Abstract
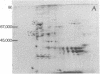
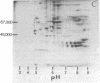
Rat liver chromatin was digested by micrococcal nuclease. More than 80% of the enzyme-digested chromatin could be recovered after centrifugation. Treatment with sodium deoxycholate and Triton X-100 at concentrations of 0.5% in the final chromatin suspension gave a higher recovery. Chromatin subunits were fractionated on a 5-30% linear sucrose density gradient. Approximately 35% of the chromatin subunits could be recovered from the gradient. Chromatin subunits and their DNA fragments were identified by gel electrophoresis and ultracentrifugation. The presence of nonhistone chromatin proteins (NHCP) in chromatin subunits was demonstrated by the following criteria: (i) Quantitative analysis showed that the mass ratio of histone to NHCP, in the presence or absence of detergents, was 1:0,25 or 1:0.1, respectively. (ii) After the removal of acid-soluble protein from the subunits, it was found that most of the phenol-soluble NHCP were similar to total chromatin NHCP. However, four major fractions of these phenol-soluble NHCP were found to be enriched in the subunits as identified by two-dimensional polyacrylamide gel electrophoresis. (iii) Experiments using an exchange of isotope-labeled and nonlabeled chromatin showed that NHCP were tightly bound to the chromatin subunits.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., Lipkin M. Chromatin monomer: absence of non-histone proteins. Biochem Biophys Res Commun. 1976 May 17;70(2):540–544. doi: 10.1016/0006-291x(76)91080-9. [DOI] [PubMed] [Google Scholar]
- Axel R. Cleavage of DNA in nuclei and chromatin with staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2921–2925. doi: 10.1021/bi00684a020. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANTRENNE H. Effects d'un inhibiteur de la catalase sur la formation induite de cet enzyme chez la levure. Biochim Biophys Acta. 1955 Mar;16(3):410–417. doi: 10.1016/0006-3002(55)90246-8. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Chemical probes of chromatin structure. Biochemistry. 1974 Aug 13;13(17):3622–3628. doi: 10.1021/bi00714a034. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Murphy R. F., Bonner J. Structure of transcriptionally active chromatin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4404–4408. doi: 10.1073/pnas.72.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. Properties of chromatin subunits from developing trout testis. J Biol Chem. 1975 Jun 25;250(12):4643–4647. [PubMed] [Google Scholar]
- Jackowski G., Suria D., Liew C. C. Fractionation of nucleolar proteins by two-dimensional gel electrphoresis. Can J Biochem. 1976 Jan;54(1):9–14. doi: 10.1139/o76-002. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Kostraba N. C., Wang T. Y. Non-histone proteins and gene activation in regenerating rat liver. Exp Cell Res. 1973 Aug;80(2):291–296. doi: 10.1016/0014-4827(73)90299-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liew C. C., Gornall A. G. Acetylation of ribosomal proteins. I. Characterization and properties of rat liver ribosomal proteins. J Biol Chem. 1973 Feb 10;248(3):977–983. [PubMed] [Google Scholar]
- Liew C. C., Liu D. K., Gornall A. G. Effects of aldosterone on RNA polymerase in rat heart and kidney nuclei. Endocrinology. 1972 Feb;90(2):488–495. doi: 10.1210/endo-90-2-488. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray A. J., Rickwood D. The heterogeneity of mouse-chromatin nonhistone proteins as evidenced by two-dimensional polyacrylamide-gel electrophoresis and ion-exchange chromatography. Eur J Biochem. 1974 Jan 3;41(1):181–190. doi: 10.1111/j.1432-1033.1974.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Fractionation of liver chromatin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2941–2944. doi: 10.1073/pnas.68.12.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky A. E. The structure of chromatin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2945–2948. doi: 10.1073/pnas.68.12.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K. Stepwise removal of histones from native deoxyribonucleoprotein by titration with acid at low temperature and some properties of the resulting partial nucleoproteins. J Mol Biol. 1969 Jan 14;39(1):125–144. doi: 10.1016/0022-2836(69)90338-6. [DOI] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Reeder R. H. Transcription of chromatin by bacterial RNA polymerase. J Mol Biol. 1973 Oct 25;80(2):229–241. doi: 10.1016/0022-2836(73)90169-1. [DOI] [PubMed] [Google Scholar]
- STEDMAN E. Cell specificity of histones. Nature. 1950 Nov 4;166(4227):780–781. doi: 10.1038/166780a0. [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Van Holde K. E. The effect of trypsin on nuclease-resistant chromatin fragments. J Biol Chem. 1974 Jan 10;249(1):152–156. [PubMed] [Google Scholar]
- Smart J. E., Bonner J. Selective dissociation of histones from chromatin by sodium deoxycholate. J Mol Biol. 1971 Jun 28;58(3):651–659. doi: 10.1016/0022-2836(71)90030-1. [DOI] [PubMed] [Google Scholar]
- Spadafora C., Geraci G. The subunit structure of sea urchin sperm chromatin: a kinetic approach. FEBS Lett. 1975 Sep 1;57(1):79–82. doi: 10.1016/0014-5793(75)80156-6. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Suria D., Liew C. C. Isolation and analysis of non-histone chromatin proteins from rat-liver nuclei by three different methods. Can J Biochem. 1974 Dec;52(12):1143–1153. doi: 10.1139/o74-159. [DOI] [PubMed] [Google Scholar]
- Suria D., Liew C. C. Isolation of nuclear acidic proteins from rat tissues. Characterization of acetylated liver nuclear acidic proteins. Biochem J. 1974 Feb;137(2):355–362. doi: 10.1042/bj1370355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T., Allfrey V. G. Studies of nuclear acidic proteins. Evidence for their phosphorylation, tissue specificity, selective binding to deoxyribonucleic acid, and stimulation effects on transcription. J Biol Chem. 1971 Jun 10;246(11):3597–3609. [PubMed] [Google Scholar]
- Weintraub H. Release of discrete subunits after nuclease and trypsin digestion of chromatin. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1212–1216. doi: 10.1073/pnas.72.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]