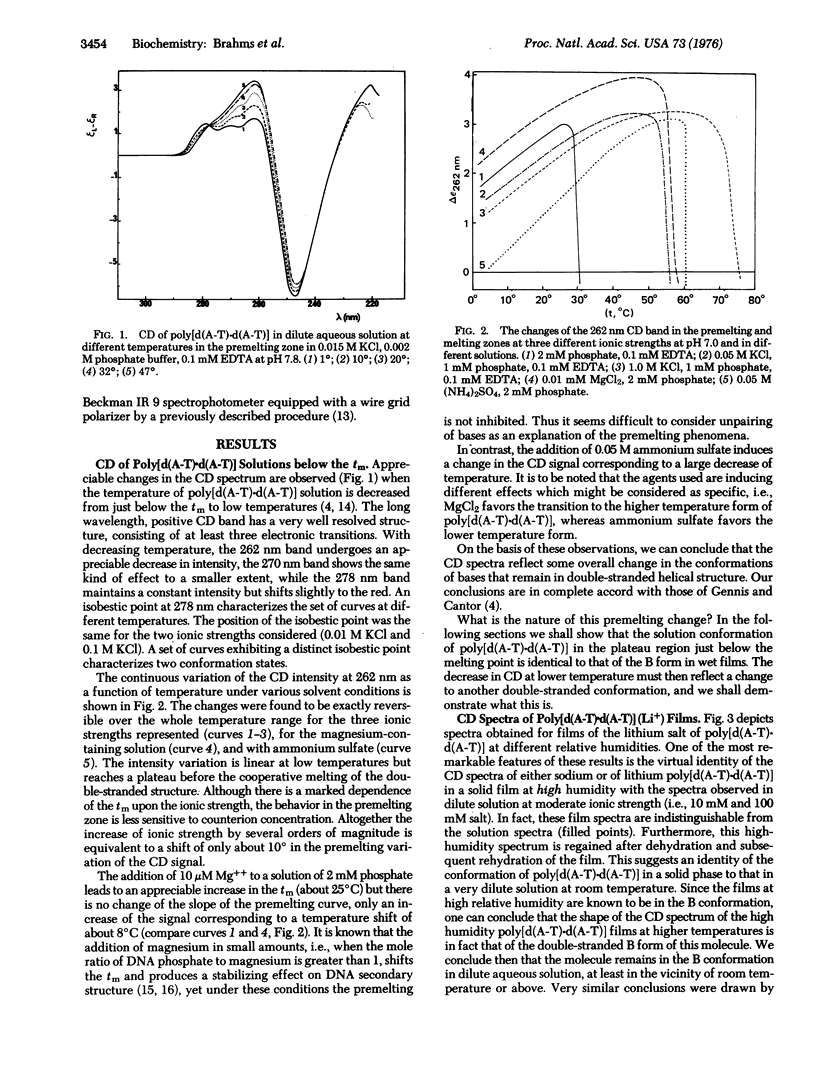
Abstract
The conformation of the synthetic DNA, poly-[d(A-T)-d(A-T)], has been investigated both in the solid state and in dilute aqueous solutions at different temperatures below its melting point. The change of the circular dichroism (CD) spectra of poly[d(A-T)-d(A-T)] solutions with decreasing temperatures from just below the melting point to 0 degrees involves a specific decrease of the intensity of the 262 nm CD band. This conformational change has been assigned to a gradual and partial transition from the B to C form, on the basis of the following results: (i) By the use of infrared dichroism measurements on oriented films we have defined humidity and salt conditions under which B and C forms of poly[d(A-T)-d(A-T](Li+) are stable. In addition, we find that ammonium salts induce the C form of poly[d(A-T)-d(A-T)] even at high relative humidity. (ii) CD studies of the films of the lithium salt of poly[d(A-T)-d(A-T)] under the same conditions have given CD spectra corresponding to the B and C forms of this polynucleotide. In addition, the CD spectrum of the ammonium salt of poly[d(A-T)-d(A-T)] in solution approaches that of the C form in films. (iii) The conformational change of poly[d(A-T)-d(A-T)] as a function of temperature can be entirely explained on the basis of changes in the double-stranded base-paired structure. Our data rule out hydrogen bond breaking and unstacking or "breathing" as an explanation of the premelting changes. Curves of the continuous variation of CD(epsilon at 262 nm) as a function of temperature (from 0 degrees to the melting zone) show similar slopes in the presence of different agents stabilizing the double-stranded structure, such as Mg++, or at different salt concentration (KCl), indicating that the nature of the process is independent of ionic strength. Some specific effects were observed in the influence of certain neutral salts; ammonium induces the C form whereas magnesium favors the B form. CD data give direct evidence that a DNA like poly[d(A-T)-d(A-T)] need not change conformation upon transition from a dilute aqueous solution to a highly hydrated (film/gel) solid state. The change of conformation begins only at a defined partial dehydration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hukins D. W., Smith P. J., Watts L. Structural details of double-helix observed for DNAs containing alternating purine and pyrimidine sequences. J Mol Biol. 1974 Sep 15;88(2):523–533. doi: 10.1016/0022-2836(74)90499-9. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. The conformation of C-DNA. J Mol Biol. 1975 Oct 15;98(1):265–269. doi: 10.1016/s0022-2836(75)80115-x. [DOI] [PubMed] [Google Scholar]
- BRAHMS J., MOMMAERTS W. F. A STUDY OF CONFORMATION OF NUCLEIC ACIDS IN SOLUTION BY MEANS OF CIRCULAR DICHROISM. J Mol Biol. 1964 Oct;10:73–88. doi: 10.1016/s0022-2836(64)80029-2. [DOI] [PubMed] [Google Scholar]
- Baba Y., Kagemoto A. Influence of magnesium ions on helix-coil transition of DNA determined by modified differential scanning calorimeter. Biopolymers. 1974;13(2):339–344. doi: 10.1002/bip.1974.360130209. [DOI] [PubMed] [Google Scholar]
- Brahms J., Pilet J., Phuong Lan T. T., Hill L. R. Direct evidence of the C-like form of sodium deoxyribonucleate. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3352–3355. doi: 10.1073/pnas.70.12.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner W. C., Maestre M. F. Circular dichroism of films of polynucleotides. Biopolymers. 1974;13(2):345–357. doi: 10.1002/bip.1974.360130210. [DOI] [PubMed] [Google Scholar]
- DAVIES D. R., BALDWIN R. L. X-ray studies on two synthetic DNA copolymers. J Mol Biol. 1963 Apr;6:251–255. doi: 10.1016/s0022-2836(63)80086-8. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfurth S. C., Peticolas W. L. Melting and premelting phenomenon in DNA by laser Raman scattering. Biopolymers. 1975 Feb;14(2):247–264. doi: 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- FREUND A. M., BERNARDI G. VISCOSITY OF DEOXYRIBONUCLEIC ACID SOLUTIONS IN THE 'SUB-MELTING' TEMPERATURE RANGE. Nature. 1963 Dec 28;200:1318–1319. doi: 10.1038/2001318b0. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Optical studies of a conformational change in DNA before melting. J Mol Biol. 1972 Apr 14;65(3):381–399. doi: 10.1016/0022-2836(72)90196-9. [DOI] [PubMed] [Google Scholar]
- Harwood S. J., Schendel P. F., Wells R. D. Micrococcus luteus deoxyribonucleic acid polymerase. Studies of the enzymic reaction and properties of the deoxyribonucleic acid product. J Biol Chem. 1970 Nov 10;245(21):5614–5624. [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962 Aug;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- Lubas B., Wilczok T. Effect of ionic strength on DNA hydration during thermal helix-coil transition. Biochim Biophys Acta. 1970 Nov 12;224(1):1–9. doi: 10.1016/0005-2787(70)90614-3. [DOI] [PubMed] [Google Scholar]
- Maestre M. F. Circular dichroism of DNA films: reversibility studies. J Mol Biol. 1970 Sep 28;52(3):543–556. doi: 10.1016/0022-2836(70)90418-3. [DOI] [PubMed] [Google Scholar]
- McConnell B., von Hippel P. H. Hydrogen exchange as a probe of the dynamic structure of DNA. I. General acid-base catalysis. J Mol Biol. 1970 Jun 14;50(2):297–316. doi: 10.1016/0022-2836(70)90194-4. [DOI] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Pilet J., Brahms J. Dependence of B-A conformational change in DNA on base composition. Nat New Biol. 1972 Mar 29;236(65):99–100. doi: 10.1038/newbio236099a0. [DOI] [PubMed] [Google Scholar]
- Raguet J. N., Brahms J. Preliminary investigation of poly (dA-dT) conformation and of its interaction with DNA polymerase. Biochimie. 1973;55(2):111–117. doi: 10.1016/s0300-9084(73)80382-7. [DOI] [PubMed] [Google Scholar]
- Rimai L., Maher V. M., Gill D., Salmeen I., McCormick J. J. The temperature dependence of Raman intensities of DNA. Evidence for premelting changes and correlations with ultraviolet spectra. Biochim Biophys Acta. 1974 Aug 29;361(2):155–165. [PubMed] [Google Scholar]
- Small E. W., Peticolas W. L. Conformational dependence of the Raman scattering intensities from polynucleotides. Biopolymers. 1971;10(1):69–88. doi: 10.1002/bip.360100107. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Tunis M. J., Hearst J. E. Optical rotatory dispersion of DNA in concentrated salt solutions. Biopolymers. 1968;6(8):1218–1223. doi: 10.1002/bip.1968.360060816. [DOI] [PubMed] [Google Scholar]
- Unis M. J., Hearst J. E. On the hydration of DNA. II. Base composition dependence of the net hydration of DNA. Biopolymers. 1968;6(9):1345–1353. doi: 10.1002/bip.1968.360060909. [DOI] [PubMed] [Google Scholar]


