Abstract
A marker of Alzheimer’s disease (AD) with a high sensitivity and specificity would facilitate a diagnosis at early stages. Blood platelets may be of particular interest in search of biomarkers, because they express amyloid-precursor protein (APP), and display a dysfunctional processing in AD. The aim of the present study is to establish and validate an assay for secreted amyloid-precursor protein (sAPP)-α and -β in platelets of AD and mild cognitively impaired (MCI) subjects, compared to healthy young and old controls. Freshly isolated platelet extracts (25 μg) were incubated with or without recombinant BACE1 (beta-site APP-Cleaving Enzyme; β-secretase, 8U) at 37°C and low pH and the levels of sAPP-α and sAPP-β were measured by specific ELISAs. Our data show that sAPP-α levels were not different between AD, MCI and control subjects. However, sAPP-β levels in MCI and AD were significantly elevated relative to controls. When recombinant BACE1 was added, no changes were seen in sAPP-α levels, but the processed sAPP-β levels were again markedly increased. The sAPP-β processing was specific and selective after 2.5 hours at 37°C, and was possibly mediated by exogenous BACE1, because it was blocked by a BACE1 inhibitor and BACE1 enzyme levels were enhanced in AD patients. Our data reveal that quantitive analysis of platelet sAPP-β assay by ELISA may be a novel diagnostic biomarker for MCI and AD.
Keywords: Alzheimer, Amyloid-precursor protein, APP, BACE1, Biomarker, ELISA, Diagnosis, Platelets
INTRODUCTION
Alzheimer’s disease (AD) is a severe chronic neurodegenerative disorder of the brain, characterized by β-amyloid (Aβ) plaques, tau pathology, inflammation, synapse loss and cholinergic neurodegeneration resulting in cognitive impairment. A probable diagnosis of AD can be established with a high confidence, based on clinical criteria, laboratory tests, neuroimaging and neuropsychological evaluation [1]. Definitive diagnosis of AD still requires a post mortem detection of Aβ plaques and tau-pathology [2, 3]. However, an early diagnosis of AD is still difficult because early symptoms of the disease are shared by a variety of disorders, including vascular dementia, mild cognitive impairment or mixed forms of dementia with or without depression. The laboratory diagnosis of AD is limited to the analysis of 3 well established biomarkers in cerebrospinal fluid: Aβ42 and total tau and phospho-tau-181 [4-7]. Several studies have been conducted in blood samples in order to establish specific changes of proteins. Despite these great efforts, no specific blood biomarker could be established as a biomarker [8, 9]. However, different biomarkers for AD diagnosis have been identified in blood cells, such as eg. monocytes [10] or platelets [11-17].
Platelets possess the proteolytic machinery to produce Aβ and fragments similar to those produced in AD [18-20], therefore they can be used as a disease model to study amyloid-precursor protein (APP) processing. Neurotoxic Aβ is generated from APP by sequential proteolytic cleavage by β-site APP cleaving enzyme (BACE1, β-secretase) and the γ-secretase complex [20-22]. Alternatively, APP can be cleaved at the α-site within the Aβ sequence by ADAM (A disintegrin and metalloenzyme) family proteases [19]. While the major population of APP is stored in α-granules in platelets as proteolyzed fragments, approx. 10% of platelets APP is associated as a full length protein in the membrane [18, 23-26]. Cleavage of APP at the α-site and β-site produces N-terminal parts of APP referred to as secreted sAPP-α and sAPP-β, respectively. Most of the serum sAPP is considered to be sAPP770, ~75% of which is derived from platelets upon activation [27-29]. It has been reported that in platelets of AD patients a decrease of a larger 130 kilo Dalton (kDa) APP fragment, a decrease of α-secretases and an increase of BACE1 are observed [13, 14, 30], possibly mediated via oxidative stress [30].
The aim of the present study was to use ELISAs and to measure secreted sAPP-α and -β in platelets of mild cognitively impaired (MCI) and AD patients compared to healthy subjects and to stimulate the sAPP processing by exogenous recombinant BACE1. We show, that sAPP-β but not sAPP-α is markedly enhanced in MCI and AD patients.
MATERIAL AND METHODS
Selection of Patients
A total number of 131 patients (healthy controls, AD and MCI) were included in this study. All subjects were recruited from the Memory Clinics at the Landeskrankenhaus Hall/Tirol in Austria. Psychiatrists clinically examined all subjects, performed a standardized neurological examination, reviewed medical records and all subjects underwent a neuropsychological assessment. The study was approved by the local ethical committee of Innsbruck Medical University and was in accordance with Helsinki Declaration and all subjects voiced understanding of the study and consent for participation.
Exclusion criteria for healthy subjects and patients suffering from MCI or AD included 1) another major psychiatric illness, such as bipolar disorder, schizophrenia, and schizoaffective disorder; 2) current or long-term alcohol or drug dependence; 3) primary neurological illness, stroke, Parkinson’s disease, seizure disorder, and multiple sclerosis; 4) current, clinically significant cardiovascular disease; 5) clinically significant hepatic, renal, pulmonary, metabolic or endocrine disturbances and infection parameters. In general, only patients who fulfilled diagnostic criteria for MCI and AD were included. Probable AD was diagnosed according to NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association) criteria [31]. MCI Patients were diagnosed according to the clinical criteria of Petersen et al. [32]. MCI was diagnosed if patients reported subjective memory complaints over the last 6 months, and impaired memory function (verbal of figural) in neuropsychological assessment > 1.5 standard deviation (SD) related to age and education. As part of standard procedures, all subjects had undergone neuropsychological assessment and performed the Mini Mental State Examination Examination (MMSE) [33] at a baseline and a follow-up visit. Further, all patients had completed the Geriatric Depression Scale (GDS) [34].
Collection of Platelets
EDTA blood was collected during normal routine clinical treatment and processed within 24 hours [30]. A total of 10 ml blood was centrifuged (250×g, 15 min, room temperature (RT)). Two-thirds of the upper phase (i.e. platelet-rich plasma) was collected, prostacyclin-2 (PGI2) (500 nM) was added to avoid activation and the samples were again centrifuged (2,300×g, 10 min, RT). Pellets were dissolved in 1 ml Tyrode’s solution (136 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgSo4, 5 mM glucose, pH 6.5) with PGI2 (500 nM), and the samples were centrifuged (1,900×g, 10 min, RT). Pellets were frozen at −80°C until use. In some cases the stability was tested on platelets isolated after 3hr or 24hr of blood collection from the same blood sample.
Platelet Extracts
Platelet pellets were thawed, resuspended in 200 μl secretase buffer (20 mM sodiumacetate, 0.2% Triton, pH 4.5), sonicated with a Branson sonifier (10 strokes, each 10 sec, on ice), then centrifuged at 14,000×g for 10 min. The supernatant was collected, an aliquot used for protein determination by Bradford and the rest frozen at −80°C until use.
BACE1 Assay
Twenty five μg of platelet extracts were incubated without or with 1 μl = 8U recombinant BACE1 (Sigma S4195) in eppendorf tubes in a total of 100 μl with secretase buffer for 150 min at 37°C. For testing the time-dependent release, some samples were incubated for additional 1-3-or 6 days.The ELISA was immediately started after incubation using 50 μl of this assay.
BACE1 Inhibitor Assay
Twenty five μg of platelet extracts were incubated without or with 10 or 100 μg/ml BACE1 inhibitor (Sigma S4562) in eppendorf tubes in a total of 100 μl with secretase buffer for 150 min at 37°C. The inhibitor (1 mg) was dissolved in 100 μl DMSO and further diluted in secretase buffer. As a control the respective amount of DMSO was tested.
sAPP ELISA
The sAPP-α ELISA (IBL, code 27734, Demetitec, Germany) and the sAPP-β ELISA (Covance SIG-38960, DCS Diagnostics, Germany) were used according to the manufacturer description. Briefly, for the sAPP-α ELISA, 100 μl sample/standard were incubated overnight at 4°C, then washed, 100 μl labelled antibody was added, incubated for 30 min at 4°C, again washed and 100 μl chromogen was added and incubated on a shaker at RT for 30 min. Then 100 μl stop solution was added and measured in a Zenyth ELISA reader at 450 nm. For the sAPP-β ELISA, plates were shortly washed, 100 μl standard/sample was added and incubated overnight at 4°C. The wells were washed, 100 μl HRP conjugate added, incubated for 1 hr at room, again washed, and after adding 100 μl substrate, the wells were measured in the Zenyth for luminescence. All values were calculated according to the standard curve. The sAPPP-α values were close to the detection limit (approx. 200 pg/ml), while sAPP-β was clearly detectable (detection limit 25 pg/ml).
BACE1 ELISA
BACE1 was determined by using the BACE1 ELISA Kit (FIVEphoton Biochemical, San Diego, USA). Briefly, samples/standards were incubated in the prepared wells for 1hr, washed, the HRP conjugate added, again incubated for 1hr, washed, then the substrate was added and after 15 min incubation at 37°C samples were measured at 450 nm.
Western Blot
To analyze the expression of APP fragments, Western blot analysis was performed as described recently [30]. Briefly, 50 μg of the platelet extracts were incubated with or without recombinant BACE1 in 10 μl secretase buffer at 37°C. Then the samples were directly loaded (non-denatured) onto 4-12% Bis-Tris polyacrylamide gel (Invitrogen) and electrophoresed for 60 min at 200 V. Samples were electrotransferred to nylon PVDF Immobilon-PSQ membranes (Millipore) for 90 min at 30V with 20% methanol blotting buffer (Invitrogen). For detection, the Western Breeze Chemiluminescent System (Invitrogen) was used. Blots were blocked for 30 min with blocking buffer, then incubated overnight at 4°C with the primary antibody rabbit-anti APP [Y188] (1:2000, abcam ab 32136) or rabbit anti-actin antibody (1:1000, Sigma-Aldrich). Blots were washed and incubated with alkaline phosphatase-conjugated anti-mouse or anti-rabbit antibodies for 30 min at room temperature. After being washed, blots were incubated in CDP-Star chemiluminescent substrate solution (Invitrogen) and the signal was visualized with a cooled CCD camera (SearchLight, Thermoscience).
Statistical Analysis
For statistical analysis a One way ANOVA has been used with a Fisher-PLSD posthoc test where p<0.05 was significant. In all cases the ratios (with or without BACE1) were calculated.
RESULTS
Subjects
The younger controls had an age of 52 years, while the older controls were 71 years old (Table 1). MCI patients were slightly and AD patients were markedly older (Table 1). The MMSE was significantly decreased in AD patients (Table 1), while the GDS was unchanged (Table 1).
Table 1. Secreted Amyloid-Precursor Protein sAPP-α and -β Levels in Platelets of Young and Old Controls, Mild Cognitively impaired (MCI) and Alzheimer Patients.
| Control Young | Control Old | MCI | Alzheimer | |
|---|---|---|---|---|
| n | 11 | 33 | 19 | 68 |
| age [years] | 52±2 *** | 71±1 | 76±2 * | 79±1 *** |
| MMSE | na | 28.2±9.4 | 27.4±0.4 | 21.0±0.5 *** |
| GDS | na | 6.4±0.7 | 4.6±1.0 | 6.0±0.6 |
| sAPP-α | ||||
| without BACE1 | 497±116 | 424±85 | 456±21 | 408±57 |
| with BACE1 | 580±104 | 393±70 | 317±28 | 479±73 |
| ratio | 1.22±0.4 | 0.74±0.1 | 0.77±0.2 | 1.00 ±0.16 |
| sAPP-β | ||||
| without BACE1 | 611±136 | 519±50 | 1,550±418* | 1,548±211** |
| with BACE1 | 1,783±498 | 2,283±273 | 5,682±1,738 | 6,054±1,307 * |
| ratio | 3.4±0.8 | 4.4±0.6 | 4.3+0.8 | 4.0±0.5 |
Platelets were isolated from blood, extracts incubated with or without recombinant BACE1 (β-secretase, 8U, 150 min, 37°C) and then directly processed for an sAPP-aor-β ELISA. Values are expressed as pg/ml × 25 μg × 150 min (except ratio). Statistical analysis was performed by one-way ANOVA with a subsequent Fisher PLSD posthoc test. Values are given as mean±SEM, n gives the number of analyzed subjects. All groups were compared against the old controls.
p<0.05;
p<0.01;
p<0.001;
p<0.1
na not applicable.
Secreted APP-α and -β Levels
All values represent APP levels in pg per 1 ml assay buffer secreted by 25 μg platelet extracts within 150 min at 37°C. The sAPP-α levels in controls were around 400-500 pg/ml*25 μg*150 min and were not different between young and old controls and also not different to MCI and AD patients (Table 1). The sAPP-β levels in controls were approx. 600 pg/ml*25 μg*150 min in young or old controls (Table 1). However, sAPP-β levels were significantly enhanced in MCI (p<0.05) and AD (p<0.01) patients (Table 1).
Effects of Recombinant BACE1 on sAPP Levels
When recombinant BACE1(8U) was added to the platelet extracts no change in sAPP-α levels was found in all tested groups (Table 1). Also the ratio of sAPP-α with or without BACE1 did not change (Table 1). However, BACE1 significantly enhanced the sAPP-β levels in young controls (3.4x) and old controls (4.4x) as well as in MCI (4.3x) and AD (4.0x) patients. Again the sAPP-β levels were significantly enhanced in AD (p<0.05) but (not significant) in MCI patients (Table 1).
Stability of sAPP
In order to test the stability of sAPP-α and sAPP-β in platelets, human blood was divided into 2 parts, one part was processed within 3 hr and the other part after 24hr. Our data show that the sAPP-α and sAPP-β levels were stable for up to 24hr in platelets (Table 2).
Table 2. Stability of Secreted Amyloid-Precursor Protein sAPP-α and -β levels in Platelets Isolated after 3hr or 24hr.
| sAPP-α | sAPP-β | |
|---|---|---|
| after 3hr | 368±79 | 605±206 |
| after 24hr | 341±46 | 657±233 |
| t value | −1.25 | −0.25 |
| t-probability | 0.23 | 0.79 |
| correlation | 0.71 | 0.59 |
| corr. probability | 0.008 | 0.032 |
Platelets were isolated from blood after 3hr or after 24hr (same blood, splitted). (n=13). Statistical analysis was performed by a paired T-test. Note that the sAPP levels were stable for up to 24 hr in blood.
Time Dependent Release
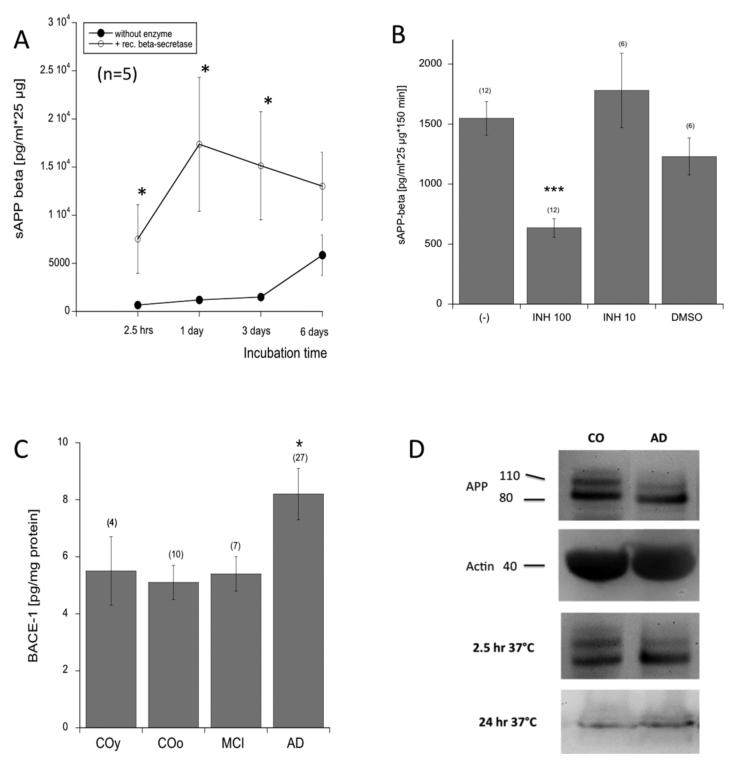
When plateletes were incubated (without enzyme) for 2.5-1-3-6 hours, a slight linear increase in sAPP-β release was seen up to 3 days which markedly increased after 6 days (Fig. 1A). When platelets were incubated with BACE1, the sAPP-β release increased after 1 day but did not change thereafter (Fig. 1A). When platelets were incubated for 2.5 hours at 37°C with 100 μg/ml (but not 10 μg/ml) BACE1 inhibitor the increase in sAPP-β was inhibited (Fig. 1B). Incubation of platelets with respective amount of DMSO slightly decreased sAPP-β release (Fig. 1B).
Fig. (1).
(A) Time course of secreted amyloid-precursor proteins APP-β release with (open circles) and without (filled circles) recombinant BACE1 (β-secretase) measured after 2.5 hours, 1-3-6 days. Values are mean±SEM pg/ml*25 μg (n=5). (B) When Alzheimers disease (AD) platelet extacts (25 μg) were incubated without (−) or with 100 or 10 μg/ml BACE1 inhibitor (INH100, INH10), the increase in sAPP-β release was significantly blocked by 100 μ/ml inhibitor. The respective amount of DMSO served as a control. (C) BACE1was significantly enhanced in AD platelets but not in platelets of young controls (COy) or patients with mild cognitive impairment (MCI) compared to old controls (COo). Values are pg/ml*25/μ*150 min (A,B) or mean±SEM pg/mg protein (C); values in parenthesis give the number of n. Statistical analysis was performed by students T-test (A) or one way ANOVA (B,C) with a subsequent Fisher LSD posthoc test (*p<0.05; *** p<0.001). (D) Western Blot analysis of platelet controls (CO) or AD extracts shows APP like immunore activity at approx. 130 and (110/106) kDa. Actin serves as an internal control as seen as a band at approx. 40 kDa. Platelet extracts incubated for 2.5hr at 37°C did not differ from controls, while the APP bands markedly disappeared after incubation with BACE1 overnight at 37°C.
BACE1 Levels
BACE1 levels in platelet extracts did not differ between young and old controls and MCI patients, but were significantly enhanced in AD patients (Fig. 1C).
Western Blot Analysis
Platelet control extracts displayed 2 strong APP-positive bands at 130 and 110/106 kDa, and in AD platelet extracts the larger band disappeared (Fig. 1D). Actin served as a control and was seen as a 40 kDa band in the western Blot (Fig. 1D). When platelet extracts were incubated for 2.5 hr, no marked changes in APP expression were visible, however, after incubation overnight with BACE1 at 37°C, the APP bands mainly disappeared (Fig. 1D).
DISCUSSION
In the present study we show that secreted sAPP-α derived from platelets was not affected in MCI and AD patients, while sAPP-β was markedly enhanced in MCI and AD patients compared to healthy subjects. Recombinant BACE1 increased sAPP-β but not sAPP-α levels. Thus, platelet-derived sAPP-β may serve as a diagnostic marker for AD.
APP in Alzheimer’s Disease
It is well established that the larger APP fragement is markedly reduced in platelets [13, 14, 30], possibly affecting Aβ processing. Platelets are the major source for plasma-derived Aβ40, while the Aβ42 mainly comes from brain. However, there is a steady-state clearance of Aβ from brain to blood and vice versa. Some authors found altered Aβ in blood of AD patients, but to date these data could not be verified in international studies and are very controversial. It seems that the blood Aβ is very sensitive to different environmental influences, such as e.g. medications [35], which could be mediated by platelets. We tried to measure Aβ42 in our human platelet samples, but failed due to low sensitivity. However, it would be extremely interesting to stimulate conditions, which may selectively induce the processing of Aβ42 from platelet-derived APP. Such a processing could have major implications in the development of the disease. Our data now show for the first time, that secreted sAPP-α was not changed in MCI and AD patients, while the sAPP-β levels were significantly increased in MCI and AD patients. The role of these sAPPs in plasma and platelets are not fully clear, but is has been suggested thats APPs may exhibit neuroprotective and synapse-promoting activities, at least in the brain [36]. In fact, sAPP770 is present in high levels in serum and its secretion from endothelial cells is increased by infammatory cytokines [27], pointing to a role during oxidative stress and inflammation. In contrast, in cerebrospinal fluid, sAPP695 has been reported to be present [27] and they suggested that this form tends to increase in the early stages of AD. More work needs to be done to explore the functional role of sAPP-α and -β in CSF and serum during the AD pathology.
Analysis of sAPP
The analysis of APP fragments by Western Blot is very insensitive and heterogenous and diagnostic accuracy is very low [37], which might be the reason that this method did not yet enter clinical routine. In order to overcome this problem we used selective and sensitive ELISAs. In our study, platelets were isolated from whole blood by a well established procedure [30], then proteins were extracted by sonification to open the cells and membranes lysed by Triton. The extraction was performed at low pH (4.5) and low temperature (4°C) to inhibit non-specific endogenous enzymatic reactions at neutral pH. After centrifugation (14,000×g) acidic platelet extracts were incubated at 37°C. Indeed, it has been shown that BACE1 works well at low pH (4.5) as given in the Sigma datasheet. When platelet extracts were incubated at 37°C for 150 min approx. 500 pg/ml*25 μg sAPP-α and equal levels of sAPP-β were detectable in controls, which seems to be mediated by endogenous released enzymes.
When exogenous recombinant BACE1(8U) was added to the platelet extracts, then no effects were seen in the processing of APP to sAPP-α, which is in full agreement, since the BACE1does not produce sAPP-α. On the other hand, exogenous BACE1 markedly enhanced the cleavage of APP to sAPP-β. The sAPP-β levels increased up to 6000 pg/ml*25 μg in AD patients, representing a 4-fold increase. This increase was always 4-fold, independent if we measured controls, MCI or AD patients. This points to the specificity of the assay that APP is cleaved into sAPP-β but not sAPP-α by exogenous BACE1. Furthermore, AD patients (and not significantly MCI patients) again displayed a significant increase in sAPP-β levels in platelets after incubation with exogenous BACE1. During our preparations and assay we recognized that the APP in extracts is very unstable. Thus, we recommend to store samples frozen and to process the ELISAs immediately after incubation. This unstability is also in agreement with Kitazume et al. [27], showing a rapid turnover of platelets APP within minutes.The same group [27] used a novel ELISA but could not differentiate between sAPP-α and -β. However, our data show, that APP seems to be stable in EDTA blood, because we did not see any marked difference between sAPP levels when platelets were isolated after 3hr or 24hr.
BACE1 in Alzheimer’s Disease
Recently, it has been reported that soluble sAPP-α decreases Aβ generation by directly associating with β-site APP-converting enzyme BACE1, whereas specifically targeting sAPP-α using antibodies enhances AP production [38]. Several reports have documented increased BACE1 protein and activity in the brain of AD patients [39-41]. However, BACE1 expression is not only affected in AD but also by many other factors such as oxidative stress [42], ischemia [43] or, hypoxia [44]. Our data provide evidence that enhanced endogenous BACE1 may indeed play a role in the processing of APP to sAPP-β. First, we show enhanced sAPP-β in normal platelet AD extracts (without exogenous enzyme), second recombinant exogenous BACE1 markedly enhanced sAPP-β and third, BACE1 levels are increased in platelet extracts of AD patients.
Study Limitations
There are some limits of this study. First, the age of the MCI and AD patients was statistically different and MCI patients were 5 years older, and AD patients 8 years older. Unfortunately, it is often difficult to get blood of age-matched older patients. Thus we cannot fully exclude a time-dependent shift of sAPP-β. On the other hand, we measured sAPP levels in younger controls (19 years younger in average), and we did not find any significant difference in sAPP-α or -β levels. Thus it is very unlikely, that the effect in MCI and AD patients is age-dependent. Second, the extraction procedure releases an endogenous enzymatic cocktail, which drives the processing of sAPP-α and -β. Third, we show that a longer incubation than 2.5 hours may markedly enhance unspecific processing of platelet APP. And fourth, our data does not tell anything about processing of Aβ, and it would be interesting to find conditions, which could force selective cleavage of Aβ peptides.
Taken together, our data show that secreted APP-β was markedly enhanced in platelet extracts of AD and MCI patients, and may provide a simple and fast assay for diagnosing AD in blood samples.We will need further longitudinal and international studies to verify these results and to establish sAPP-β as a novel biomarker.
ACKNOWLEDGEMENTS
This study has been supported by the Austrian Science Funds (P24734-B24). We thank Karin Albrecht and Ursula Kirzenberger-Winkler for excellent technical help.
Footnotes
CONFLICT OF INTEREST The authors confirm that this article content has no conflicts of interest.
ETHICAL STATEMENT The study was approved by the local ethical committee of Innsbruck Medical University and was in accordance with Helsinki Declaration and all subjects voiced understanding of the study and consent for participation.
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
REFERENCES
- [1].Desai AK, Grossberg GT. Diagnosis and treatment of Alzheimer’s disease. Neurology. 2005;64:34–9. doi: 10.1212/wnl.64.12_suppl_3.s34. [DOI] [PubMed] [Google Scholar]
- [2].McKeel DW, Price JL, Miller JP, et al. Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. J Neuropathol Exp Neurol. 2004;63:1028–37. doi: 10.1093/jnen/63.10.1028. [DOI] [PubMed] [Google Scholar]
- [3].Fradinger EA, Bitan G. En route to early diagnosis of Alzheimer’s disease - are we there yet? Trends Biotechnol. 2005;23:531–3. doi: 10.1016/j.tibtech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [4].Blennow K. CSF biomarkers for Alzheimer’s disease: use in erarly diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 2005;5:661–72. doi: 10.1586/14737159.5.5.661. [DOI] [PubMed] [Google Scholar]
- [5].Zetterberg H, Blennow K, Hanse E. Amyloid β and APP as biomarkers for Alzheimers disease. Exp Gerontol. 2010;45:23–9. doi: 10.1016/j.exger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- [6].Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol. 2010;45(1):30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Blasko I, Lederer W, Oberbauer H, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement Geriatr Cogn Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- [8].Humpel C. Identifying and validating biomarkers for diagnosing Alzheimer’s disease. Trends Biotechnol. 2010;29(1):26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pelech S. Biomarker identification for diagnosis of Alzheimers disease. Expert Opin Med Diagn. 2008;2:577–91. doi: 10.1517/17530059.2.5.577. [DOI] [PubMed] [Google Scholar]
- [10].Hochstrasser T, Marksteiner J, Defrancesco M, Deisenhammer EA, Kemmler G, Humpel C. Two blood monocytic biomarkers (CCL15 and p21) combined with Mini-Mental State Examination discriminates Alzheimer’s disease patients from healthy controls. Dement Geriatr Cogn Disord Extra. 2011b;1(1):297–309. doi: 10.1159/000330468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hochstrasser T, Ehrlich D, Marksteiner J, Sperner-Unterweger B, Humpel C. Matrix metalloproteinase-2 and epidermal growth factor are decreased in platelets of Alzheimer patients. Curr Alzheimer Res. 2011a;9(8):982–9. doi: 10.2174/156720512803251156. [DOI] [PubMed] [Google Scholar]
- [12].Padovani A, Pastorino L, Borroni B, et al. Amyloid precursor protein in platelets: a peripheral marker for the diagnosis of sporadic AD. Neurology. 2001;57:2243–8. doi: 10.1212/wnl.57.12.2243. [DOI] [PubMed] [Google Scholar]
- [13].Borroni B, Agosti C, Marcello E, Di Luca M, Padovani A. Blood cell markers in Alzheimer disease: amyloid precursor protein form ratio in platelets. Exp Gerontol. 2010;45:53–6. doi: 10.1016/j.exger.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [14].Borroni B, Perani D, Broli M, et al. A. Pre-clinical diagnosis of Alzheimer disease combining platelet amyloid precursor protein ratio and rCBF spect analysis. J Neurol. 2005;252:1359–62. doi: 10.1007/s00415-005-0867-z. [DOI] [PubMed] [Google Scholar]
- [15].Borroni B, Di Luca M, Padovani A. Predicting Alzheimer dementia in mild cognitive impairment patients. Are biomarkers useful? Eur J Pharmacol. 2006;545:73–80. doi: 10.1016/j.ejphar.2006.06.023. [DOI] [PubMed] [Google Scholar]
- [16].Vignini A, Sartini D, Morganti S, et al. Platelet amyloid precursor protein isoform expression in Alzheimer’s disease: evidence for peripheral marker. Int J Immunopathol Pharmacol. 2011;24:529–34. doi: 10.1177/039463201102400229. [DOI] [PubMed] [Google Scholar]
- [17].Casoli T, Di Stefano G, Balietti M, Solazzi M, Giorgetti B, Fattoretti P. Peripheral inflammatory biomarkers of Alzheimer’s disease: the role of platelets. Biogerontology. 2010;11:627–33. doi: 10.1007/s10522-010-9281-8. [DOI] [PubMed] [Google Scholar]
- [18].Bush Al, Martis RN, Rumble B, et al. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J Biol Chem. 1990;265:15977–83. [PubMed] [Google Scholar]
- [19].Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL. Secretion of Alzheimer’s disease Aß-amyloid peptide by activation of human platelets. Lab Invest. 1998;78:461–9. [PubMed] [Google Scholar]
- [20].Chen M, Inestrosa NC, Ross GS, Fernandez HL. Platelets are the principal source of amyloid β-peptide in human blood. Biochem Biophys Res Commun. 1995;213:96–103. doi: 10.1006/bbrc.1995.2103. [DOI] [PubMed] [Google Scholar]
- [21].De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol. Rev. 2010;90:465–94. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- [22].Li QX, Fuller SJ, Beyreuther K, Masters CL. The amyloid precursor protein of Alzheimer disease in human brain and blood. J Leukoc Biol. 1999;66:567–74. doi: 10.1002/jlb.66.4.567. [DOI] [PubMed] [Google Scholar]
- [23].Evin G, Zhu A, Holsinger RM, Masters CL, Li QX. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein in brain and platelets. J Neurosci Res. 2003;74:386–92. doi: 10.1002/jnr.10745. [DOI] [PubMed] [Google Scholar]
- [24].Li QX, Berndt MC, Bush AI, et al. Membrane-associated forms of the β A4 amyloid protein precursor of Alzheimer’s disease in human platelet and brain: surface expression on the activated human platelet. Blood. 1994;84:133–42. [PubMed] [Google Scholar]
- [25].Tang K, Hynan LS, Baskin F, Rosenberg RN. Platelet amyloid precursor protein processing: a bio-marker for Alzheimer’s disease. J Neurol Sci. 2006;240:53–8. doi: 10.1016/j.jns.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Skovronsky DM, Lee VM, Praticò D. Amyloid precursor protein and amyloid β peptide in human platelets Role of cyclooxygenase and protein kinase C. J Biol Chem. 2001;20:17036–43. doi: 10.1074/jbc.M006285200. [DOI] [PubMed] [Google Scholar]
- [27].Kitazume S, Yoshihisa A, Yamaki T, et al. Soluble amyloid precursor protein 770 is released from inflamed endothelial cells and activated platelets: a novel biomarker for acute coronary syndrome. J Biol Chem. 2012;287(48):40817–25. doi: 10.1074/jbc.M112.398578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Di Luca M, Pastorino L, Bianchetti A, et al. Differential level of plateletamyloid β precursor protein isoforms: an early marker for Alzheimer disease. Arch Neurol. 1998;55:1195–2000. doi: 10.1001/archneur.55.9.1195. [DOI] [PubMed] [Google Scholar]
- [29].Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer’s disease β A4 amyloid precursor protein in human platelets. J Biol Chem. 1995;270:14140–7. doi: 10.1074/jbc.270.23.14140. [DOI] [PubMed] [Google Scholar]
- [30].Ehrlich D, Hochstrasser T, Humpel C. Effects of oxidative stress on amyloid precursor protein processing in rat and human platelets. Platelets. 2013;24(1):26–36. doi: 10.3109/09537104.2012.661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [32].Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 1992;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- [33].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [34].Yesavage JA, Brink TL, Rose TL, et al. Development and Validation of A Geriatric Depression Screening Scale - A Preliminary-Report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- [35].Blasko I, Jungwirth S, Jellinger K, et al. Effects of medications on plasma amyloid beta (Abeta) 42: longitudinal data from the VITA cohort. J Psychiatr Res. 2008;42(11):946–55. doi: 10.1016/j.jpsychires.2007.10.010. [DOI] [PubMed] [Google Scholar]
- [36].Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Role of the APP non-amyloidogenic signaling pathway and targeting α-secretase as an alternative drug target for treatment of Alzheimer’s disease. Curr Med Chem. 2007;14:2848–64. doi: 10.2174/092986707782360060. [DOI] [PubMed] [Google Scholar]
- [37].Zainaghi IA, Talib LL, Diniz BS, Gattaz WF, Forlenza OV. Reducedplatelet amyloid precursor protein ratio (APP ratio) predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Neural Transm. 2012;119(7):815–9. doi: 10.1007/s00702-012-0807-x. [DOI] [PubMed] [Google Scholar]
- [38].Obregon D, Hou H, Deng J, et al. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation. Nat Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–9. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- [40].Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor β-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–6. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- [41].Li R, Lindholm K, Yang LB, et al. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2004;101:3632–7. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tamagno E, Bardini P, Obbili A, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–88. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- [43].Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased β-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- [44].Zhang X, Zhou K, Wang R, et al. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J Biol Chem. 2007;282:10873–80. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]