Abstract
Over the last two decades in particular there has been a remarkable increase in the number of solid organ transplants being performed worldwide alongside improvements in long-term survival rates. However, the infrastructure at transplant centres has been unable to keep pace with the current volume of the transplant patient work load. These pressures on transplant specialist centres has led to calls for an increased role of the general practitioner (GP) managing particular aspects of transplant patients’ medical care. Indeed, many aspects of follow-up care such as screening for malignancies, preventing infection through immunisation programmes, and managing cardiovascular risk factors are already important aspects of family practice medicine. This paper aims to review some of the aspects of transplant patient care that is important for healthcare workers in family practice to manage.
Keywords: Health promotion, immunosuppression, primary care, transplant medicine
Introduction
The first successful solid-organ transplant took place in 1954, when Dr Joseph Murray performed a kidney transplant on Richard Herrick using a kidney from Ronald Herrick (Richard's identical twin).[1] Since the first transplant, surgical techniques, postoperative care, management of potential organ donors and understanding of transplant medicine has improved dramatically and now patients may undergo transplantation of many solid body organs, often with excellent results.[2]
Over the last two decades in particular there has been a remarkable increase in the number of solid organ transplants being performed worldwide[3,4,5] alongside improvements in long-term survival rates. Indeed, 1- and 5-year survival rates for liver transplant patients are 86.9% and 73.6%, respectively,[6] and are approximately 90%[7] and 70%[8] for kidney transplant patients, respectively. This increase in survival outcomes for transplant patients has uncovered numerous complications of being on long-term potent immunosuppressants and the impact of prolonged survival with disease processes that led to patient transplantation in the first instance.[3,4] Indeed, cardiovascular, neoplastic and metabolic bone disease are all associated with considerable morbidity and mortality in the post-transplant patient group[2] and as such are becoming an important part of transplant follow-up care.[9]
However, the infrastructure at transplant centres has been unable to keep pace with the current volume of the transplant patient work-load. These pressures on transplant specialist centres has led to calls for an increased role of the general practitioner (GP) managing particular aspects of transplant patients’ medical care.[3] Indeed, many aspects of follow-up care such as screening for malignancies, preventing infection through immunisation programmes and managing cardiovascular risk factors are already important aspects of family practice medicine. GP's can help play a pivotal role in managing many aspects of care that will enable transplant centres to focus upon the more difficult and complicated aspects of transplant medicine.[3,4]
This paper aims to review some of the aspects of transplant patient care that is important for healthcare workers in family practice to manage.
General practitioner and the management of transplant patients in context
GP's will commonly manage patients in the community who have had a solid organ transplant.[10] However, GP's will generally not play a very active role in managing transplant patients in the first year post-op, apart from prescribing with input from the specialist centre and providing a point of contact for less serious problems or issues not relating to the transplant.[9]
This is because in the first year, transplant centres have regular patient contact and are actively monitoring transplant function and treatment side-effects. Indeed, in this period specialists in transplant medicine stress to patients that they should feel able to contact a transplant centre directly if they encounter any problems or complications with their transplant function. Patients who survive the first year are followed-up regularly throughout their lives with the number of transplant clinic visits varying between 3-monthy to annually depending upon the type of transplant, overall patient health and graft function. It is after the first year that the role of the GP becomes more important and more active in managing patients.
Transplant patients have numerous post-transplant complications that will be seen regularly in clinical practice. In 2000, a paper reported all medical complications in patients who received a liver transplant and survived for at least 5 years.[11] The study reported that compared to the US general population, hypertension and diabetes were significantly more prevalent. Hypertension was present in 60% of all patients, with half requiring more than one antihypertensive agent to manage their blood pressure.[11] Diabetes was six times more prevalent compared to the general population, with 60% requiring treatment.[11] Interestingly although cholesterol levels were high (70% of patients had an increase in cholesterol from pretransplant readings) there was no difference from control groups.[11] Similar issues are seen in patients’ who have received a kidney transplant, with Aakhus demonstrating that cardiovascular disease appears up to 20 years earlier in this patient group compared to the general population.[12] It should be remembered that there are clear benefits to receiving a transplant. In addition to the numerous benefits to quality of life,[13] receiving a kidney transplant reduces cardiovascular risk compared to remaining on dialysis with the annual risk of death from cardiovascular disease decreasing from 9% per year to between 3.5–%.[14,15]
Aspects of patient care managed mainly in family practice
General preventative measures
In the United Kingdom, the Quality and Outcomes Framework (QOF) was introduced as part of the new GP contract in 2004 and has attempted to promote effective healthcare screening and maintenance and reward good clinical practice with increase payments.[16] It has already been noted that transplant patients are at a higher risk of developing cardiovascular risk factors compared to the general population and therefore they stand to benefit from regular assessment and treatment of reversible cardiovascular risk factors including hypertension, diabetes, and dyslipdaemia. Table 1 summarises the US Preventative Services Task Force[17] and the QOF and NHS screening guideline[18,19] recommendations for general preventative medicine in family practice and slight differences for transplant patients based on studies in this patient group.[3,9,17,18,19,20,21,22,23,24]
Table 1.
Summary of preventative management in family practice
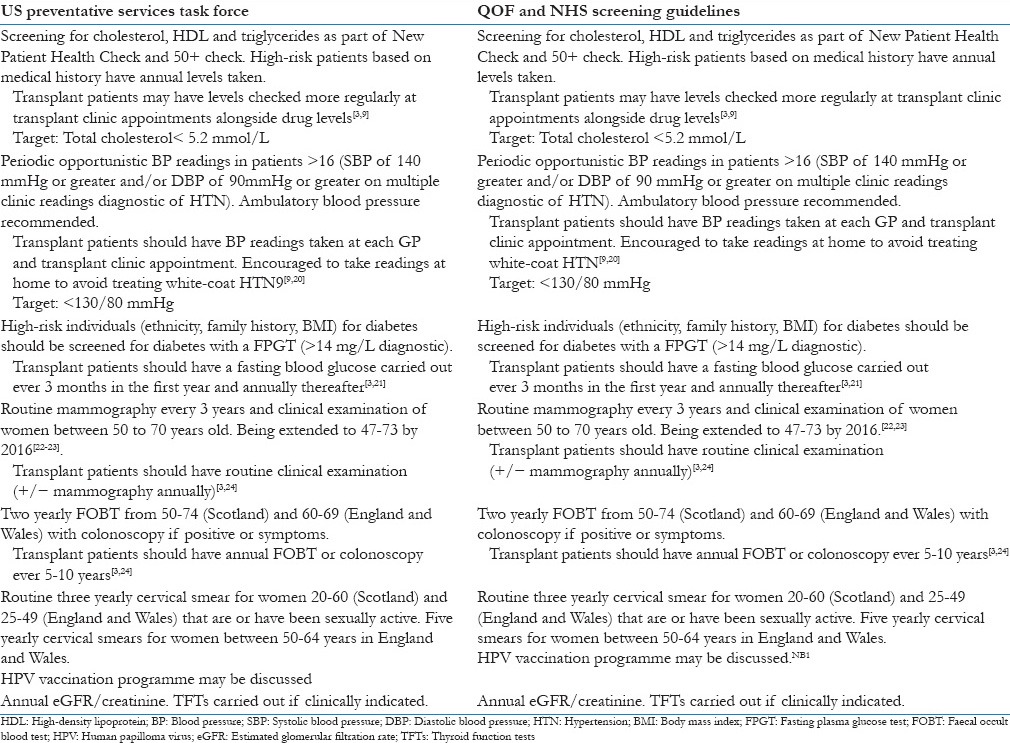
It is well established that transplant patients are at increased risk of developing human papilloma virus (HPV)-related anogenital and cervical cancers alongside cervical intraepithelial neoplasia.[22,25] Although, the vaccine has not yet been demonstrated in clinical trials to work in the transplant population the fact the vaccine is not a live vaccine and other vaccines work in this population is reassuring and should prompt discussion about its use in high-risk patients.[22,25]
Infections are a major cause of morbidity and mortality in transplant patients as a result of potent immunosuppression. For example, liver transplant patients are 100 times more likely to die of sepsis following liver transplant compared to the general population.[9] Therefore, another important area of preventative medicine is effective vaccination. Vaccinations, wherever possible, should be administered before the transplant as the post-transplant immune response to vaccination is generally reduced. Thus, transplant patients should have regular boosters to ensure that a clinically safe immune response is evoked. It should be noted that live vaccines are generally contra-indicated in the transplant patient (eg., measles mumps rubella and varicella-zoster vaccines). Transplant patients should be offered the following (if not already immune): Influenza vaccine (annually), tetanus and pertussis vaccine (5 yearly booster), diptheria vaccine (booster 2 years after vaccine and then every 10 years), hepatitis A and B vaccines.[3,9] Family members should be vaccinated in line with the UK vaccination programme recommendations (and stay away from transplant recipients for 1 to 2 weeks after being administered with a live vaccine).[3]
Most opportunistic infections arise between 2 and 6 months post-transplant when the patient is under close follow-up in secondary care.[3,5,10] However, patients are vulnerable to atypical organisms throughout the period they are on immunosuppressant medications and GPs should always consider this when approaching this patient group. Antibiotic prophylaxis against Pneumocystits jirovecii with co-trimoxazole is common up until 1 year with other antibiotic or antifungal prophylactic agents selected by the transplant centre if there are specific concerns.[9]
Patient presenting with fevers should be clinically examined meticulously with the addition of urine, blood, and sputum samples. Chest radiographs alongside routine blood tests should be the first line investigations with a lower index for hospitalising a patient. Any clinical signs of sepsis (clinical symptoms of infection; temperature >38°C or <36°C; tachycardia >90 bpm; tachypnoea RR >20/min; WCC <4 × 109/L or >12 × 109/L) should prompt hospital referral or advice from secondary care specialist. Urinary tract infections (UTI) are a particular problem in the renal transplant group, and any patient with suspected UTI should undergo urine analysis and urine culture and treatment started accordingly.
Prescribing challenges: A barrier to primary care management of transplant patients?
The diagnosis of co-morbid medical conditions in the transplant patient often merits the prescription of further medications and follow-up. Prescribing further medications on top of an already complicated drug regime is a difficult and challenging clinical scenario.
Calcineurin inhibitors are the mainstay of maintenance therapy in the majority of transplants, with steroid free regimes being more commonly used (20% of patients are off corticosteroids at 1-year post-transplant).[26] Both cyclosporine and tacrolimus are extensively metabolised by cytochrome P4503A, meaning that numerous medications may affect drug levels. Table 2 lists the common drug interactions relating to immunosuppressant medications.[27]
Table 2.
Common drug interactions relating to immunosuppressant medications

Public health: Smoking and obesity
During the 20th century, it has been estimated that 100 million people died of tobacco-related illnesses worldwide with this number is expected to increase 10-fold during the 21st century.[28] In addition to the increased risk of cardiovascular events and invasive malignancy that all smokers have,[28] transplant patients that smoke have a 30% increased risk of graft failure.[29] Smoking cessation in transplant patients is associated with improvements in mortality and smoking-related damage may dissipate after 5 years of smoking cessation.[29] Research looking smoking behaviour reported that receiving a kidney transplant acts as a strong incentive for patients’ to stop smoking, particularly in females and patients under 55.[30] Postulated reasons for this included regular contact with medical staff and psychological support after a transplant, alongside concern about potentially losing the transplanted organ.[30] GPs are particularly well placed to talk to patients about smoking cessation, and it is therefore imperative that GPs approach this area in a patient centred way considering how the patient themselves feels about their smoking status.[31] GPs should feel comfortable using psychological and pharmacological approaches to help transplant patients stop smoking as they are equally efficacious in this patient group.[3,9,30] Nicotine replacement therapy can be used in transplant patients as in the general population,[32] with varenicline being reported as effective and safe without drug–drug interactions in some centres.[33] Bupropion may reduce clinically effective concentrations of cyclosporine.[34] Discussions about potential side effects of smoking cessation medications should be no different from the general population, and should include psychiatric side effects of varenicline and bupriopion.
Weight gain is commonly reported in transplant patients.[3,4,5,10,35] This is multifactorial and relates to freedom from pretransplantation dietary restrictions, decreased anorexia (such as patients on dialysis), improvements in patients psychological well being and appetite stimulating medications such as corticosteroids.[3,4,5,10,35] Obesity (BMI > 30) is associated with an increased risk of developing metabolic syndrome, atrial fibrillation, and reduced graft survival.[36] GPs are again well placed to have a thoughtful and sensitive discussion about weight loss and can provide lifestyle advice. Furthermore, GPs in some areas may be able to refer patients directly to local exercise classes and gymnasiums.[37] Life-style alterations including a healthy diet and increased levels of activity or exercise is first-line therapy and is effective.[37] GPs may be able to help patients’ sustain weight loss by helping the patient make lifestyle alterations in conjunction with changes in their behaviours and attitudes towards exercise and nutrition.[37] Nondrug approaches to weight loss are effective, with studies noting improvements in low-density lipoprotein levels with dietary changes alone[37,38] and improvements in high-density lipoprotein levels with an exercise intervention.[37,39] Surgical options and pharmacotherapy medications, such as orlistat, should be discussed with a transplant centre as they may interfere with the absorption of immunosuppressant medications.
Gout
Gout is a common morbidity seen most commonly in liver and renal transplants. Calcineurin inhibitors can impair the excretion of uric acid.[10,27] In addition, all transplant patients are vulnerable to acute kidney injury during the perioperative period, which increases the risk of developing chronic kidney disease (CKD).[40,41] CKD disease may further reduce the clearance of uric acid and increase the risk of a patient developing gout.
An acute attack of gout can be managed in a number of ways. Firstly, gout affecting a single and easily accessible joint can be best managed with an intra-articular steroid injection, once a diagnosis of septic arthritis has been ruled out.[42] For patients where an intra-articular infection is not feasible or gout is in multiple sites there are other options. Nonsteroidal anti-inflammatories (NSAIDs) should be avoided[9] or used cautiously for a very limited duration (<5 days) in patients with good renal function[3] although the use of other options may be prudent. In patients who are not in end stage renal failure colchicine is often used as first line [0.15–0.6 mg] although a myo-neuropathy may develop as an adverse reaction.[9,27,42] Prednisolone may be used as second-line [40 mg initially and tapering down over 4–7 days].[9,43]
Allopurinol should be dose adjusted according to kidney function tests when prescribed for prophylaxis [eg, 100 mg/day] alongside periodic monitoring of urate levels.[44] It is important that the combination of azathioprine and allopurinol is never prescribed due to the risk of bone marrow suppression.[27]
Compliance
Quality healthcare outcomes depend fundamentally upon patients being compliant with medication(s) and/or following advocated interventions.[45] Problems with patient compliance affect all medical specialties from respiratory medicine[46] to psychiatry,[47] and transplantation is no different.[9] Patients who are poorly compliant with therapeutic interventions are more likely to experience worsening or progression of their disease,[48] and transplant patients may reject their graft.[3,4,9] In addition, patients who are noncompliant without the knowledge of their doctor are at risk of being harmed by the use of drugs (and doses of drugs) that would not have been prescribed if their doctor had known about their noncompliance.[45]
Unfortunately, there are not accurate predictors of poor compliance in transplant patients although clinical judgement and knowledge of patients is useful in clinical practice.[49] Despite the lack of accurate predictors,[49] there are both practical and psychological steps that can be taken to try and improve patient compliance in family practice. Complex drug regimens should be simplified if at all possible so the patient can fit taking tablets easily into their everyday lives. Dosette boxes can be used to make taking tablets easier. Such approaches have been shown to improve compliance in many medical specialities.[45]
GPs may be perceived as nonthreatening by patients in addressing sensitive areas around their care such as poor compliance with medications and may be well placed to have an open and meaningful discussion.[9] GPs may provide a realistic assessment of a patients understanding of a drug regimen and its importance to the patients’ health. In addition, GPs can be a reassuring and educational healthcare figure to help lead a patient through their complicated medical drug regimen. Providing a therapeutic supportive relationship in family practice is extremely valuable in improving patient compliance as the importance of taking medications can be made clear and patients questions about medications can be answered.[9,45]
Depression
Depression appears in patients at any stage of the transplant process due to psychological stressors, medications (such as steroids) and physiological disturbances.[50] A retrospective study (n = 47,889) based upon American Medicare claims reported cumulative incidences of depression of 5%, 7.3%, and 9.1% at 1, 2, and 3 years post-transplant, respectively.[51] Diabetes, female gender, obesity, and younger age at time of transplantation (<65) were all associated with higher rates of depression.[51] A smaller Japanese retrospective study (n = 116) noted a prevalence of depression of 41.4%.[52] The study found that patients without a regular income, living alone, who did not receive the desired kidney transplantation and those who experienced a rejection episode were more likely to develop depression.[52] The authors reported that the single best predictor of future depression was living alone, with subjects living alone being 2.51 times more likely to be depressed as those living with others.[52]
Early recognition and treatment of depression is necessary to prevent impact on patient compliance. Dobbels et al. noted that a diagnosis of depression was associated with almost a two-fold increase risk in graft failure and death.[51]
GPs can play a vital role in comprehensive screening of patients felt to be at high risk of developing depression alongside more opportunistic assessment for depression at routine visits of lower risk patients.[3] Cognitive behavioural therapy and other forms of psychotherapy are efficacious[3] and should be used at an early stage for moderate and mild depression for maximal benefit.[5,10] Drug therapy with selective serotonin reuptake inhibitors is well tolerated by patients and is efficacious for depression, with citalopram and escitalopram having the lowest risk of drug–drug interactions with commonly used immunosuppressant agents.[50] Dose adjustments are required when renal or hepatic impairment is present with advice sought from specialist centres if impairment is severe and there are queries about antidepressant dosing.[50]
Headaches
Headaches are common in the immediate postoperative period, and rarely can persist for months to years and present to GPs in family practice.[53] The most common causes of headaches include hypertension, high tacrolimus, or cyclosporine levels but it is common for a cause not to be found.[9,53] Headaches that are transient in nature can often be well managed with as required paracetamol.[53,54] NSAID medications are best avoided for use in this setting, and patients should be advised not to use over the counter (OTC) NSAIDs without informing their GP.[54] Medication over-use headaches should also be explored as a differential if the patient has had long-standing transient headaches whilst taking regular prescribed or OTC pain relief.[54]
Persistent headaches, any signs of raised intracranial pressure or patients with any focal neurology should be referred to hospital for imaging with CT or MRI.[53,54] A lumbar puncture should be carried out if there is any suggestion of meningism.[53] This immunosuppressed patient group may develop neurological symptoms secondary to infections of atypical organism including Cryptococcus, Listeria, or herpes viruses in addition to more common organisms (N. meningitidis and S. pneumoniae).[9]
Aspects of patient care managed in family practice and transplant centres
Cardiovascular disease risk factors
Hypertension
The blood pressure values for being diagnosed as having hypertension are the same as the general population, a systolic blood pressure of 140 mmHg or greater and/or diastolic blood pressure of 90 mmHg or greater[17,18,19] The incidence of hypertension in the transplant population is high, with between 55% to 85% of liver transplant patients being hypertensive.[55,56] There are numerous mechanisms proposed for this high incidence including side effects of immunosuppressant medications (eg, corticosteroids inducing hypervolaemia and calcineurin inhibitors leading to vasoconstriction of the afferent renal arteriole), alongside increased rates of weight gain and cardiovascular disease in the post-transplant population. Hypertension in this patient group is associated with the same cardiovascular complications as the general population (stroke, myocardial infarction etc.[57]) alongside an incremental association between hypertension and graft failure.[58] Therefore, treatment of hypertension is imperative in this patient group with a target blood pressure of <130/80 mmHg.[59]
The management of hypertension should include lifestyle modifications alongside the prescription of antihypertensive medications.[60] Lifestyle recommendations are very similar to the general population and include weight loss in patients who are overweight (BMI > 25),[37] regular moderate exercise, reduced alcohol consumption, and a balanced diet with sodium restriction.[60] Due the increase risk of graft failure in the transplant patient, patients with regular high blood pressure readings in secondary or primary clinics should be treated with medication as there is a risk of target organ damage.[60] Ambulatory blood pressure monitoring should be offered to all patients and may help promote patient control over their own condition and isolate cases of white-coat hypertension. Ambulatory blood pressure monitoring is particularly relevant for kidney transplant patients as there may be a circadian ‘nondipping’ pattern of their blood pressure, which is associated with a greater risk of cardiac death.[61]
Drug and dosing regimens for hypertension should be agreed and arranged in collaboration with the secondary care centre.[3,62] Diuretic treatment with furosemide if often considered first line and is well tolerated, with potassium sparing diuretics best avoided in the transplant patient group.[10,62] Calcium channel blockers (CCBs) can be a useful second line therapy and act as vasodilators to increase renal blood flow and decrease mean arterial pressure. However, selection of a CCB must be done with caution as several including diltiazem and verapamil interact with calcineurin inhibitors and may increase their levels [Table 2]. Amlodipine is used in some centres due to lower rates of drug interactions,[10] but regular levels of calcineurin inhibitors should be monitored in all cases.[62] Angiotensin-coverting enzyme inhibitors (ACEIs) and angiotensin receptor blocks (ARBs) are generally third line in transplant patients, but are first line for patients with proteinuria (>1g/day). Beta-blockers and alpha-blockers are not used regularly and would most commonly be prescribed by a specialist if clinically indicated.
Close follow-up upon the initiation of a new antihypertension medication (in particular ACEIs and ARBs) is recommended with blood pressure readings, creatinine, and electrolytes readings carried out. This may include weekly readings for two weeks and then monthly.[3] Patients not responding to optimal medical therapy should be considered for referral to secondary care for a comprehensive assessment, as there may be a secondary cause for their hypertension such as graft artery stenosis after renal transplant. In addition, poor patient compliance with antihypertensive medications should also be considered as a potential explanation for a poor treatment response.
Dyslipidaemia
Hyperlipidaemia is an established risk factor for the development of cardiovascular disease in transplant patients[63] and prompt recognition and treatment is important. Lifestyle modification with dietary and exercise interventions should be attempted and are associated with improvements in lipid profile levels with and without the addition of statins, the mainstay pharmacotherapy.[37,38,39,64] Calcineurin inhibitors increase the effective concentrations of statins, so there is an increased risk of statin-induced myopathy. Although, this does cease upon the cessation of the statin medication patients should be informed of this increased risk and told to contact their family doctor if they feel that they may be getting muscular pain(s). Fluvastatin has the lowest risk of causing statin-induced myopathy when used in combination with calcineurin inhibitors compared to other drugs in the statin group.[65]
The largest trial in this area (n = 2,102) in 2003 reported that fluvastatin is safe at a clinically effective dose in renal transplant patients against placebo, and reduced the risk of fatal and nonfatal myocardial infarction by 35%.[65] However, the study found no significant difference between groups in the primary end points (major adverse cardiac event, defined as cardiac death, non-fatal myocardial infarction (MI), or coronary intervention procedure).[65] There is also data noting that atorvastatin and pravastatin have good safety profiles in conjunction with tacrolimus, so statins can be used in this patient group at normal dose.[66,67] Other statins should be prescribed initially at a lower dose before being titrated up to reduce the risk of statin-induced myopathy. Hypertriglyceridaemia may be treated with fibrates, but bile acid sequestrants should be avoided as they significantly reduce absorption of calcineurin inhibitors.[66]
Diabetes mellitus type 2
The high incidence of diabetes in the transplant population has already been outlined[11] and its presence has numerous macrovascular and microvascular complications alongside worsening graft survival for transplant patients.[68] The high incidence relates in part to diabetogenic medications (such as corticosteroids) alongside psychosocial factors including the removal of dietary restrictions.
As transplant patients are at high risk of developing diabetes, primary care clinicians should regularly screen asymptomatic patients for diabetes and clearly should investigate any symptomatic patients.
When screening patient, patients usually receive fasting plasma glucose testing at least once a week during the first month after transplant, with an oral glucose tolerance test arranged if levels are abnormally raised.[69] This may be arranged and carried out in the secondary care centre or in the community depending upon individual patient factors. It should be noted that the HbA1c is not recommended before three months following transplantation, as the test may not be valid until new hemoglobin has been synthesized and glycated.[70]
All transplant recipients should receive fasting plasma glucose testing at three, six, and twelve months, and then annually thereafter. Detection of diabetes should promptly lead to treatment alongside intensive preventive strategies that should include a secondary review care of immunosuppressant regimen.
Diabetes is diagnosed in transplant patients the same as in in the general population, by one of the following:[71,72]
+ a random venous plasma glucose concentration >=11.1 mmol/l
+ OR a fasting plasma glucose concentration >=7.0 mmol/l (whole blood >=6.1 mmol/l)
+ OR 2 h plasma glucose concentration >=11.1 mmol/l = 2 h after 75g anhydrous glucose in an oral glucose tolerance test (OGTT)
The management of diabetes in the transplant patient has been based upon research in the nontransplant population, as there is limited evidence of how to manage diabetes in the transplant population.
Once diagnosed with diabetes mellitus type 2, a patient should have a HbA1c checked regularly (3-monthly in some centres) and kidney transplant patients should have annual spot urine protein: creatinine ratios [<20 mg/mmol] calculated alongside usual regular kidney function tests. Patients should be educated about their condition and how to manage and check their blood sugars.[72] Following the ACCORD trial that found higher levels of mortality in the patient cohort with intensive glycaemic control (<6%),[73] HbA1c levels of between 7-7.5% are considered the recommended goal.[72,73,74] However, the International Diabetes Federation recommends a goal of HbA1c <6.5%.[75] The exact target may change slightly depending upon the individual patients ability to manage their diabetes, their risk of developing complications of diabetes, and their experience treatment side effects (such as hypoglycaemia).[72,74] Patients should be referred to the usual diabetes screening programmes and followed up by a diabetes specialist.[72]
Lifestyle changes including improved diet and exercise are of paramount importance to ensure optimal glucose levels and to reduce the risks of diabetic complications.[72] Medical treatment is similar to the nontransplant population and includes oral hypoglycaemic agents and insulin regimens. Preferred agents for the transplant population (based on expert consensus) are sulfonylureas (glipizide), meglitinides (repaglinide), and insulin.[3] Thiazolidinediones should be avoided in patients with congestive cardiac failure and/or coronary heart disease.[27] Metformin should be used with caution in patients with CKD and have their doses adjusted. Finally, a reduction in steroid medications may be appropriate for some patients and can be considered by the transplant centre.[10]
Malignancy
Transplant patients are at a higher risk of developing cancer due to long-term immunosuppression and exposure to oncogenic viral infections. Indeed, a 2011 study of 175,732 transplant patients reported that compared with the general population, recipients of solid organ transplants are at an increased risk of a diverse range of cancers (infection and noninfection related).[76] In an American study of liver transplant patients that died 1 year or more after being transplanted (n = 656), de novo malignancy was the second largest cause of death (16%), only behind recurrent liver disease (20%).[9]
There have been calls for guidelines to recommend best practice to monitor for the development of malignancy in this high-risk group. In the absence of national screening guidelines for immunosuppressed patients, local guidelines have been developed by local health boards. For GP's it is imperative that on routine clinical visits the patient is clinically assessed (through history and or examination) for signs of malignancy and that the GP should have a low index of suspicion for malignancy in this patient cohort.
Cutaneous malignancies are a major clinical concern in the transplant patient population.[77,78,79] Indeed, the relative risk of a transplant patient developing non-melanoma skin cancer was 108.6 for men and 92.8 for women,[78] and another study noted that accuracy of diagnosis from expert Dermatologists was poor without a biopsy.[79] Therefore, regular assessment of the skin and patient education about sun exposure remains very important. Any patient with suspicious lesions should be referred urgently to a dermatologist. Table 1 already outlines how transplant patients could be screened and monitored for the development of colon, breast and cervical cancer. Clinical examination is of great use when considering post-transplant lymphoproliferative disease as a differential diagnosis and patients should be regularly asked about any skin changes that they have noticed in the proceeding few months/years (including the oral cavity). Prostate and bladder malignancy should be investigated in the presence of clinical signs and symptoms.
Bone disease
Osteoporosis (an absolute decrease in the mass of bone) is common in the transplant patient due to long-term corticosteorid medications, immobility postoperatively and poor nutritional status, alongside the general population risk factors (such as menopause and increasing age). It should be noted that patients who have undergone a renal transplant are most at risk of developing osteoporosis. Renal transplant patients have commonly had previous bone loss secondary to dialysis and prior CKD.[3] Hay reported that the first 3 to 6 months sees the most rapid loss of bone mass, between 2 to 15 times the normal population rate.[80] Although there is recovery of bone loss after the first 6 months that may continue for 7 years,[81] the risk of transplant patients maintained on long-term steroids experiencing a fracture is four times greater compared to the general populations risk.[82]
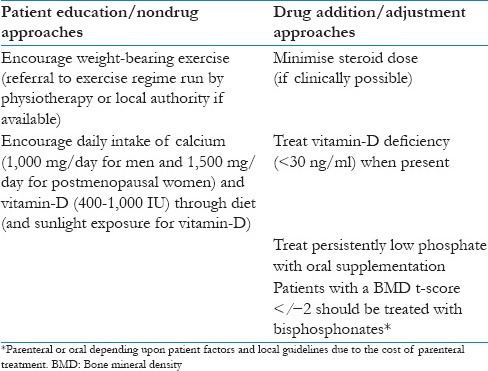
Serum calcium and phosphorus levels can be measured every three months for the first year post-transplant and then annually.[83] Bone mineral density (BMD) and parathyroid hormone levels should be carried out at 0, 1, and 2 years post transplant as this will help guide medical therapy. Recommendations for the management of osteoporosis in the transplant patient based on the current clinical guideline and recent review in Table 3.[3,82,83,84,85] Bisphosphonates are currently the most promising approach for the management of transplantation osteoporosis with vitamin D helping to reduce hyperparathyroidism (mainly in kidney transplantation).[85]
Table 3.
Recommendations for the management of osteoporosis in the transplant patient
Summary
With advances in surgical techniques, postoperative care and immunosuppressant regimes transplant patient survival has increased enormously. However, the increase in patient survival has uncovered increased rates of cardiovascular disease, bone disease and malignancy in this patient group. It has been proposed by secondary specialists that primary care physicians are well placed to identify, and manage the general medical challenges and complications of transplant patients in the community as many of these complications are a fundamental part of family practice. Indeed, this appears likely to happen as in the coming years with more patients receiving transplants and the overall survival of transplant patients improving transplant centres are going to be under enormous pressure to provide comprehensive patient care. Communication between primary and secondary care in transplant medicine will help make this transition smooth and beneficial for patients’ as well as healthcare professionals.
Footnotes
Source of Support: I would like to thank Professor Jeremy Hughes (Chair of Experimental Nephrology and Honorary Consultant Nephrologist, University of Edinburgh) and Dr Simon Watson (Clinical Lead Scottish Patient Safety Fellowship Programme and Consultant Nephrologist, NHS Lothian) for their advice and recommendations in developing this clinical review.
Conflict of Interest: None declared.
References
- 1.Professor Joseph Murray: Surgeon who performed the first successful kidney transplant (2012). Independent Online. [Last accessed on 2013 Oct 01]. Available from: http://www.independent.co.uk/news/obituaries/vprofessor-joseph-murray-surgeon-whoperformed-the-first-successful-kidney-transplant-8360442.html .
- 2.Kirby RR, Taylor RW, Civetta JM. 1st ed. USA: JB Lippincott; 1990. Pocket Companion of Critical Care Immediate Concerns. [Google Scholar]
- 3.Gupta G, Unruh ML, Nolin TD, Hasley PB. Primary care of the renal transplant patient. J Gen Intern Med. 2010;25:731–40. doi: 10.1007/s11606-010-1354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller JC, Prochazka AV, Everson GT, Forman LM. Long-term management after liver transplantation: Primary care physician versus hepatologist. Liver Transpl. 2009;15:1330–5. doi: 10.1002/lt.21786. [DOI] [PubMed] [Google Scholar]
- 5.Kumar K, Clark M. 6th ed. Philadelphia: Saunders Ltd; 2005. Kumar and Clark Clinical Medicine. [Google Scholar]
- 6.The 2007 US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients Annual Report: Transplant Data 1997-2006. Published by the Health Resources and Services Administration. [Last accessed on 2014 Dec 17]. Available from: http://www.srtr.org/annual_reports/archives/2007/2007_Annual_Report/default.htm .
- 7.Cecka JM. The OPTN/UNOS renal transplant registry. Clin Transpl. 2005:1–16. [PubMed] [Google Scholar]
- 8.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–12. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 9.McCashland TM. Posttransplantation care: Role of the primary care physician versus transplant center. Liver Transpl. 2001;7(Suppl 1):S2–12. doi: 10.1053/jlts.2001.28513. [DOI] [PubMed] [Google Scholar]
- 10.Colledge NR, Walker BR, Ralston SH. 21st ed. Philadelphia: Elsevier Ltd; 2010. Davidson's Principles and Practice of Medicine. [Google Scholar]
- 11.Shiener PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JA, et al. Long-term complications in patients surviving >5 years after liver transplant. Transplantation. 2000;69:781–9. doi: 10.1097/00007890-200003150-00018. [DOI] [PubMed] [Google Scholar]
- 12.Aakus S, Dahl K, Wideroe TE. Cardiovascular morbidity and risk factors in renal transplant patients. Nephrol Dial Transplant. 1999;14:648–54. doi: 10.1093/ndt/14.3.648. [DOI] [PubMed] [Google Scholar]
- 13.Lavelle-Jones M, Dent J. 3rd ed. Philadelphia: Elsevier Ltd; 2008. Master Medicine-Surgery. [Google Scholar]
- 14.Aakus S, Dahl K, Wideroe TE. Cardiovascular disease in stable renal transplant patients in Norway: Morbidity and mortality during a 5-year follow-up. Clin Transplant. 2004;18:596–604. doi: 10.1111/j.1399-0012.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- 15.Foley RN, Parfey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S2–13. [PubMed] [Google Scholar]
- 16.Tidy C. Quality and Outcomes Framework (QOF) 2013- 2014. Patient.co.uk Online. 2013. [Last accessed on 2013 Oct 01]. Available from: http://www.patient.co.uk/doctor/quality-and-outcomes-framework-qof-2013-2014 .
- 17.US Preventative Services Task Force. Guide to clinical preventative medicine. 2012. [Last accessed on 2013 Oct 01]. Available from: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/guide/index.html .
- 18.QOF clinical indicators 2013/14: Summary tables. eGuidelines.co.uk. [Last accessed on 2013 Oct 01]. Available from: http://www.eguidelines.co.uk/eguidelinesmain/external_guidelines/qof.php#.UkvZl4XfYd0 .
- 19.Health checks for ages 30 to 64 (2012). NHS Livewell Online. [Last accessed on 2013 Oct 01]. Available from: http://www.nhs.uk/Livewell/Screening/Pages/Checks30to64.aspx .
- 20.Tomson CR. Ambulatory blood pressure measurement in kidney transplantation: An overview. Transplantation. 2003;76:1643–4. doi: 10.1097/01.TP.0000091289.03300.1A. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 22.Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen TH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer, from a randomized controlled trial from the Breast Screening Programme in England. J Med Screen. 2010;17:25–30. doi: 10.1258/jms.2009.009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.What does the NHS Breast Screening Programme do? NHS Screening Online. [Last accessed on 2013 Oct 01]. Available from: http://www.cancerscreening.nhs.uk/breastscreen/screening.programme.html .
- 24.Fischereder M. Cancer in patients on dialysis and after renal transplantation. Nephrol Dial Transplant. 2008;23:2457–60. doi: 10.1093/ndt/gfn183. [DOI] [PubMed] [Google Scholar]
- 25.Pafefsky JM, Gillison ML, Strickler HD. Chapter 16: HPV vaccines in immunocompromised women and men. Vaccine. 2006;24(Suppl 3):S3/140–6. doi: 10.1016/j.vaccine.2006.05.120. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, et al. Immunosuppression: Evolution in practice and trends, 1994-2004. Am J Transplant. 2006;6:1111–31. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 27.BNF. London: BMJ Publishing Group Ltd and RPS Publishing; 2012. British National Formulary 63. [Google Scholar]
- 28.White WB. Smoking-related morbidity and mortality in the cardiovascular setting. Prev Cardiol. 2007;10(2 Suppl 1):1–4. doi: 10.1111/j.1520-037x.2007.06050.x. [DOI] [PubMed] [Google Scholar]
- 29.Kasiske BL, Klinger D. Cigarette smoking in renal transplant recipients. J Am Soc Nephrol. 2000;11:753–9. doi: 10.1681/ASN.V114753. [DOI] [PubMed] [Google Scholar]
- 30.Banas MC, Banas B, Wolf J, Hoffmann U, Krüger B, Böger CA, et al. Smoking behaviour of patients before and after renals transplantation. Nephrol Dial Transplant. 2008;23:1442–6. doi: 10.1093/ndt/gfm764. [DOI] [PubMed] [Google Scholar]
- 31.The basics – Smoking cessation (2010) GPOnline.com. [Last accessed on 2013 Oct 01]. Available from: http://www.gponline.com/Clinical/article/976430/the-basics-smoking-cessation/
- 32.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist vs placebo or sustained release bupriopion for smoking cessation: A randomised controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Lewis BR, Aoun SL, Bernstein GA, Crow SJ. Pharmacokinetic interactions between cyclosporine and bupropion or methylphenidate. J Child Adolesc Psychopharmacol. 2001;11:193–8. doi: 10.1089/104454601750284117. [DOI] [PubMed] [Google Scholar]
- 35.Hasse J. From malnutrition to obesity. Changes in nutritional status associated with liver transplantation. Nutrition. 1999;15:507–8. doi: 10.1016/s0899-9007(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 36.Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–63. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Health and Clinical Excellence. London: National Institute for Health and Clinical Excellence; 2006. Obesity: Guidance on the prevention, identification, assessment and management of overweight adults and children. [PubMed] [Google Scholar]
- 38.Lopes IM, Martin M, Errasti P, Martinez JA. Benefits of a dietary intervention on weight loss, body composition, and lipid profile after renal transplantation. Nutrition. 1999;15:7–10. doi: 10.1016/s0899-9007(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 39.Painter PL, Hector L, Ray K, Paul SM, Dodd M, Tomlanovich SL, et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2005;42:362–9. doi: 10.1016/s0272-6386(03)00673-5. [DOI] [PubMed] [Google Scholar]
- 40.Karthikeyan V, Karpinski J, Nair RC, Knoll G. The burden of chronic kidney disease in renal transplant recipients. Am J Transplant. 2004;4:262–9. doi: 10.1046/j.1600-6143.2003.00315.x. [DOI] [PubMed] [Google Scholar]
- 41.Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18:3031–41. doi: 10.1681/ASN.2007040394. [DOI] [PubMed] [Google Scholar]
- 42.Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British health Professionals in Rheumatology Guideline Management of Gout. Rheumatology (Oxford) 2007;46:1372–4. doi: 10.1093/rheumatology/kem056a. [DOI] [PubMed] [Google Scholar]
- 43.Treatment of Acute Gout. General Practice Notebook. [Last accessed on 2013 Oct 02]. Available from: http://www.gpnotebook.co.uk/simplepage.cfm?ID=-46858210 .
- 44.Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: Walking the tightrope between adequate urate lowering and adverse events. Semin Dial. 2007;20:391–5. doi: 10.1111/j.1525-139X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 45.Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–99. [PMC free article] [PubMed] [Google Scholar]
- 46.Barr RG, Somers SC, Speizer FE, Camargo CA, Jr National Asthma Education and Prevention Program (NAEPP) Patient factors and medication guideline adherence among older women with asthma. Arch Intern Med. 2002;162:1761–8. doi: 10.1001/archinte.162.15.1761. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell AJ, Selmes T. Why don’t patients take their medicines? Reasons and solutions in psychiatry. Adv Psychiatr Treat. 2007;13:336–46. [Google Scholar]
- 48.Miller NH. Review Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102:43–9. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 49.Bunzel B, Laederach-Hofmann K. Solid Organ Transplantation: Are there predictors for posttranplantation noncompliance? A literature overview. Transplantation. 2000;70:711–6. doi: 10.1097/00007890-200009150-00001. [DOI] [PubMed] [Google Scholar]
- 50.Crone CC, Gabriel GM. Treatment of anxiety and depression in transplant patients: Pharmacokinetic considerations. Clin Pharmacokinet. 2004;43:361–94. doi: 10.2165/00003088-200443060-00002. [DOI] [PubMed] [Google Scholar]
- 51.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, MacLean JR, Kasiske BL. Depressive disorder in renal transplantation: Analysis of Medicare claims. Am J Kidney Dis. 2008;51:819–28. doi: 10.1053/j.ajkd.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Tsunoda T, Yamashita R, Kojima Y, Takahara S. Risk factors for depression after kidney transplantation. Transplant Proc. 2010;42:1679–81. doi: 10.1016/j.transproceed.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 53.Stein DP, Lederman RJ, Vogt DP, Carey WD, Broughton TA. Neurological complications following liver transplantation. Ann Neurol. 1992;55:1078–82. doi: 10.1002/ana.410310612. [DOI] [PubMed] [Google Scholar]
- 54.Storr E, Nicholls G, Lee A, Leigh M, McMain S. Germany: Wiley-Blackwell; 2008. Clinical Cases Uncovered - General Practice. [Google Scholar]
- 55.Textor SC. De-novo hypertension after liver transplantation. Hypertension. 1993;22:257–67. doi: 10.1161/01.hyp.22.2.257. [DOI] [PubMed] [Google Scholar]
- 56.Textor SC, Canzanello VJ, Taler SJ, Schwartz L, Augustine J. Hypertension after liver transplantation. Liver Transpl. 1995;1:20–8. [PubMed] [Google Scholar]
- 57.Wang W, Lee ET, Fabsitz RR, Devereux R, Best L, Welty TK, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: The Strong Heart Study. Hypertension. 2006;47:403–9. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- 58.Manage KC, Cizman B, Joffe M, Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–8. doi: 10.1001/jama.283.5.633. [DOI] [PubMed] [Google Scholar]
- 59.K/DOQI. Clinical practice guidelines on hypertension and anti hypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S14–290. [PubMed] [Google Scholar]
- 60.National Institute for Health and Clinical Excellence. London: National Institute for Health and Clinical Excellence; 2011. Hypertension: Clinical management of primary hypertension in adults. [Google Scholar]
- 61.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olyaei AJ, deMattos AM, Bennet WM. A practical guide to the management of hypertension in renal transplant recipients. Drugs. 1999;58:1011–27. doi: 10.2165/00003495-199958060-00005. [DOI] [PubMed] [Google Scholar]
- 63.Jindal RM, Sidner RA, Hughes D, Pescovitz MD, Leapman SB, Milgram ML, et al. Metabolic problems in recipients of liver transplant. Clin Transplant. 1996;10:213–7. [PubMed] [Google Scholar]
- 64.National Institute for Health and Clinical Excellence. London: National Institute for Health and Clinical Excellence; 2010. Lipid modification: Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. [PubMed] [Google Scholar]
- 65.Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: A multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–31. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 66.Kasiske B, Cosio FG, Beto J, Bolton K, Chavers BM, Grimm R, Jr, et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: A report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant. 2004;4(Suppl 7):13–53. doi: 10.1111/j.1600-6135.2004.0355.x. [DOI] [PubMed] [Google Scholar]
- 67.Lemahieu WP, Hermann M, Asberg A, Verbeke K, Holdaas H, Vanrenterghem Y, et al. Combined therapy with atorvastatin and calcineurin inhibitors: No interactions with tacrolimus. Am J Transplant. 2005;5:2236–43. doi: 10.1111/j.1600-6143.2005.01005.x. [DOI] [PubMed] [Google Scholar]
- 68.Jindal RM, Hjelmesaeth Impact and management of post-transplant diabetes. Transplantation. 2000;70(Suppl):S58–63. [PubMed] [Google Scholar]
- 69.Madhira BR, Bunnapradist S. Posttransplant Diabetes Mellitus: Early Screening and Intervention Are Key. Nephrology Times. 2008;1(5):4–650. [Google Scholar]
- 70.Wilkinson A, Davidson J, Dotta F, et al. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin Transplant. 2005;19(3):291. doi: 10.1111/j.1399-0012.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 71.Diagnosis of Diabetes Mellitus. General Practice Notebook. [Last accessed on 2013 Oct 03]. Available from: http://www.gpnotebook.co.uk/simplepage.cfm?ID=1745223718 .
- 72.National Institute for Health and Clinical Excellence. London: National Institute for Health and Clinical Excellence; 2008. Type 2 diabetes: National clinical guideline for management in primary and secondary care (update) [Google Scholar]
- 73.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–7. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 74.Tkác I. Effect of intensive glycemic control on cardiovascular outcomes and all-cause mortality in type 2 diabetes: Overview and metaanalysis of five trials. Diabetes Res Clin Pract. 2009;86(Suppl 1):S57–62. doi: 10.1016/S0168-8227(09)70011-7. [DOI] [PubMed] [Google Scholar]
- 75.Adamu AN. Comparative performance of HbA1c 6.5% for FPG≥7 vs 2hr PG≥111 criteria for diagnosis of Type 2 diabetes. Afr Health Sci. 2011;11:421–6. [PMC free article] [PubMed] [Google Scholar]
- 76.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta AK, Cardella CJ, Haberman HF. Cutaneous malignant neoplasms in patients with renal transplants. Arch Dermatol. 1986;122:1288–93. [PubMed] [Google Scholar]
- 78.Lindelöf B, Sigurgeirsson B, Gäbel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–9. [PubMed] [Google Scholar]
- 79.Cooper SM, Wojnarowska F. The accuracy of clinical diagnosis of suspected premalignant and malignant skin lesions in renal transplant recipients. Clin Exp Dermatol. 2002;27:436–8. doi: 10.1046/j.1365-2230.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 80.Hay JE. Bone disease after liver transplantation. Liver Transpl Surg. 1995;1:55–63. [PubMed] [Google Scholar]
- 81.Hamburg SM, Piers DA, van der Berg AP, Sloof MJ, Haagsma Bone mineral density in the long term after liver transplantation. Osteoporosis Int. 2000;11:600–6. doi: 10.1007/s001980070081. [DOI] [PubMed] [Google Scholar]
- 82.Weisinger JR, Carlini RG, Rojas E, Bellorin-Font E. Bone disease after renal transplantation. Clin J Am Nephrol. 2006;1:1300–13. doi: 10.2215/CJN.01510506. [DOI] [PubMed] [Google Scholar]
- 83.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–201. [PubMed] [Google Scholar]
- 84.Roy D, O’Neill Adebajo WO, Dickson JD, editors. Corticosteroid-Induced Osteoporosis: Prevention And Treatment. Collected Reports on the Rheumatic Diseases Series 4 (REVISED). Arthritis Research Campaign [Google Scholar]
- 85.Ebeling PR. Transplantation osteoporosis. Curr Osteoporos Rep. 2007;5:29–37. doi: 10.1007/BF02938620. [DOI] [PubMed] [Google Scholar]


