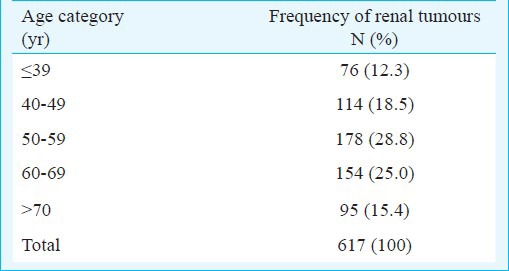
Table II.
Histopathological findings of clinically diagnosed renal tumour (N=617)
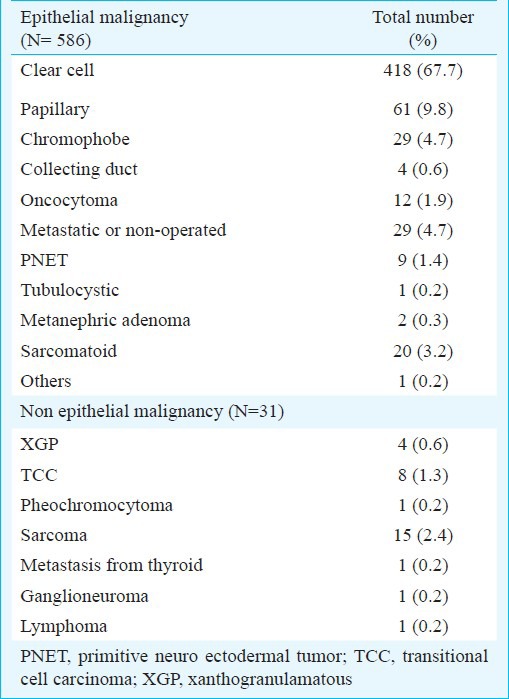
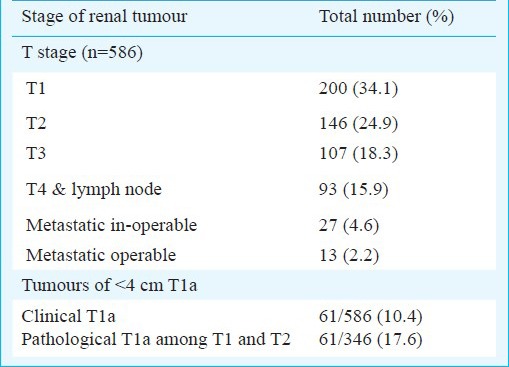
The mean size of the tumours was 8.08 ± 3.5 cm (median 7, range 1-25 cm). The mean age at diagnosis was 55.15±13.34 (median 56, range 14-91) years. The percentage distribution of renal tumours in various age groups was 12.3, 18.5, 28.8, 25.0 and 15.4 per cent, respectively. A total of 190 (30.03%) patients presented at age younger than 50 yr with renal tumours (Table I). The disease was organ confined (pT1 and pT2) in 346 (59%) of the patients and among those, pT1a (tumour <4 cm) was present in only 61/346 (17.6%), but clinically the percentage of T1a 61/586 (10.4%) was low (Table IV). Male preponderance was seen in patients of all age groups.
Table IV.
Distribution of stage of RCC at presentation (N= 586)
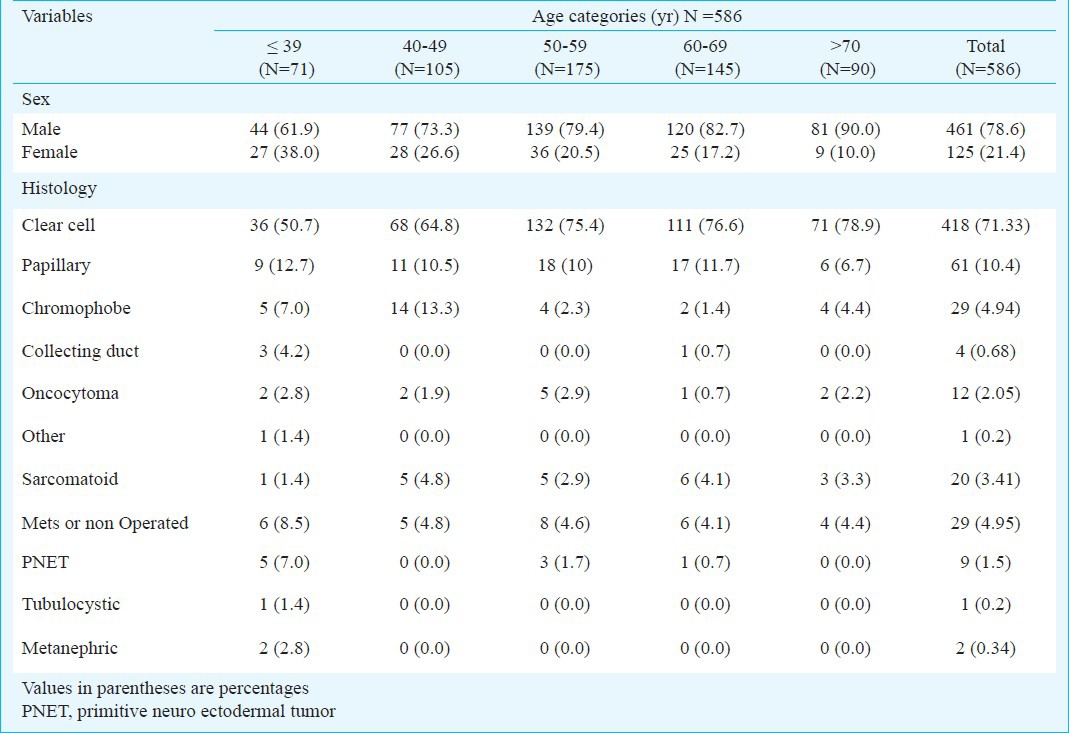
There was no difference in clinical stage of the tumour, tumour size, tumour grade, presence of lymph nodes and IVC thrombus in different age groups. The relationship between age categories and histopathological subtypes are described in Table III. Apart from the sex ratio, histological subtypes were significantly different among the age categories. Overall, the proportion of patients presenting with clear cell carcinoma was 71.33 per cent. This proportion was even less (48.59%) in patients younger than 50 yr of age.
Table III.
Relationship between age categories and histopathological subtypes of renal cell carcinoma (RCC)
Mean follow up was 27.8±21.2 (range 6-121) months. Though age per se did not affect the survival, the mean value of cancer specific survival in younger patients of <39 yr was lower than the older patients of >70 yr, Hazard ratio (HR) of 1.7 (0.8-3.2). (Table V, Figure) Five year cancer specific survival rates in patients of ≤39, 40-49, 50-59, 60-69 and more than 70 yr of age were 53.3, 75, 60, 65 and 80 per cent, respectively.
Table V.
Mean survival of patients with renal cell malignancy with hazard ratio (N=551)
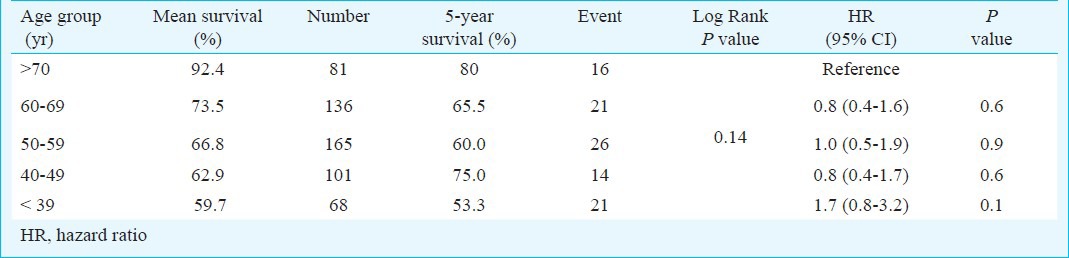
Fig.
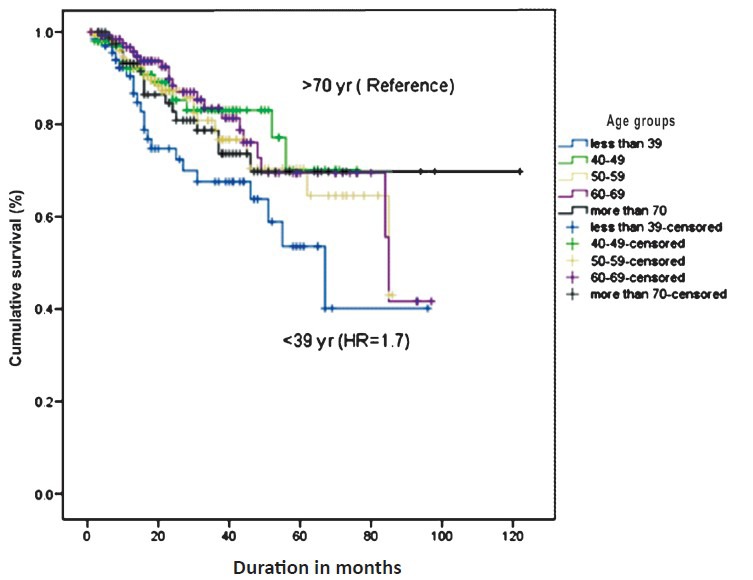
Kaplan Meier's survival curve depicting the survival of patients of renal cell malignancy based on different age groups.
Discussion
Renal cell carcinoma, based on the data from developed countries, is considered to be the malignancy of 6th and 7th decade of life but there are no data available from the developing country to support this. It has been observed that in India, more and more patients are presenting at a younger age and in advanced stage of the disease. In contrast to the western countries where more than 60 per cent of the RCC has been reported to present as less than 4 cm size (T1a), only 10.4 per cent of our patients were picked up as T1a, which is an ideal size for partial nephrectomy7.
One third of the total patients with RCC were younger than 50 yr of age at the time of presentation. Data from the larger epidemiological studies from West have shown that around 3.4 to 5 per cent of patients with the renal tumour were of less than 40 yr of age8, while in our study 12.3 per cent of patients were below the age of 40 yr.
In a study from Surveillance Epidemiology and End Results (SEER) database, majority of RCC cases at presentation were between 60-69 or 70-79 yr of age and only 42 per cent of patients presented in < 60 yr of age9. In the present study, 60.2 per cent of patients were below the age of 60 yr.
Unlike in West where the male to female ratio is 2:1, males were 4-times likely to present with renal tumours in our population10,11,12. Proportion of female patients was more in younger age group, while in a study from West, proportion of female patients increased towards older age, where about 44.7 per cent of patients were females10. This difference in sex ratio may reflect the difference in perception in seeking health care for a male and a female member of the family looking at the limited financial resources in a developing country like India.
Another difference observed was the incidence of clear cell RCC. Contrary to most of the western literature9,12,13,14 where clear cell RCC is present in around 85 per cent of the patients, clear cell RCC in the current study was present only in 71.33 per cent of the patients9,12,13,14. Clear cell RCC was uniformly higher in all age groups ranging from 82 to 88 per cent in (SEER) data9 but in the present study younger patients had less proportion of clear cell carcinoma ranging from 50.7-64.8 per cent.
Our study demonstrated a lower survival rate in younger patients and also highlighted the fact that more patients presented at younger age in comparison to patients from the West. Though the known risk factors for RCC such as smoking, hypertension, obesity, and diet have not been studied in different geographical area, this data set may provide a basis to find out the reasons for this difference in presentation at the younger age and poor survival15,16. As RCC is an immunogenic malignancy where body immunity plays an important role, the plausible hypothesis we put forth for younger age at presentation is the comparatively poorer nutritional status of younger population in developing countries, which could be analogous to the nutritional status of elderly people in a developed country. This needs to be validated prospectively by assessing nutritional status by standardized instruments to measure nutritional status.
Though we did not observe variation in the incidence of localized versus metastatic RCC at the time of presentation, the average size of the tumour at presentation was 8.08 cm. This could be explained by a higher proportion of men presenting with symptom. We have earlier reported that only 28 per cent of RCC were detected incidentally on imaging i.e. ultrasonography or CECT done for other causes like nonspecific abdominal symptoms, lower urinary tract symptoms, pyrexia of unknown origin, etc17. Another finding was that only 10.4 per cent of our patients presented with the tumour size of less than 4 cm, whereas in the West, almost two third of the kidney tumours were small in size7. Apart from the difference in proportion of clear cell RCC and sex ratio in various age groups, no difference was found in Fuhrman's grade, stage of the tumour, lymph node metastases, involvement of IVC and distant metastasis, which are the important prognosticating factors for the treatment outcome.
In conclusion, one third of our patients with RCC presented at less than 50 yr of age with relatively higher mean tumour size of 8.08 cm. Though there was no difference in Fuhrman's grade, stage of the tumour, lymph node metastases, involvement of IVC and the distant metastasis in various age groups, the proportion of clear cell RCC was relatively small in younger age group patients. The survival was much lower in younger age group. The reason for difference in presentation at the younger age from the western population is worth exploring.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Pantuck AJ, Zisman A, Beldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–23. [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphological parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 6.lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. WHO classification of renal tumors of the adults. Eur Urol 2006. 2004;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Salagierski M, Akdogan B, Brookman-May S, Dobrowdska-Glazar B, Ficarra V, Langenhuijsen JF, et al. What is the contemporary role of radiofrequency ablation in the management of small renal masses? Are small lesions the radiologist's tumors? Eur Urol. 2013;63:493–5. doi: 10.1016/j.eururo.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Kantor AL, Meigs JW, Heston JF, Flannery JT. Epidemiology of renal cell carcinoma in Connecticut, 1935-1973. J Natl Cancer Inst. 1976;57:495–500. doi: 10.1093/jnci/57.3.495. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Abdollah F, Bianchi M, Trinh Q, Jeldres C, Tian Z, et al. A stage-for-stage and grade-for grade analysis of cancer specific mortality rates in renal cell carcinoma according to age: A competing-risks regression analysis. Eur Urol. 2011;60:1152–9. doi: 10.1016/j.eururo.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 10.Taccoen X, Valeri A, Descostes JL, Morin V, Stindel E, Doucet L, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer-specific survival. Eur Urol. 2007;51:980–7. doi: 10.1016/j.eururo.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Jun C, Zhishun X, Xianzhou J, Qiang F, Jin W. Association between age and clinical characteristics of renal cell carcinoma in adult patients. Int J Urol. 2006;13:515–9. doi: 10.1111/j.1442-2042.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 12.Verhost G, Veillard D, Guille F, De La Taille A, Salomon L, Abbou CC, et al. Relationship between age at diagnosis and clinicopathological features of renal cell carcinoma. Eur Urol. 2007;51:1298–304. doi: 10.1016/j.eururo.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 13.Patard JJ, Tazi H, Bensalah K, Rodriguez A, Vincendeau S, Riox-Leclercq N, et al. The changing evolution of renal tumors: A single center experience over a two decade period. Eur Urol. 2004;45:490–3. doi: 10.1016/j.eururo.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Ortiz RF, Rosser J, Lydia T, David A, Wood G. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J Urol. 2004;171:2160–5. doi: 10.1097/01.ju.0000125487.96469.2e. [DOI] [PubMed] [Google Scholar]
- 15.Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol. 2006;176:2353–8. doi: 10.1016/j.juro.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 16.Amling CL. The association between obesity and the progression of prostate and renal cell carcinoma. Urol Oncol. 2004;22:478–84. doi: 10.1016/j.urolonc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Jain P, Surdas R, Aga P, Jain M, Kapoor R, Srivastava A, et al. Renal cell carcinoma: Impact of mode of detection on its pathological characteristics. Indian J Urol. 2009;25:479–82. doi: 10.4103/0970-1591.57919. [DOI] [PMC free article] [PubMed] [Google Scholar]


