Abstract
Matrix metalloproteinases (MMPs), a family of extracellular soluble or membrane bound endopeptidases, are implicated in many physiological and pathophysiological functions—based on their capability to cleave all protein components of the extracellular matrix. Recent studies have implicated several forms of MMPs in chronic neurodegenerative diseases like Alzheimer’s disease (AD), vascular dementia (VD), and Parkinson’s disease (PD). The aim of the present study was to analyse eight MMPs (MMP-1, -2, -3, -7, -8, -9, -10, -13) in the human cerebrospinal fluid (CSF) and to correlate with the well established biomarkers beta-amyloid1–42 (Aβ), total-tau and phospho-tau-181. Our data show a significant decrease of MMP-2 and MMP-3 levels in the CSF in samples with significantly reduced Aβ levels. It is concluded that MMP-2 and MMP-3 are directly linked to Aβ in the brain and a dysfunction may influence the processing of Aβ.
Keywords: Matrix metalloproteinases, Cerebrospinal fluid, Beta-amyloid
Matrix metalloproteinases (MMPs) are a family of extracellular soluble or membrane bound and structurally related zinc-dependent endopeptidases. Subdivided on the basis of protein-domain structure and substrate preference metalloproteinases constitute a family of 24 (human) MMPs [26,27]. Four main subgroups are divided: collagenases, gelatinases, stromelysins, and membrane type MMPs. Collagenases (MMP-1, -8, -13) degrade triple helical fibrillar collagens of bones and cartilage. Gelatinases (MMP-2/gelatinase A, MMP-9/gelatinase B) degrade molecules in the basal lamina around capillaries, enable angiogenesis and neurogenesis, participate in inducing cell death and play a prominent role in injury and repair [21]. Stromelysines (MMP-3, -7, -10, -11) metabolise components of the extracellular matrix, although not the triple helical fibrillar collagens. Membrane bound MMPs act at the cell surface and have several functions, including activation of other proteases and growth factors. MMPs are secreted and cleave all protein components of the extracellular matrix and regulate growth factors, receptors and adhesion molecules. Because of the high potential of tissue destruction MMPs are highly controlled at the gene expression, pro-enzyme activity and secretion to prevent tissue damage [26].
MMPs have been associated with important pathophysiological functions and play a detrimental role in blood-brain barrier (BBB) dysfunction, demyelination, neuroinflammation, CNS injuries and diseases, multiple sclerosis and stroke [26]. Recent studies have implicated MMPs in chronic neurodegenerative diseases associated with Alzheimer’s disease (AD), vascular dementia (VD) and Parkinson’s disease (PD) [1,21]. In fact MMPs are involved in beta-amyloid1–42 (Aβ) degradation and MMPs have been found in AD plaques [2,9]. Several subtypes of MMPs (MMP-2, -3, -7, -8, -9) have been detected in cerebrospinal fluid (CSF) [1,5,7,11,13,19] as well as different subtypes (MMP-1, -2, -9) in plasma [14,15,17]. Since Aβ clearance at the BBB may be dysregulated in AD and MMPs may be involved in the breakdown of the BBB, an interaction of MMPs with Aβ seems to be likely. Thus, the aim of the present study was to measure eight MMPs in CSF and to correlate their expression levels to Aβ, total-tau (t-tau) and phospho-tau-181 (p-tau).
Our sample set included only patients with AD, mild cognitive impairment (MCI), VD and healthy controls, all with or without depression. Subjects with neurological disorders or other psychiatric diseases or other organic diseases or cancer were excluded. CSF was obtained by lumbar puncture in all patients for routine diagnostic procedures at the Innsbruck Medical University hospital (Austria). CSF was collected in polypropylene tubes (Falcon) and frozen within 3 days at −80 °C until analysis. Aβ, t-tau and p-tau were determined by commercial ELISAs (Innogenetics NV, Gent, Belgium). Only clear CSF samples with a total protein (Bradford) < 800 μg/ml were included in this study. The INNOTEST Aβ1–42, INNOTEST Total-tau and INNOTEST Phospho-tau-181 allow for the specific and reliable measurement of these parameters in CSF. The present study was approved by the ethical committee of the hospital [3,16].
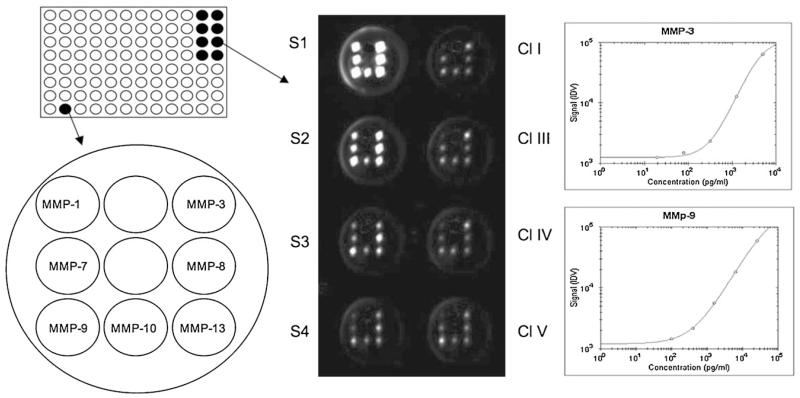
The detection of 8 MMPs (MMP-1, -2, -3, -7, -8, -9, -10, -13) was performed using the Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Vienna). This method is a multiplexing sandwich-ELISA system based on chemiluminescent detection of analytes whose respective capture-antibodies are spotted in arrays within each well of a 96-well microplate (Fig. 1). Briefly, 50 μl calibrated protein standards or 25 μl CSF mixed with 25 μl diluent were added to coated wells, incubated for 60 min at room on a shaker, then washed three times, and then the biotinylated antibodies (50 μl) were added that specifically bind to the captured proteins. After incubation for 30 min at room on a shaker, the wells were again washed (3×) and then incubated with streptavidin-horseradish peroxidase conjugate (50 μl), again washed (3×) and the SuperSignal ELISA Femto Chemiluminescent Substrate (50 μl) was added. The entire plate was imaged using a compatible CCD imaging system to capture the chemiluminescent signal from each spot within each well (Fig. 1). The concentration of each analyte in the array was quantified by comparing the spot intensities for each unknown sample to the corresponding standard curves calculated from the standard sample results by the SearchLight Array Analyst Software. Integrated density values were proportional to the concentrations of bound proteins. Individual analytes were identified by the position of each specific capture (Fig. 1) antibody within the well. Standard curves, raw data and final pg/ml concentrations for each analyte and each sample were reviewed in the array software and exported to Microsoft Excel Software for further statistical analysis. The detection limits for each MMPs are given in Table 2. To reduce interassay variations, all runs of the same multiplex assay were performed on the same day. Each plate contained an own standard curve. The controls and patient group samples were randomly distributed across all plates in duplicates.
Fig. 1.
Analysis of matrix metalloproteinases (MMPs) using Thermo Scientific SearchLight Protein Array Technology. A single well of a 96-well plate is spotted with different primary antibodies against MMPs. After processing of the wells the chemiluminescence is detected by a CCD camera: the spots in the wells S1–S4 correlate to standards of the respective MMPs, which are plotted as standard curves (e.g. for MMP-3 and MMP-9). The unknown biomarker in the wells of clusters I, III, IV, V are calculated from the respective standard curves.
Table 2.
Analysis of 8 matrix metalloproteinases (MMPs) in CSF of seven clusters (CLs).
| DL | Intra-assay CV | CL I | CL II | CL III | CL IV | CL V | CL VI | CL VII | |
|---|---|---|---|---|---|---|---|---|---|
| MMP-1 | 49 | - | <DL | <DL. | <DL | <DL | <DL | <DL | <DL |
| MMP-2 | 62 | 3.9% | 59.022 ± 6.228 | 39.745 ± 2.572* | 55.309 ± 3.475 | 47.846 ± 4.364 | 43.871 ± 2.913 | 60.870 ± 7.096 | 50.869 ± 8.750 |
| MMP-3 | 20 | 15.2% | 511 ± 195 | 61 ± 30* | 332 ± 84 | 214 ± 54 | 172 ± 57 | 386 ± 131 | 490 ± 157 |
| MMP-7 | 7 | 10.8% | 109 ± 37 | 52 ± 9 | 83 ± 16 | 65 ± 7 | 56 ± 11 | 60 ± 16 | 87 ± 22 |
| MMP-8 | 90 | - | <DL | <DL | <DL | 122 ± 18 | <DL | <DL | <DL |
| MMP-9 | 90 | 34.2% | 563 ± 165 | 145 ± 44 | 766 ± 370 | 482 ± 145 | 895 ± 462 | 930 ± 571 | 200 ± 83 |
| MMP-10 | 5 | 9.6% | 43 ± 20 | <DL | 24 ± 10 | 37 ± 13 | 21 ± 12 | 35 ± 19 | <DL |
| MMP-13 | 20 | - | <DL | <DL | <DL | <DL | < DL | <DL | <DL |
MMPs were analysed in CSF by multiplex ELISA. Values are given as mean ± SEM in pg/ml. The detection limit (DL) of the assay is given. Statistical analysis was performed by one-way ANOVA with a subsequent Dunnett post hoc test. CV, coefficient of variation.
p <0.05, compared to cluster I.
Statistical analysis was performed by one Way ANOVA with a subsequent Fisher PLSD or Dunnet post hoc test, where p < 0.05 was significant. Analysis and sample measurement were performed blinded by two individual researchers.
Out of 504 CSF samples, seven clusters (with 82 samples) were included in the assay based on age, Aβ, t-tau and p-tau levels (Table 1). All subjects were older than 62 years. Subjects from clusters IV and V were slightly younger in age (Table 1). The control levels in cluster I of Aβ were 733 ± 29 pg/ml, of t-tau were 311 ± 15 pg/ml and of p-tau were 38 ± 3 ±pg/ml (Table 1). Aβ levels were significantly reduced in clusters II and VII, and enhanced in clusters III and V (Table 1). T-tau levels were significantly enhanced in clusters VI and VII (Table 1). P-tau levels were significantly reduced in clusters IV and V and enhanced in clusters VI and VII (Table 1).
Table 1.
Clustering of samples dependent on CSF beta-amyloid1-42 (Aβ), total-tau (t-tau) and phospho-tau-181 (p-tau) levels.
| Cluster | n | Age | Aβ | t-tau | p-tau | Suggested diagnosis |
|---|---|---|---|---|---|---|
| I | 15 | 74 ± 2 (62–85) | 733 ± 29 (541–950) | 311 ± 15 (257–474) | 38 ± 3 (8–50) | Controls |
| II | 9 | 77 ± 2 (67–86) | 137 ± 7*** (65–194) | 363 ± 25 (249–463) | 69 ± 4 (52–91) | Neuroinflammation, neuroinfection |
| III | 14 | 75 ± 2 (66–85) | 1275 ± 62*** (1015–1670) | 312 ± 15 (258–432) | 35 ± 3 (21–47) | Aβ dysfunction (high) |
| IV | 18 | 72 ± 1** (65–90) | 737 ± 47 (532–997) | 129 ± 17 (40–238) | 17 ± 3*** (10–59) | t-tau dysfunction (low) |
| V | 14 | 69 ± 2*** (66–86) | 1360 ± 95*** (1048–2180) | 182 ± 13 (100–243) | 31 ± 3*** (14–50) | Aβ (high) and t-tau (low) dysfunction |
| VI | 7 | 78 ± 2 (70–88) | 736 ± 57 (515–994) | 1244 ± 131*** (1032–2000) | 122 ± 12*** (64–169) | Neurodegeneration (high t-tau) |
| VII | 5 | 82 ± 2 (77–90) | 187 ± 5*** (167–197) | 1439 ± 242*** (1117–2400) | 151 ± 13*** (120–190) | Alzheimer’s disease (severe) |
Values are given as mean ± SEM and range. n gives the number of analysed samples. Statistical analysis was performed by one-way ANOVA with a subsequent Fisher PLSD post hoc test.
p<0.001.
p<0.01.
SearchLight multiplex ELISA of eight MMPs revealed that MMP-1 and MMP-13 were not detectable in CSF (Table 2). MMP-8 was only detectable in cluster IV with very low p-tau and low t-tau levels (Table 2). MMP-10 was below the detection limit in clusters II and VII, but detectable in all other clusters (Table 2). MMP-2 levels were very high in CSF and significantly reduced in cluster II (Table 2). MMP-3 was detectable in all samples and significantly reduced in cluster II (Table 2). MMP-7 as well as MMP-9 were detectable in all clusters but not changed (Table 2). No correlation was found between age and MMP-2/-3 (R < 0.03), Aβ and MMP-2/-3 (R < 0.05), t-tau and MMP-2/-3 (R < 0.15) and p-tau and MMP-2/-3 (R < 0.05).
In the present study we show a significant decrease of MMP-2 and MMP-3 levels in CSF with significantly reduced Aβ levels. The accuracy of the CSF biomarkers Aβ, t-tau and p-tau to diagnose AD has been assessed in several studies. It is well established that CSF t-tau and p-tau are increased in AD while Aβ levels are reduced in AD [4,16,23]. CSF levels of Aβ, t-tau and p-tau allow distinction of subjects with MCI, who are likely to converse to AD. These three CSF biomarkers therefore indicate different stages of dementing illnesses [4,23]. Furthermore t-tau is significantly enhanced in different neurodegenerative processes and dramatically increased in Creutzfeldt-Jakob disease [22]. In the present study we divided seven different clusters with different changes in Aβ, t-tau and p-tau. Based on well established CSF control levels (Cluster I) [4], we defined six clusters (II–VII) based on low (<194 pg/ml) and high (>1015 pg/ml) Aβ levels, low (<59 pg/ml) and high (>64 pg/ml) p-tau levels and high (>1032 pg/ml) t-tau levels. Recently it has been reported that clusters of CSF biomarker levels are related to cognitive profiles of AD [24]. We decided to use clusters which might allow more restrictive sample analysis in order to perform biochemical homogenous subgroups, since large variations in the levels of these biomarkers may exist among patients with AD and MCI.
MMP-2 (72-kDa gelatinase A) is constitutively expressed and found in healthy brains and CSF [15,21]. It has been detected in various brain structures, and reported to be preferably of astroglial origin. Additionally it has been detected in neurons from cortex and cerebellum [17]. MMP-2 specifically cleaves type IV collagen, the major structural component of basement membranes. MMP-2 causes a disruption of the BBB and increases its permeability. MMP-2 is also important in remodelling the basal lamina, in regeneration of axons and remyelination as well as in angiogenesis and neurogenesis [21]. For AD MMP-2 is important because it has the capability of degrading Aβ1–40 and Aβ1–42 in vitro [20] and in vivo [18]. It is well established that MMP-2 is detected in normal CSF [19]. CSF samples from patients with AD, VD [1] as well as subjects with mild cognitive impairment yielded MMP-2 values that were similar to those of the controls [1,17,18]. In the CSF of patients with primary and metastatic brain tumors (malignant astrocytoma, meningeal carcinomatosis) and patients with neuroborreliosis MMP-2 has been detected [7,10,19]. Patients with amyotrophic lateral sclerosis exhibited high levels of MMP-2 in plasma and CSF [2,17]. In association with the human immunodeficiency virus dementia the MMP-2 level in the CSF has been reported to be elevated [5]. The plasma activities of MMP-2 in relation with oxidative stress in patients with MCI and AD were not different in comparison with healthy controls [17]. Our data show that MMP-2 is reduced in CSF of patients with low Aβ levels, indicating a role in Aβ processing.
MMP-3 (stromelysin-1) is expressed in brain cells (astrocytes, microglia, endothelial cells) and immune cells (T-cells and macrophages). The main physiological substrates of MMP-3 are fibronectin, laminin and various collagens [12]. Furthermore an important extracellular role for MMP-3 is the proteolytic activation of pro-MMPs [27]. An intracellular role for MMP-3 in cell death of dopaminergic neurons has been recently identified [21]. MMP-3 is normally present in the brain at low concentrations, but rapidly increases after inflammatory stimuli [21]. It has been shown that in brain tissue of multiple sclerosis the production of MMP-3 in and around plaques is increased, but the CSF level is not changed [12]. A functional polymorphism in MMP-3 in combination with apolipoprotein epsilon4 non-carriers leads to an increased risk of dementia [8]. MMP-3 increases the permeability of the BBB and it enhances after cerebral hypoxia-ischemia [21]. MMP-3 was detected in low concentrations in the CSF in patients with neuroborreliosis, but not in controls [10]. Our data show that MMP-3 is reduced in CSF of patients with low Aβ levels, and may play a role in Aβ processing.
The accumulation of Aβ in AD derives from an imbalance between the biosynthesis of Aβ and its degradation and removal from the brain [23]. It has been shown that in the CNS MMP-2 and -3 are capable to degrade Aβ in vivo [18]. It is therefore plausible that the decrease of MMP-2 and MMP-3 in CSF may contribute to the accumulation of insoluble Aβ peptides in plaques. On the other hand decreased concentrations of MMP-2 and MMP-3 may protect the BBB and may counteract dysfunctional diffusion of Aβ through the BBB [21]. An accumulation of MMPs in AD brains in the proximity of extracellular amyloid plaques has been shown [2] and a reduction of MMPs may contribute to Aβ accumulation and consequently to AD development. Both MMP-2 and MMP-3 are upregulated by copper, and it is known that the copper homeostasis in AD is altered [6]. A rapid degradation of Aβ by up-regulation of MMP-2 and -3 after addition of the metal ligand clioquinol and copper has been shown in cell culture [25]. Taken together, there is clear evidence that reduced MMP-2 and -3 levels correlate with reduced Aβ levels in CSF.
In summary we show that MMP-2 and -3 levels are reduced in CSF in cases with low Aβ levels. Since low Aβ levels may be associated with neuroinflammation and/or neuroinfection it seems likely that MMPs may also be affected in these disorders. Furthermore, a dysfunctional Aβ clearance at the BBB may directly involve MMPs. A dysregulated Aβ-MMPs interaction may play a role in AD.
Acknowledgements
The study was supported by the Austrian Science Funds (L429-B05). We thank Univ.-Prof. Dr. Josef Marksteiner (LKH Klagenfurt) for help with CSF collections.
References
- [1].Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, Stetler-Stevenson WG, Rosenberg GA. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke. 2004;35:e159–162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- [2].Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40) J. Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blasko I, Jungwirth S, Jellinger K, Kemmler G, Krampla W, Weissgram S, Wichart I, Tragl KH, Hinterhuber H, Fischer P. Effects of medications on plasma amyloid beta (Abeta) 42: longitudinal data from the VITA cohort. J. Psychiatr. Res. 2008;42:946–955. doi: 10.1016/j.jpsychires.2007.10.010. [DOI] [PubMed] [Google Scholar]
- [4].Blennow K. CSF biomarkers for Alzheimer’s disease: use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 2005;5:661–672. doi: 10.1586/14737159.5.5.661. [DOI] [PubMed] [Google Scholar]
- [5].Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann. Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [6].Filiz G, Price KA, Caragounis A, Du T, Crouch PJ, White AR. The role of metals in modulating metalloprotease activity in the AD brain. Eur. Biophys. J. 2008;37:315–321. doi: 10.1007/s00249-007-0244-1. [DOI] [PubMed] [Google Scholar]
- [7].Friedberg MH, Glantz MJ, Klempner MS, Cole BF, Perides G. Specific matrix metalloproteinase profiles in the cerebrospinal fluid correlated with the presence of malignant astrocytomas, brain metastases, and carcinomatous meningitis. Cancer. 1998;82:923–930. doi: 10.1002/(sici)1097-0142(19980301)82:5<923::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [8].Helbecque N, Cottel D, Hermant X, Amouyel P. Impact of the matrix metalloproteinase MMP-3 on dementia. Neurobiol. Aging. 2007;28:1215–1220. doi: 10.1016/j.neurobiolaging.2006.05.030. [DOI] [PubMed] [Google Scholar]
- [9].Helbecque N, Hermant X, Cottel D, Amouyel P. The role of matrix metalloproteinase-9 in dementia. Neurosci. Lett. 2003;350:181–183. doi: 10.1016/s0304-3940(03)00905-4. [DOI] [PubMed] [Google Scholar]
- [10].Kirchner A, Koedel U, Fingerle V, Paul R, Wilske B, Pfister HW. Upregulation of matrix metalloproteinase-9 in the cerebrospinal fluid of patients with acute Lyme neuroborreliosis. J. Neurol. Neurosurg. Psychiatry. 2000;68:368–371. doi: 10.1136/jnnp.68.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 2000;31:80–84. doi: 10.1086/313922. [DOI] [PubMed] [Google Scholar]
- [12].Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res. Brain Res. Rev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- [13].Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, Beal MF. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J. Neurol. Sci. 2003;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- [14].Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003;43:191–196. doi: 10.1016/s0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- [15].Lorenzl S, Buerger K, Hampel H, Beal MF. Profiles of matrix metalloproteinases and their inhibitors in plasma of patients with dementia. Int. Psychogeriatr. 2008;20:67–76. doi: 10.1017/S1041610207005790. [DOI] [PubMed] [Google Scholar]
- [16].Marksteiner J, Pirchl M, Ullrich C, Oberbauer H, Blasko I, Lederer W, Hinterhuber H, Humpel C. Analysis of cerebrospinal fluid of Alzheimer patients. Biomarkers and toxic properties. Pharmacology. 2008;82:214–220. doi: 10.1159/000156487. [DOI] [PubMed] [Google Scholar]
- [17].Martin-Aragon S, Bermejo-Bescos P, Benedi J, Felici E, Gil P, Ribera JM, Villar AM. Metalloproteinase’s activity and oxidative stress in mild cognitive impairment and Alzheimer’s disease. Neurochem. Res. 2009;34:373–378. doi: 10.1007/s11064-008-9789-3. [DOI] [PubMed] [Google Scholar]
- [18].Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Perides G, Charness ME, Tanner LM, Peter O, Satz N, Steere AC, Klempner MS. Matrix metalloproteinases in the cerebrospinal fluid of patients with Lyme neuroborreliosis. J. Infect. Dis. 1998;177:401–408. doi: 10.1086/514198. [DOI] [PubMed] [Google Scholar]
- [20].Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem. Biophys. Res. Commun. 1994;205:1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- [21].Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- [22].Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Saanchez-Valle R, Mitrovaa E, Stoeck K, Sklaviadis T, Kulczycki J, Hess K, Bodemer M, Slivarichova D, Saiz A, Calero M, Ingrosso L, Knight R, Janssens AC, van Duijn CM, Zerr I. CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2006;67:637–643. doi: 10.1212/01.wnl.0000230159.67128.00. [DOI] [PubMed] [Google Scholar]
- [23].Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JC, Montine TJ. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol. 2008;7:704–714. doi: 10.1016/S1474-4422(08)70162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van der Vlies AE, Verwey NA, Bouwman FH, Blankenstein MA, Klein M, Scheltens P, van der Flier WM. CSF biomarkers in relationship to cognitive profiles in Alzheimer disease. Neurology. 2009;72:1056–1061. doi: 10.1212/01.wnl.0000345014.48839.71. [DOI] [PubMed] [Google Scholar]
- [25].White AR, Du T, Laughton KM, Volitakis I, Sharples RA, Xilinas ME, Hoke DE, Holsinger RM, Evin G, Cherny RA, Hill AF, Barnham KJ, Li QX, Bush AI, Masters CL. Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J. Biol. Chem. 2006;281:17670–17680. doi: 10.1074/jbc.M602487200. [DOI] [PubMed] [Google Scholar]
- [26].Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- [27].Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]