Abstract
Pregnancy is associated with complex of endocrinological, immunological, metabolic, and vascular changes that may influence the skin and other organs in various ways. Pregnancy is a period in which more than 90% women have significant and complex skin changes that may have great impact on the woman's life. The dermatoses of pregnancy represent a heterogeneous group of skin diseases related to pregnancy and/or the postpartum period. The dermatoses of pregnancy can be classified into the following three groups: Physiologic skin changes in pregnancy, pre-existing dermatoses affected by pregnancy, and specific dermatoses of pregnancy. Though most of these skin dermatoses are benign and resolve in postpartum period, a few can risk fetal life and require antenatal surveillance. Most of the dermatoses of pregnancy can be treated conservatively but a few require intervention in the form of termination of pregnancy. Correct diagnosis is essential for the treatment of these disorders. This article discusses the current knowledge of various skin changes during pregnancy and the evaluation of the patient with pregnancy dermatoses with special emphasis on clinical features, diagnostic tests, maternal and fetal prognosis, therapy, and management.
Keywords: Dermatoses, pregnancy, skin
Introduction
Pregnancy is a period in which more than 90% women have significant and complex skin changes that may have great impact on the woman's life.[1] These changes are mainly due to a number of complex endocrinological, immunological, metabolic, and vascular changes occurring in pregnancy that may influence the skin in various ways. Some of these changes are physiological due to endocrinological changes[2] and there may be changes in the course of pre-existing skin diseases which could show improvement or exacerbations. Apart from these there is an ill-defined heterogeneous group of dermatoses that are specific to pregnancy and seen only in pregnancy and/or the postpartum period referred to as specific dermatoses of pregnancy. Many physicians are familiar with the normal physiologic changes in pregnancy; knowledge on the rarer pregnancy-specific dermatoses is often lacking. This may lead to misdiagnosis and mistreatment of the patients.
Immunological changes during pregnancy
During pregnancy, the maternal immune response is extensively altered to allow the fetus to attach to the mother.[3] Cytokine profile is altered, leading to a tendency toward the Th2 cytokines (IL-4, IL-5, IL-10, IL-13), which favour the maintenance of fetal survival. In postpartum period, there is elevation in the levels of Th1 cytokines (IL-2, TNF-a, IFN-c).
Hormonal changes during pregnancy also lead to an overall preference of the Th2 cytokine profile. Estrogen suppresses IL-2 production while progesterone promotes the production of Th2 cytokines like IL-4, IL-5, and IL-10.
Progesterone has an inhibitory effect on TNF-a secretion and glucocorticoid levels increase steadily during pregnancy. It inhibits the IL-1, IL-2, TNF-a, and IFN-c productions and stimulates IL-10, IL-4, and IL-13 synthesis.
Dermatological conditions modified during pregnancy
Physiological changes in pregnancy
Pregnancy is a time of significant and complex physiological changes. Some of these changes are due to the de novo production of a variety of protein and steroid hormones by the feto-placental unit as well as by increased activity of the maternal pituitary, thyroid, and adrenal glands [Table 1].[2,3]
Table 1.
Classification of skin changes occurring during pregnancy

Pigmentary changes
Hyperpigmentation is most common presentation of pregnancy due to elevated serum levels of MSH, estrogen or progesterone. Estrogen increases the output of melanin by the melanocytes and effect of estrogen is augmented by progesterone, resulted from melanin deposition into epidermal and dermal macrophages. It starts from the first trimester of pregnancy, and occurs in areas that are already pigmented particularly nipples, areola, and genital areas. Freckles, nevi, and recent scars become dark and even enlarge during pregnancy.[3] Generalized hyperpigmentation occur in people with Fitzpatrick skin type 1or2. Linea nigra is hyperpigmented line, found on the abdomen in pregnant women and noticed in the second trimester. Its vertical line typically runs from the pubic bone to the belly button, but can run all the way up to the chest and usually disappears a few months after delivery. This is often accompanied by the displacement of the umbilicus to the right, known as the “ligamentum teres sign.”[3] It is hypothesized that folic acid reduces the formation of linea nigra seen in foods such as leafy green vegetables, oranges, and whole wheat bread. Chloasma or melasma is also known as mask of pregnancy, seen in 45–75% of women in pregnancy[4] presented with irregular sharply demarcated brownish pigmentation of the face mainly over centrofacial or malar region. Striae distensae (striae gravidarum) develop in up to 90% of women during the sixth and seventh month of pregnancy[5] and are partial tears in the structures of the skin, which appear as reddish or bluish depressed streaks, usually on the abdomen but also on the breasts and thighs.[6] Soft-tissue fibromas (skin tags) can occur on the face, neck, upper chest, and beneath the breasts during late pregnancy. These fibromas generally disappear during postpartum.[7]
Hair changes
A mild to moderate hirsutism and hypertrichosis is seen during pregnancy. After delivery it usually resolves. There is an increased proportion of anagen growing hairs due to estrogen and androgen stimulation in the second half of pregnancy. After the end of pregnancy the follicles in which anagen has been prolonged rapidly enters catagen followed by telogen and increased hair shedding is evident in 6-16 weeks, called telogen effluvium, more marked in the frontal and temporal regions but may be generalised.[5] Spontaneous recovery of hair takes in 3–12 months. Rarely male-pattern baldness or hypotrichosis are seen especially in women with a tendency toward androgenetic alopecia.[3]
Nail changes
Nails often turn brittle during pregnancy. Distal onychomycosis occur in some women.[3,5,7] Nail changes are benign. Reassurances and promotion of good care, avoiding of any external nail sensitizer will take care of problems.
Vascular changes
Multiple vascular growth factors mediated by increased pituitary, adrenal, and placental hormone secretion stimulate vascular growth as well as vascular changes. The placenta is a rich source of basic fibroblast growth factor, a very active angiogenic factor in pregnancy. Microvascular endothelial cells grow in vitro with lower concentrations of human serum from pregnant women compared to the concentration used from nonpregnant individuals because of the amount of vascular growth factors released during pregnancy.[8] Spider angiomas is reddish elevations on the skin, particularly common on the face, neck, upper chest, and arm with radicles branching out from a vascular body. The condition is often designated as nevus, angioma, or telangiectasia. Palmar erythema is noticed in two-third of white women and one-third of black women.[9] Capillary hemangioma is seen in about 5% of women during pregnancy especially in the head and neck region. Epulis of pregnancy (granuloma gravidarum) are typical granuloma pyogenicum which are found in the oral cavity, often arise from the gingival papillae (gingival hypertrophy), often regress spontaneously during postpartum.
Glandular changes during pregnancy
Increased eccrine glands function lead to miliaria, hyperhidrosis, dyshidrotic eczema, and decreased apocrine gland function lead to improvement in hidradenitis suppurativa, fox-fordyce disease. Increased sebaceous function in third trimester lead to Acne (variant pruritic folliculitis of pregnancy) and enlargement of sebaceous glands on the areola (called montgomery's gland or tubercles).[5]
Pre-Existing Skin Diseases and Pregnancy
Diseases potentially improved during pregnancy
Allergic contact dermatitis,[3] fox–fordyce disease, and hidradenitis suppurativa potentially improve during pregnancy because of decreases in the apocrine gland function. During the pregnancy,[5] Psoriasis is more likely to improve than worsen, 40% to 63% of pregnant women with psoriasis improve during pregnancy, whereas only 14% worsen.[10] This may be attributed to the high levels of interleukin-10 in pregnancy.[11] Psoriatic arthritis has been reported to develop or worsen during pregnancy and 30% to 45% of women had onset of psoriatic arthritis either postpartum or perimenopausal.[12]
Diseases potentially worsened during pregnancy
Infections: Candida vaginitis is more frequent during pregnancy, up to 50% neonates born to infected mothers are positive for candida. Infants may present with oral thrush and diaper rash. Condyloma acuminate can attain large size during pregnancy. Human papilloma virus may produce laryngeal papilloma in infant. Herpes Simplex infection can be transmitted during pregnancy and delivery. Leprosy will experience an exacerbation of disease during pregnancy or within the first 6 months of lactation. The type-1 lepra reaction increases in frequency during the first trimester of pregnancy, then declines until delivery, whereupon it again increases sharply. The type-2 reaction increases in frequency with peaks in the first and third trimesters and the first 9 months of lactation. Trichomoniasis is detected in 60% of pregnancy.[5] Varicella zoster virus can also infect pregnant women. Varicella pneumonia in about 14% of affected individual, 3% of them have a fatal outcome. Congenital varicella syndrome can occasionally occur if primary maternal infection occurs during the first trimester.
Immune-mediated diseases: Systemic lupus erythematosus (SLE) in pregnancy is well tolerated by mothers in remission for at least 3 months before conception, except those with nephropathy or cardiomyopathy. If conception occurs during the active stage of SLE, approximately 50% of patients will worsen during pregnancy and a few will die or experience permanent renal damage. Patients whose SLE first appears during pregnancy have a high frequency of severe manifestations.[5] Neonatal lupus may occur in the baby of a mother with lupus.
Dermatomyositis and polymyositis in some patients may experience deterioration.[13] Pemphigus may be exacerbated during or after pregnancy, but generally to a mild degree. Although the rate of stillbirth was not as high as previously reported, the rate of abortion was considerable. Pregnancy may have an uneventful course, especially in patients in clinical remission; nevertheless, careful monitoring of the high risk mother and fetus is mandatory.[14]
Impetigo herpetiformis occurs during last trimester of pregnancy without prior history of psoriasis. This shows that irregular erythematous patches with superficial pustules arranged in groups begin in intertriginous areas slowly involve the whole body with sparing of face, hand, and feet.[5]
Metabolic diseases: Acrodermatitis enteropathica flares during pregnancy[3] as serum zinc levels decline early in gestation. In some patients, the disease is recognized in pregnancy, but it may be misdiagnosed as impetigo herpetiformis or herpes gestationis, unless serum zinc is measured. Women with Porphyria cutanea tarda may experience chemical and biochemical deterioration.[5]
Connective tissue disorders: Ehlers-Danlos syndrome Type 1 and 4 may experience excessive bleeding, wound gaping and uterine laceration. Pseudoxanthoma elasticum can lead major gastrointestinal bleeding during pregnancy.
Specific dermatoses of pregnancy
The first classification of specific dermatoses of pregnancy was proposed by Holmes and Black[15] in 1983 included four skin conditions [Table 2a]:
Table 2a.
The first classification of specific dermatoses of pregnancy

The second, proposed by Shornick[16] in 1998, included intrahepatic cholestasis of pregnancy (ICP) in addition to Pemphigoid gestationis (PG), Polymorphic eruption of pregnancy (PEP), and Prurigo of pregnancy (PP) [Table 2b].
Table 2b.
The second classification of specific dermatoses of pregnancy

The most recent rationalized classification has been proposed by Ambros-Rudolph et al.[17] in 2006 after their retrospective two-centre study on 505 pregnant patients. They introduced a new entity “Atopic eruption of pregnancy (AEP)” as AEP was observed to be the most common pruritic skin condition in pregnancy in their study. It was noted in almost 50% of patients affected with pregnancy specific dermatoses. They included three conditions – eczema in pregnancy, PP, and pruritic folliculitis of pregnancy (PF) under AEP due to their overlapping features.
While AEP starts significantly earlier PEP, PG and ICP present late in pregnancy.
Atopic Eruption of Pregnancy
AEP is the most common specific dermatoses in pregnancy, accounting for 50% of patients, starts early in 75% before the third trimester, and tends to recur in subsequent pregnancies.[18] It is a benign pruritic disorder of pregnancy which includes eczematous and/or papular lesions in patients with a personal and/or family history of atopy and/or elevated IgE levels after exclusion of the other dermatoses of pregnancy. The pathogenesis of AEP is thought to be triggered by pregnancy-specific immunological changes; reduced cellular immunity and reduced production of Th1 cytokines (IL-2, interferon gamma, IL-12); and dominant humoral immunity and increased secretion of Th2 cytokines (IL-4, IL-10).[19] It has no maternal or fetal risk. Infant may develop atopic skin changes later in life.[20]
It includes three entities: Eczema in pregnancy, Prurigo of pregnancy/prurigo gestationis, and Pruritic folliculitis.
Eczema in pregnancy
More commonly occurs in primigravida in first and second trimester [Figure 1].[21] Widespread eczematous changes affect typical atopic sites such as face, neck, upper chest, and the flexural surfaces of the extremities with raised total serum IgE levels.
Figure 1.
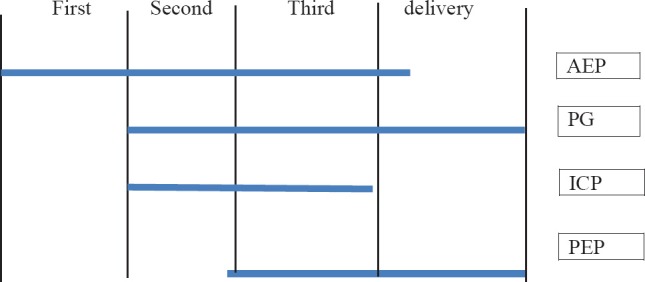
Coincidence of specific dermatoses of pregnancies. AEP: Atopic eruption of pregnancy, PG: Pemphigoid gestationis, ICP: Intrahepatic cholestasis of pregnancy, PEP: Polymorphic eruption of pregnancy.
Prurigo of pregnancy
Prurigo of pregnancy has been reported to occur in approximately 1 in 300 pregnancies.[22] Onset at 25 to 30 weeks of pregnancy [Figure 1], may persist for 3 months after delivery. It presented with 1–5 mm size erythematous pruritic papules and nodules over the extensor surfaces of the extremities, occasionally on the abdomen with excoriation mark over the affected areas. A key finding is the often extreme dryness of the skin.
Pruritic folliculitis of pregnancy
It is occur in about 1 in 3,000 pregnancies[22] usually in second and third trimester of pregnancy [Figure 1]. Contrary to its name, pruritus is not a major feature. Generalized erythematous, follicular papule usually occurs on shoulders, upper back, arms, chest, and abdomen. It resolves spontaneously 1–2 months following delivery.[23]
Polymorphic Eruptions of Pregnancy
PEP usually occurs in 1 in 160 to 200 pregnant women and the condition is associated with excessive maternal weight gain and multiple pregnancies.[24,25] It is benign, self-limited pruritic inflammatory disorder affects primigravidae in the last weeks of pregnancy or immediately postpartum [Figure 1]. The main pathogenesis is the rapid, late, excessive abdominal distension resulting in collagen and elastic fibre damage in the striae, with subsequent conversion of nonantigenic molecules to antigenic ones, which may act as a trigger for the inflammatory skin changes[26] substance released from placenta into the maternal circulation triggers fibroblast proliferation.
IT typically starts on the abdomen, within striae distensae, with severely pruritic urticarial papules that coalesce into plaques, spreading to the buttocks and proximal thighs. The eruption remains located to these sites but can quickly generalize in severe cases sparing umbilical region. A polymorphous vesicle but never bullae, nonurticarial erythema, targetoid eczematous lesions appear in more than half of patients. The rash usually resolves within 4 to 6 weeks.[26] Histopathology is nonspecific.[20] Immunofluorescence is negative.
Resolution usually occurs 7–10 days after delivery. Fetal prognosis is unaffected, but there might be a risk of developing atopic skin changes in the infant, later-on.[25]
Pemphigoid Gestationis
Dermatitis herpetiformis of pregnancy was first described in 1811, was named herpes gestationis by Milton in 1872.[27] PG is a rare, self-limited autoimmune bullous disorder that presents mainly in late pregnancy or the immediate postpartum period but can appear in any of the three trimesters [Figure 1]. The incidence of herpes gestationis is 1 in 50,000 pregnancies.[21]
Pathogenesis of PG involves the production of auto-antibodies, mainly IgG1 subclass, that bind to the extracellular NC16A domain of the carboxyl terminus of the 180 kDa bullous pemphigoid antigen (BPAg2). Once bound, the antigen-antibody complex fixes complement via the classical complement pathway. Complement activation leads to chemotaxis of eosinophils to the site of the antigen-antibody complex on the BMZ and eosinophils degranulate lead to damage the dermal-epidermal junction, leading to blister formation.[28] Production of autoantibody with potential cross reactivity between placental tissue and skin is also seen. It is linked with anti HLA-DR3 and DR4 antibodies.[3] The primary site of autoimmunity seems the placenta, because antibodies also bind to that of chorionic and amniotic epithelia.[18]
Clinically PG presents with intense pruritic erythematous urticarial papules and plaques develops typically on the abdomen. The umbilical region is almost always involved. Sites such as face, mucous membranes, palms, and soles are spared. In the “prebullous” stage, differentiation between PG and polymorphic eruption of pregnancy is somewhat difficult but investigation solves the problem. It occurs in second or third trimester and improves during the latter phase of pregnancy sometime it may flare at the time of delivery or postpartum. Complete resolution is seen in weeks to months after postpartum. The antenatal risks are small for-date babies and pre mature birth 5% to 10% of babies will develop lesions from passive transplacental transmission of antib odies. Pemphigoid gestationis is rarely associated with hydatidiform moles and choriocarcinoma.[29]
Histopathological examination depends on the stage. Direct IF of perilesional skin shows C3 with or without IgG (IgG1, IgG3, or IgG4) in a linear band along the BMZ. The antibody localizes to the roof of the blister. Indirect IF studies shows circulating IgG antibody in patients serum detected in 30-100% cases. The enzyme-linked immunosorbent assay (ELISA) shows circulating BPAg2 autoantibodies.[28]
Intrahepatic Cholestasis of Pregnancy
Obstetric cholestasis is manifested by pruritus in pregnancy with or without laboratory evidence of cholestasis. Incidence is 1 in 50 to 5,000 pregnancies.[3] The disorder is a genetically linked oestrogen-dependent condition, which results in cholestasis with or without jaundice. It usually begins in the latter half of pregnancy [Figure 1]. There is a family history of jaundice in 50% cases. Recurrence in subsequent pregnancy occurs in 60-70% of cases.[30,31]
Pathophysiologically there is a defect in the excretion of bile salts resulting in elevated bile acids in the serum that leads to severe pruritus in the mother. Toxic bile acids can pass into fetal circulation, may have deleterious effects on the fetus due to acute placental anoxia and cardiac depression. The reason for this defect seems to be multifactorial with genetic, hormonal, and exogenous factors being involved.[32,33] Recently, mutation of certain genes encoding for transport proteins necessary for bile excretion (e.g. the ABCB4 [MDR 3] gene) have been identified in some ICP patients. Association with the presence of HLA-A31 and HLA-B8 is seen. A high prevalence of the HLA haplotype Aw31B8 in patients with ICP was found in one, but not confirmed. Thus, the genetic base of ICP is still under investigation.[32,34,35]
Clinical features usually start in the third trimester and typically presents with sudden onset of severe pruritus that may start on the palms and soles but quickly becomes generalized [Figure 2]. It is not associated with primary skin lesions. Secondary skin lesions develop due to scratching that range from subtle excoriations to severe prurigo nodules. Symptoms are worsened at night. Extensor surfaces of the limbs, abdomen, and back are mainly involved. Jaundice, clay colored stools, dark urine seen in 10% of patients. Steatorrhea and malabsorption can lead to vitamin K deficiency and prolongation of prothrombin time lead to risk of hemorrhage. Primigravidae with ICP have a 2.7-fold increased risk for gallstones compared to pregnant women without cholestasis.[36]
Figure 2.
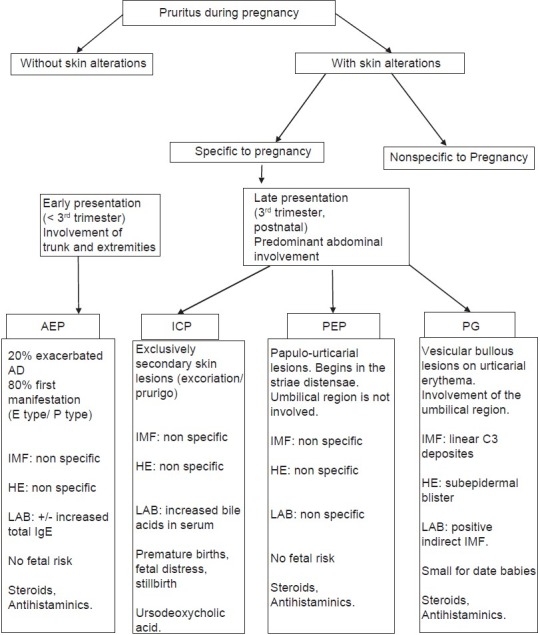
Flow chart of pruritus during pregnancy. AEP: Atopic eruption of pregnancy, PG: Pemphigoid gestationis, ICP: Intrahepatic cholestasis of pregnancy, PEP: Polymorphic eruption of pregnancy, IMF: Immunoflorescence, HE: Histopathological examination, LAB: Laboratory investigations
Laboratory investigations: The most sensitive indicator for the diagnosis of ICP is a rise of serum bile acid levels, mild–moderate elevation of transaminases, marked elevation in alkaline phosphatase and bilirubin levels.[32,36] Close surveillance of prothrombin time and an ultrasound examination of the liver may be necessary. Histopathology is nonspecific. Direct and indirect immunofluorescence: Negative – Atopic dermatitis, scabies, and other causes of hepatitis, most importantly, viral hepatitis must be ruled out be diagnosing ICP.
Early diagnosis, prompt treatment, and close obstetric surveillance are mandatory in the cases of ICP. Fetal risk such as distress, still birth, preterm delivery, meconium-stained amniotic fluid, neonatal respiratory distress syndrome, placental anoxia common due to vasoconstriction of placental chorionic veins from toxic bile acids, and meconium.[37] This can be prevented by early treatment and delivery between 36–38 weeks with favorable lung maturity.[36]
Conclusion
Along with the many physiological changes occurring in skin during pregnancy, there is lack of knowledge regarding specific dermatosis of pregnancy; also there are few conditions which might get worsened during pregnancy.
The most common pregnancy-associated skin changes observed in India are pigmentary changes and pruritus. Pruritus in pregnancy should never be neglected and should always lead to a precise work-up of the patient, which may be the symptom of the specific dermatoses of pregnancy. Careful history taking and examination will help to identify each condition clinically. The physical examination should focus on the distribution and morphology of the lesions. Involvement of striae is seen commonly in pruritic urticarial papules and plaques of pregnancy (PUPPP) but not in other dermatoses. Nodular lesions on the limbs are commonly seen in PP, whereas a follicular distribution is characteristic for PF or acne. Urticarial lesions suggest PUPPP or HG, whereas vesicular lesions can be seen in HG, herpes simplex/zoster, eczema, and occasionally in PUPPP.
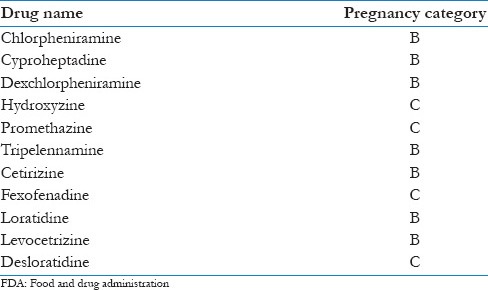
Most skin conditions resolve postpartum and only require symptomatic treatment. Liberal application of topical emollients and antihistamines [Table 3][3] should be advised in pregnancy associated pruritus. However, there are specific treatments for some conditions. Antepartum surveillance is recommended for patients with intrahepatic cholestasis of pregnancy, impetigo herpetiformis, and pemphigoid gestationis.
Table 3.
FDA pregnancy category classification for antihistamines
Such dermatosis should be managed jointly with Obstetricians, Pediatritians and Dermatologists.
This article highlights the need for Primary care phsycians to know about the dermatoses that may present in pregnancy as it may get unnoticed and might have serious implications for the expectant, fetus and newborn. Furthermore, appropriate advice for future pregnancies will depend on the correct diagnosis of the eruption. A discussion with the pregnant woman about the nature of her skin condition and the possible fetal risks associated with it is imperative.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kumari R, Jaisankar TJ, Thappa DM. A clinical study of skin changes in pregnancy. Indian J Dermatol Venereol Leprol. 2007;73:141. doi: 10.4103/0378-6323.31910. [DOI] [PubMed] [Google Scholar]
- 2.Ambros-Rudolph CM, Shornick JK. Pregnancy dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, Callen JP, Cerroni L, editors. Bolognia Dermatology. 3rd ed. Vol. 1. London: Elsevier; 2012. pp. 439–49. [Google Scholar]
- 3.Kar S, Krishnan A, Shivkumar PV. Pregnancy and skin. J Obstet Gynecol India. 2012;62:268–75. doi: 10.1007/s13224-012-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236–40. doi: 10.1177/120347540200600308. [DOI] [PubMed] [Google Scholar]
- 5.Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1–19. doi: 10.1067/mjd.2001.114595. [DOI] [PubMed] [Google Scholar]
- 6.Thomas RG, Liston WA. Clinical associations of striae gravidarum. J Obstet Gynaecol. 2004;24:270–1. doi: 10.1080/014436104101001660779. [DOI] [PubMed] [Google Scholar]
- 7.Tunzi M, Gray GR. Common skin conditions during pregnancy. Am Fam Physician. 2007;75:211–8. [PubMed] [Google Scholar]
- 8.García-González E, Ahued-Ahued R, Arroyo E, Montes-De-Oca D, Granados J. Immunology of the cutaneous disorders of pregnancy. Int J Dermatol. 1999;38:721–9. doi: 10.1046/j.1365-4362.1999.00810.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin AG, Leal-Khouri S. Physiologic skin changes associated with pregnancy. Int J Dermatol. 1992;31:375–8. doi: 10.1111/j.1365-4362.1992.tb02662.x. [DOI] [PubMed] [Google Scholar]
- 10.Ceović R, Lipozencić J, Pasić A, Kostović K. Psoriasis in pregnancy: A review of most important literature data. Acta Dermatovenerol Croat. 2009;17:193–7. [PubMed] [Google Scholar]
- 11.Trautman MS, Collmer D, Edwin SS, White W, Mitchell MD, Dudley DJ. Expression of interleukin-10 in human gestational tissues. J Soc Gynecol Investig. 1997;4:247–53. [PubMed] [Google Scholar]
- 12.McHugh NJ, Laurent MR. The effect of pregnancy on the onset of psoriatic arthritis. Br J Rheumatol. 1989;28:50–2. doi: 10.1093/rheumatology/28.1.50. [DOI] [PubMed] [Google Scholar]
- 13.Ambros-Rudolph CM. Disorders of pregnancy. In: Burgdorf WH, Plewig G, Wolff HH, Landthaler M, editors. Braun-Falco's Dermatolog. 3rd ed. Heidelberg: Springer Medizin Verlag; 2009. pp. 1160–9. [Google Scholar]
- 14.Daneshpazhooh M, Chams-Davatchi C, Valikhani M, Aghabagheri A, Mortazavizadeh SM, Barzegari M, et al. Pemphigus and pregnancy: A 23-year experience. Indian J Dermatol Venereol Leprol. 2011;77:534. doi: 10.4103/0378-6323.82404. [DOI] [PubMed] [Google Scholar]
- 15.Holmes RC, Black MM. The specific dermatoses of pregnancy. J Am Acad Dermatol. 1983;8:405–12. doi: 10.1016/s0190-9622(83)70046-0. [DOI] [PubMed] [Google Scholar]
- 16.Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172–81. doi: 10.1016/s1085-5629(98)80011-4. [DOI] [PubMed] [Google Scholar]
- 17.Ambros-Rudolph CM, Müllegger RR, Vaughan-Jones SA, Kerl H, Black MM. The specific dermatoses of pregnancy revisited and reclassified: Results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol. 2006;54:395–404. doi: 10.1016/j.jaad.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Pãunescu MM, Feier V, Pãunescu M, Dorneanu F, Sisak A, Ambros-Rudolph CM. Dermatoses of pregnancy. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:4–11. [PubMed] [Google Scholar]
- 19.Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 20.Ambros-Rudolph CM. Dermatoses of pregnancy-clues to diagnosis, fetal risk and therapy. Ann Dermatol. 2011;23:265–75. doi: 10.5021/ad.2011.23.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdeva S. The dermatoses of pregnancy. Indian J Dermatol. 2008;53:103–5. doi: 10.4103/0019-5154.43203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roger D, Vaillant L, Fignon A, Pierre F, Bacq Y, Brechot JF, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Arch Dermatol. 1994;130:734–9. [PubMed] [Google Scholar]
- 23.Kroumpouzos G, Cohen LM. Pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2000;43:132–4. doi: 10.1067/mjd.2000.105568. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph CM, Al-Fares S, Vaughan-Jones SA, Müllegger RR, Kerl H, Black MM. Polymorphic eruption of pregnancy: Clinicopathology and potential trigger factors in 181 patients. Br J Dermatol. 2006;154:54–60. doi: 10.1111/j.1365-2133.2005.06856.x. [DOI] [PubMed] [Google Scholar]
- 25.Ambros-Rudolph CM, Black MM. Polymorphic eruption of pregnancy. In: Black MM, Ambros-Rudolph CM, Edwards L, Lynch P, editors. Obstetric and Gynecologic Dermatology. 3rd ed. London: Elsevier Limited; 2008. pp. 49–56. [Google Scholar]
- 26.Aronson IK, Bond S, Fiedler VC, Vomvouras S, Gruber D, Ruiz C. Pruritic urticarial papules and plaques of pregnancy: Clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933–9. doi: 10.1016/s0190-9622(98)70265-8. [DOI] [PubMed] [Google Scholar]
- 27.Diamond WJ. Herpes gestationis. S Afr Med J. 1976;50:739–40. [PubMed] [Google Scholar]
- 28.Cobo MF, Santi CG, Maruta CW, Aoki V. Pemphigoid gestationis: Clinical and laboratory evaluation. Clinics (Sao Paulo) 2009;64:1043–7. doi: 10.1590/S1807-59322009001100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giam YC. Dermatoses of pregnancy. J Pediatr Obstet and Gynecol. 2003;29:22–7. [Google Scholar]
- 30.Yakasai IA, Thomson AJ, Fitzsimons C. Specific dermatoses of pregnancy: A review. West Afr J Med. 2011;30:239–44. [PubMed] [Google Scholar]
- 31.Kroumpouzos G, Cohen LM. Specific dermatosis of pregnancy: An evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083–92. doi: 10.1067/mob.2003.129. [DOI] [PubMed] [Google Scholar]
- 32.Lammert F, Marschall HU, Glantz A, Matern S. Intrahepatic cholestasis of pregnancy: Molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012–21. doi: 10.1016/s0168-8278(00)80139-7. [DOI] [PubMed] [Google Scholar]
- 33.Reyes H, Báez ME, González MC, Hernández I, Palma J, Ribalta J, et al. Selenium, zinc and copper plasma levels in intrahepatic cholestasis of pregnancy, in normal pregnancies and in healthy individuals, in Chile. J Hepatol. 2000;32:542–9. doi: 10.1016/s0168-8278(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 34.Holzbach RT, Sivak DA, Braun WE. Familial recurrent intrahepatic cholestasis of pregnancy: A genetic study providing evidence for transmission of a sex-limited, dominant trait. Gastroenterology. 1983;85:175–9. [PubMed] [Google Scholar]
- 35.Hirvioja ML, Kivinen S. Inheritance of intrahepatic cholestasis of pregnancy in one kindred. Clin Genet. 1993;43:315–7. doi: 10.1111/j.1399-0004.1993.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 36.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049–66. doi: 10.3748/wjg.15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid R, Ivey KJ, Rencoret RH, Storey B. Fetal complications of obstetric cholestasis. Br Med J. 1976;1:870–2. doi: 10.1136/bmj.1.6014.870. [DOI] [PMC free article] [PubMed] [Google Scholar]


