Abstract
Increased homocysteine (Hcy) levels contribute to a variety of cardiovascular and cerebrovascular diseases including stroke and Alzheimer’s disease. Recent data has shown that elevated levels of Hcy can lead to blood-brain barrier (BBB) dysfunction and activation. However, the mechanism for Hcy-mediated dysfunction remains unclear. The aim of this study is to characterize the effects of moderate Hcy administration in rat brain capillary endothelial cells (BCECs), which serve as a simple model to study blood-brain barrier functions. This present study shows that addition of 20 μM Hcy for 6 days does not significantly affect BCEC survival, as measured by acridine orange staining, propidium iodide staining, and nitrite content. However, addition of 20 μM Hcy for 6 days does elevate lactate dehydrogenase (LDH) activity released into the supernatant of BCECs, as well as significantly enhances the transmigration of monocytes across the BCEC in a time-dependent manner. In addition, TNFα levels in BCEC are also elevated by Hcy, whereas inflammatory markers MIP3α and RANTES are significantly reduced. Finally, this study shows that intercellular adhesion molecule-1 (ICAM-1) expression is significantly enhanced by 20 μM Hcy treatment compared to control conditions. These results suggest that moderate levels of homocysteine can affect proinflammatory patterns expressed by BCECs, ultimately leading to BBB activation and dysfunction through enhanced monocyte transmigration and ICAM-1 expression.
Keywords: Homocysteine, blood-brain barrier, intracellular adhesion molecule-1, monocyte transmigration
INTRODUCTION
An elevated level of plasma homocysteine (or hyperhomocysteinemia, HHcy) has been implicated in a number of cardiovascular and cerebrovascular diseases [1,2]. These diseases can result in health problems ranging from mild cognitive impairment, to severe dementia, atherosclerosis, seizure and stroke [3,4]. Despite evidence that HHcy serves as a strong indicator and independent risk factor for these diseases [5-8], its pathological role in disease onset and progression, particularly blood-brain barrier (BBB) dysfunction and activation, remains unclear. Homocysteine (Hcy) is a sulphur containing amino acid that circulates in plasma upon its generation from the metabolism of methionine [9,10]. At elevated levels, this non-essential amino acid has been associated with BBB toxicity and dysfunction [1,11,12].
HHcy-mediated dysfunction may also result in enhanced recruitment of leukocytes into the central nervous system (CNS) by increased chemokine and cell adhesion molecule expression. A recent study demonstrated the presence of inflammation in mild HHcy mice accompanied by increased leukocyte adherence and endothelial activation [11]. HHcy treatment of cultured aortic endothelial cells results in enhanced expression of chemokines [13] and adhesion molecules [14,15]. Studies have further demonstrated that HHcy can promote the differentiation of monocytes into macrophages [16] and stimulate chemokine production [reviewed in 17]. Monocytes circulate throughout the bloodstream eventually giving rise to macrophages. Considerable evidence has shown that elevated levels of Hcy can promote and enhance monocyte adhesion. Postea et al. [18] showed a time and dose-dependent increase in monocyte adhesion by human acute monocytic leukemia cell line (THP-1) cells to human endothelial cells or primary human umbilical vein endothelial cells when treated with L-Hcy. In addition, Silverman et al. [14] demonstrated that human aortic endothelial cells incubated with Hcy for 24 hrs increases monocyte adhesion by 3.5 – 4.5 fold to endothelial cells. Another study showed that Hcy at pathophysiological levels in vitro and in vivo leads to enhanced adhesion and migration of human neutrophils/leukocytes to mesenteric venules and endothelial cells [19]. Furthermore, superfusing the mesentery with 1-5 mmol/l Hcy enhances leukocyte rolling, adherence and extravasation significantly in a time and dose-dependent manner [20]. These previous studies provide evidence that Hcy leads to enhanced monocyte adhesion and migration in the peripheral system and cell lines, however further studies are needed to better understand the role of Hcy in primary cells of the CNS, as well as the role of Hcy and inflammatory markers in promoting monocyte adhesion and transmigration during BBB activation.
Taken together, these studies illustrate the potential role that HHcy may play in the dysfunction, degradation, and activation of the BBB. Characterizing the role of Hcy in a brain capillary endothelium model may result in a better understanding of its involvement in BBB toxicity, specifically addressing its role in the activation of inflammatory and adhesion molecules that promote monocyte adhesion and transmigration. The aim of the present study is to explore the acute (1 day) and chronic (6 days) effects of moderate Hcy (20 μM) administration on the integrity of an in vitro rat BBB composed of a simple brain capillary endothelial cells (BCEC) monolayer. Furthermore, we are interested in observing whether Hcy-stimulated BCEC induces an enhanced transmigration of primary rat monocytes through specific cell adhesion molecules.
METHODS
Isolation of Primary Rat Monocytes
Primary rat monocytes were freshly isolated as previously described [21-24]. In brief, Sprague–Dawley rats (250g, Himberg, Austria) were anaesthetized by an intraperitoneal injection of 40 mg/kg body weight thiopental (Biochemie Kundl, Austria) and perfused with 500 ml of 4°C pre-chilled 10 mM phosphate-buffer saline (PBS)/2.7 mM EDTA/25 mg/ml heparin, pH 7.3 through the left ventricle. The collected effluent was centrifuged for at 550×g for 10 min at 20°C. The perfusate pellet was resuspended in 100 ml of 10 mM PBS/2.7 mM EDTA/1% bovine serum albumin (BSA; Serva), pH 7.3 and carefully overlaid on a Percoll working solution [25]. After centrifugation at 500×g for 30 min at 20°C, the peripheral blood mononuclear cells (PBMC) were harvested from the interface and washed twice with 50 ml of PBS. Following washes 2 × 107 PBMC were resuspended in 100 μl of PBS/EDTA/BSA. Monocytes were purified from PBMC by negative magnetic selection: PBMC were incubated in a cocktail consisting of four different affinity purified monoclonal antibodies (20 μg of each: CD8 (clone OX-8), CD5 (clone OX-19), CD45RA (clone OX-33), PAN-T (clone OX-52); all from Cedarlan, Szabo, Austria) for 10 min at 4°C on a shaker. PBMC were washed once with PBS and resuspended in 100 μl of PBS/EDTA/BSA and 40 μl of Goat Anti-Mouse-IgG Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). PBMC were incubated for 15 min at 4°C on a shaker and following incubation washed once with PBS. The cells were resuspended in 500 μl of PBS/EDTA/BSA and then applied to MS-MACS columns fixed to a strong magnet. PBS/EDTA/BSA (800 μl) was added to the supernatant containing purified monocytes and the resuspended cells were centrifuged and pooled for further experiments. Approximately 1 × 107 cells were isolated from one adult rat. The described isolation procedure yields approximately 90% ED-1-positive and CD68-positive monocytes [22,24,25]. During this preparation monocytes were counted using the Cell Coulter Counter (COULTER®Z™ Series, Fischerlehner & Kucera, Austria) in a range from 4 to 11 μm. All animal experiments were approved by the Austrian Ministry of Science and conformed to the Austrian guidelines on ethical animal use.
Preparation and Culture of Brain Capillary Endothelial Cell (BCEC) Monolayers
Culturing of the rat BCEC-monolayer was performed as described previously with some modifications [22,23]. Briefly, the cortex of 10 day old rats was minced and digested with 0.1% collagenase type 2 (Worthington, US) + 0.1% dispase + 2 μg/ml DNAse for 90 min at 37°C. After centrifugation (1000×g, 5 min), capillaries were resuspended in bovine serum albumin-solution (250 mg/ml BSA in optimem; Serva) and centrifuged (1000×g, 15 min). Capillaries were then resuspended and again incubated with collagenase + dispase for 60 min. Finally, capillaries were washed, resuspended in full BCEC medium (DMEM, Sigma; 20% plasma-derived serum, First Link, UK; 100 μg/ml heparin, Sigma; 2 ng/ml rat FGF-2, Peprotech) including 4 μg/ml puromycin (Sigma; Perriere et al., 2005) and plated onto rat tail collagen I (Roche) coated Petri dishes. After 2 days medium was changed and cells were incubated for 5-7 days without puromycin. BCEC were then trypsinized and replated onto collagen I-coated 24 well plate (CDM, nitrite, cell death, cell adhesion molecule expression) or 12 mm 3 μm pore membrane inserts (Millipore, PITP01250) (transmigration) in astrocyte-conditioned medium containing 500 ng/ml hydrocortisone (Sigma) + 2 ng/ml FGF-2. After 1-2 weeks the BCEC displayed a fully confluent monolayer. BCECs were incubated for 1 or 6 days with or without 20 μM Hcy and then used for further experiments.
Cell Death Assay
BCEC cell death was evaluated by in situ staining of non-fixed cells with nuclear dyes: acridine orange (AO) and propidium iodide (PI) as previously described [26]. In brief, cells were washed twice with PBS and incubated in 10 μg AO [27] and 2 μg/ml PI in PBS for 5 min at 20°C shaking. After incubation, cells were washed twice with PBS and evaluated immediately by fluorescent microscopy.
LDH Detection
Cell viability was assessed by lactate dehydrogenase (LDH) cytotoxicity assay (Roche). Briefly, the supernatant of BCEC was centrifuged (250×g) for 5 min and 100 μl (1:4 and 1:2) was mixed with 100 μl of reaction mixture and incubated at 20°C in the dark for 30 min. Light absorbance was measured at 490 nm using an ELISA reader.
Nitrite Assay
Levels of nitrite released by BCEC and cellular oxidative stress were evaluated using the Griess reaction. Nitrite content of BCEC supernatant was determined photometrically after reaction with Griess reagent solution (Sigma). Briefly, 100 μl of conditioned media were mixed with equal amounts of Griess reagent solution at 20°C. After 15 min, the light absorbance was measured photometrically at 540 nm using an ELISA reader. Values were compared to a standard curve using NaNO2.
Transmigration Experiments
The adhesion and transmigration of primary monocytes through an in vitro BBB was explored using 3 μm inserts with a fully confluent Hcy-pretreated BCEC monolayer. To verify cell confluency, a percentage of cells were stained for nuclear DAPI (30 min, 1:10,000; Sigma) the day prior to experiment. Corresponding cells that were unstained but plated in parallel were used for further experiments. Only fully confluent inserts were used. The inserts were placed into 24-well plates with 500 μl of the BCEC medium + 1ng/ml monocyte chemotactic protein-1 (MCP-1, Peprotech, UK). Then 100 μl medium containing 1 million monocytes were applied to the apical side of the monolayer. After incubation for 90 min or 18 hours at 37°C respectively, the basolateral medium was collected and the small inserts were fixed for 3 hr with 4% paraformaldehyde. Some inserts were fixed and stained by F-actin phalloidin TRITC (45 min; 1:2,000; Fluka) after transmigration studies; others were processed for immunohistochemistry. The number of transmigrated cells was counted with a Cell Coulter Counter (COULTER®Z™ Series; Fischerlehner & Kucera; Austria) in a range from 4-11 μm.
Immunohistochemistry
Immunohistochemistry was performed as previously described [23,26]. After fixation for 30 min in 4% paraformaldehyde (PFA) at 4°C, cells were washed 3× with PBS and then incubated in PBS + 0.1% Triton (T-PBS) for 30 min at 20°C shaking. After incubation, T-PBS was removed and replaced with PBS/5% Methanol/1% H2O2 for 20 min at 20°C shaking. The cells were then washed 3× with PBS and blocked in T-PBS/20% Horse Serum/0.2% BSA for 30 min at 20°C shaking. Following incubation, the primary antibody against Laminin (1:1000; Sigma) or monocytic markers (clone ED1, 1:750; Millipore) in T-PBS + 0.2% BSA at 1:500 was added and incubated overnight at 4°C. The cells were then washed and incubated with secondary anti-rabbit (Laminin) or anti-mouse ED1 antibody in T-PBS + 0.2% BSA at 1:200 for 1 hr at 20°C shaking. After rinsing 3× with PBS, cells were incubated in avidin-biotin complex solution (ABC) for 1 hrs at 20°C shaking. Finally, the cells were washed 3× with 50 mM Tris-buffered saline (TBS) and then incubated in 0.5 mg/ml 3, 3′diaminobenzidine (DAB)/TBS/0.003% H2O2 at 20°C in dark until signal was detected. Once DAB staining was visible, the reaction was stopped by adding TBS to cells. Cells were rinsed 3× with TBS and then evaluated by microscopy. Alternatively, monocytes were detected by fluorescence using secondary Alexa 488 antibodies (Invitrogen).
Western Blot
Western Blot was performed as previously described [21,23,24]. In brief, BCECs were centrifuged, resuspended in 150 μl of PBS + protease inhibitor cocktail, and sonicated for 10 sec at 4°C. Following sonication, cells were centrifuged again and 20 μl of supernatant were loaded with sample buffer. Samples were separated in 10% Bis-Tris SDS-polyacrylamide gels for 35 min at 200V and then electrotransferred to nylon-PVDF Immobilon-PSQ membranes for 90 min at 30V in 20% methanol blotting buffer. The Western Breeze Chromogenic System was used for the detection of adhesion molecules in BCEC. Briefly, blots were blocked for 30 min in blocking buffer, incubated in 1:200 primary intercellular cellular adhesion molecule-1 (ICAM-1; I-12/sc-74097), platelet endothelial cell adhesion molecule-1 (PECAM-1; M-20/sc-1506 and V-16/sc-31045), or vascular cell adhesion molecule-1 (VCAM-1; H-276/sc-8304 and D-20/sc-31048) antibodies (all Santa Cruz, USA) for 90 min, washed, and then incubated in alkaline phosphatase conjugated anti-mouse, anti-rabbit, or anti-goat IgG for 30 min. After washing, bound antibodies were visualized by p-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate.
Searchlight ELISA
The detection of inflammatory proteins (IL-1α, interleukin-1α; IL-6, interleukin-6; IL-10, interleukin-10, MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1 α; MIP-3α, macrophage inflammatory protein-3α; MIP-2, macrophage inflammatory protein-2; MMP-2, matrix metalloproteinase-2; PDGF-BB, platelet-derived growth factor-BB; TNFα, tumor necrosis factor-α) was performed using the Thermo Scientific SearchLight Protein Array Technology (THP Medical Products, Vienna). This method is a multiplexing sandwich-ELISA system based on chemiluminescent detection of analytes. Ligands bind to a defined variety of antibodies which are coated to each well of a 96-well microplate. Briefly, BCEC medium treated with Hcy for 6 days (diluted 1:2 in diluent) or calibrated protein standards were added to the wells of the plate (50 μl), incubated for 60 min at 20°C on a shaker, and then washed 3 times. Following washes, the biotinylated antibodies (50 μl cocktail) were added to specifically bind to the captured proteins. After incubation for 30 min at 20°C on a shaker, the wells were again washed 3×, incubated with streptavidin-horseradish peroxidase conjugate (50 μl), and washed 3×. Following washes, the SuperSignal ELISA Femto Chemiluminescent Substrate (50 μl) was added. After incubation for 1-5 minutes the entire plate was imaged using a compatible CCD imaging system to capture the chemiluminescent signal from each spot within each well. The concentration of each analyte in the array was quantified by comparing the spot intensities for each unknown sample to the corresponding standard curves calculated from the standard sample results by the SearchLight Array Analyst Software. Integrated density values were proportional to the concentrations of bound proteins. Individual analytes were identified by the position of each specific capture antibody within the well. Standard curves, raw data and final pg/ml concentrations for each analyte and each sample were reviewed in the array software and exported to Microsoft Excel Software for further statistical analysis.
Quantitative Analysis and Statistics
Monocyte adherence to the BCEC monolayer was measured by counting the number of ED-1-positively stained fluorescent cells over F-actin rhodamine-phalloidin BCEC in a complete insert well. Multistatistical analysis was obtained by one-way AN0VA with Fisher LSD post hoc test, comparing controls against respective treatments in which p<0.05 represents statistical significance.
RESULTS
Effects of Homocysteine on BCEC Survival
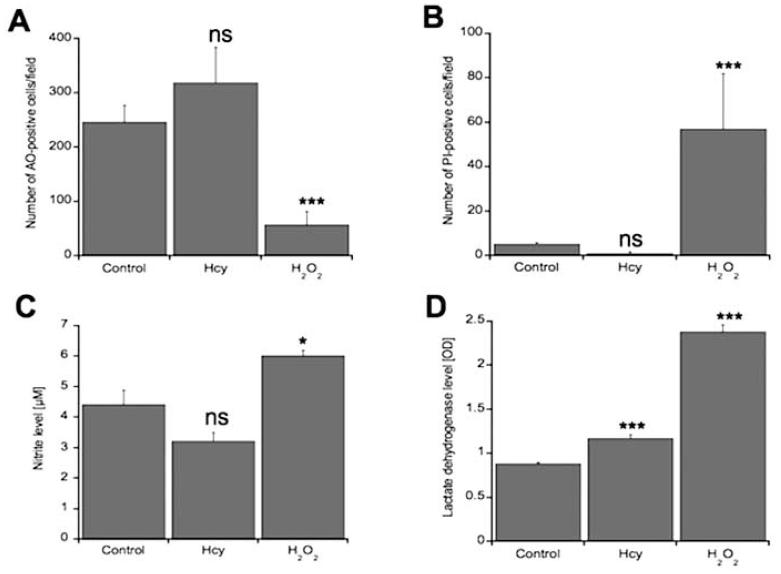
To evaluate the effects of 20 μM Hcy treatment on BCEC cell viability, cells were examined for propidium iodide (PI) staining, acridine orange (AO) staining, nitrite content and LDH activity. Hcy treatment for 6 days did not significantly affect BCEC cell death compared to negative controls as assessed by AO staining (Fig. 1, Fig. 2A), PI staining (Fig. 1, Fig. 2B), and nitrite content (Fig. 2C). BCECs displayed green homogenous nuclei by AO staining indicative of healthy and intact cells (Fig. 1). PI staining, nitrite levels, and LDH release were all significantly enhanced in positive controls treated with hydrogen peroxide on BCECs (Fig. 2). AO staining was significantly decreased in these positive controls (Fig. 1, Fig. 2A). Laminin staining also indicates that Hcy did not have a significant effect on BCEC cell viability compared to hydrogen peroxide, which displayed significant cell loss (Fig. 1). However, LDH activity, a more sensitive measure for cell death and cell membrane lysis, released into the supernatant of BCECs was significantly enhanced in BCEC treated with Hcy compared to negative controls (Fig. 2D).
Fig. (1).
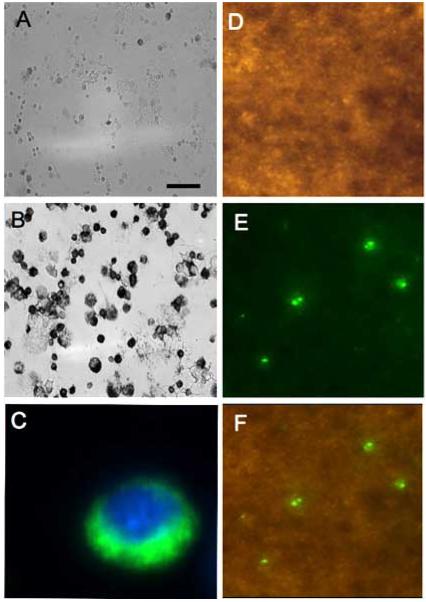
Effects of homocysteine (Hcy) on the survival of brain capillary endothelial cells (BCEC). BCEC were cultured for 6 days in the presence (E-H) or absence (A-D) of 20 μM HCy in BCEC medium. Prior to imaging, some BCEC without Hcy treatment were incubated for 24 hrs with 10 mM H2O2 (I-L). Cell death was assessed by acridine orange (A, E, I), propidium iodide (B, F, J), or Laminin staining (D, H, L). Green staining is indicative of healthy and living cells, whereas yellow and/or red staining indicates dead or dying cells. Fig. C-G-K display the merged pictures of acridine orange and propidium iodide staining. Scale bar = 30 μm (A-C, E-G, I-L), 60 μm (D, H, L).
Fig. (2).
Quantitative analysis of effects of homocysteine (Hcy) on brain capillary endothelial cells (BCEC) survival. BCEC were cultured for 6 days with or without 20 μM Hcy in BCEC medium. Prior to counting, some BCEC without Hcy treatment were incubated for 24 hrs with 10 mM H2O2. Cell death was assessed by acridine orange staining (A) and propidium iodide staining (B). AO-positive cells are indicative of healthy living cells (A). PI-positive cells are indicative of dead or dying cells (B). Cells were also evaluated for other signs of cytotoxicity including nitrite content (C) and lactate dehydrogenase (LDH) activity (D). Values are given as mean±SEM number of AO-positive cells/field (A), number of PI-positive cells/field (B), μM of nitrite released into BCEC supernatant, or amount of LDH released into BCEC supernatant as measured by optical density (D). Statistical analysis was performed by one-way ANOVA with Fisher’s LSD posthoc test (*p < 0.05; ***p < 0.001; ns not significant; n=5-7).
Effects of Homocysteine on the Secretion of Inflammatory Markers by BCEC
After evaluating BCEC cell viability upon Hcy treatment, BCEC were subsequently screened for inflammatory markers to evaluate the effects of Hcy on proinflammatory expression patterns in BCEC. The secretion of MIP3α and RANTES into the cell culture supernatant by BCECs was significantly reduced by 6 day (chronic) 20 μM Hcy treatment (Table 1). Hcy-treated cells secreted approx. 1600 pg/ml of MIP3α compared to approx. 2000 pg/ml of MIP3α in controls (Table 1). RANTES secretion by BCECs was reduced by 3-fold with Hcy treatment; controls secreted approx. 5000 pg/ml of RANTES whereas Hcy-treated cells secreted approx. 1400 pg/ml (Table 1). TNFα secretion was significantly enhanced with 20 μM Hcy treatment (Table 1). TNFα secretion was enhanced 4-fold with Hcy treatment; controls secreted approx. 15 pg/ml of TNFα whereas Hcy-treated cells secreted approx. 60 pg/ml (Table 1). A tendency of decreased levels of IL-6, MCP-1, and MIP-2 after chronic Hcy treatment was observed (Table 1), however, no changes were observed for IL-1α, IL-10, MIP-1α, MMP-2 or PDGF-BB secretion (Table 1).
Table 1. Effects of Homocysteine on the Secretion of Inflammatory Markers in BCEC.
| Supernatant [pg/ml*6d] | Control (n=10) | Hcy (n=9) |
|---|---|---|
| IL-1α | 1,474±244 | 1,861±195 ns |
| IL-6 | 522±96 | 286±60 p=0.059 |
| IL-10 | 45±6 | 42±11 ns |
| MCP-1 | 52,511±4162 | 42,425± 3167 p=0.075 |
| MIP-1α | 307±48 | 331±23 ns |
| MIP-3α | 2,279±293 | 1,573±129 p=0.049 * |
| MIP-2 | 187±17 | 145±15 p=0.093 |
| MMP-2 | 197,660±18,074 | 187,170±26,496 ns |
| PDGF-BB | 738±77 | 866±100 ns |
| RANTES | 4,777±1,336 | 1,408±405 p=0.034 * |
| TNFα | 14±3 | 62±21 p=0.035 * |
Brain capillary endothelial cells (BCEC) were cultured in medium for 6 days in the presence or absence (control) of 20 μM homocysteine (Hcy). Following incubation, supernatants from confluent BCEC were collected and analyzed by Searchlight Multiplex ELISA for various inflammatory markers (IL-1α, interleukin-1α; IL-6, interleukin-6; IL-10, interleukin-10, MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1α; MIP-3α, macrophage inflammatory protein-3α; MIP-2, macrophage inflammatory protein-2; MMP-2, matrix metalloproteinase-2; PDGF-BB, platelet-derived growth factor-BB; TNFα, tumor necrosis factor-α). Values represent the total inflammatory marker secretion (pg/ml) in collected supernatant. Values are given as mean ± SEM (n indicates the number of experiments). Statistical analysis was performed by one-way ANOVA with Fisher’s LSD postdoc test.
p < 0.05
ns not significant
Effects of Homocysteine on Monocyte Adhesion and Migration
Monocytes were immunohistochemically stained and displayed strong monocytic marker ED1 staining (Fig. 3B) compared to control sections (Fig. 3A). Monocytes were fluorescently labelled and displayed strong cytoplasmic staining (Fig. 3C). During transmigration studies, monocytes adhered to a BCEC-monolayer and displayed strong ED-1 staining, as seen in co-localization studies with F-actin phalloidin and nuclear DAPI staining (Figs. 3D-F). After 90 min of monocyte application, approx. 700 cells adhered to the BCEC-monolayer under control conditions compared to approx. 600 cells that adhered to the BCEC-monolayers when pre-treated with Hcy (20 μM) for 24 hrs and 6 days (Table 2). After 18 hrs of monocyte application, approx. 700 cells adhered to a BCEC-monolayer under control conditions (Table 2). In addition, approx. 500 cells adhered after 18 hrs to BCEC-monolayers when treated with Hcy for 24 hrs or 6 days (Table 2). Furthermore, approx. 15,000 cells migrated through the BCEC-monolayer after 90 min under normal conditions compared to approx. 10,000 and 18,000 cells pre-treated with Hcy for 24 hrs and 6 days respectively (Table 2). However, after 90 min of monocyte application the migration of monocytes was significantly enhanced by 6 day treatment of 20 μM Hcy compared to controls (Table 2). Although after 18 hrs of monocyte application, approx. 14,000 cells migrated through the BCEC-monolayer under normal conditions compared to 11,000 and 19,000 migrated cells following Hcy pre-treatment for 24 hrs and 6 days respectively (Table 2).
Fig. (3).
Adhesion of primary monocytes on BCEC-monolayer. Primary monocytes were isolated, spotted onto glass slides, and immunohistochemically stained for monocyte specific marker ED1 (B). As a control cells were incubated without primary antibody (A). Monocytes were also fluorescently stained using Alexa 488 secondary antibodies and counterstained with nuclear DAPI (C). Monocytes were added to a fully confluent BCEC monolayer and incubated for 24 hrs. Following incubation the BCEC monolayer was labelled with F-Actin phalloidin (D) and adhered monocytes were stained for ED1 (E). Fig. (F) shows the merged image of fluorescent adhered monocytes on the BCEC monolayer. Scale bar = 60 μm (A), 30 μm (B), 3 μm (C), and 15 μm (D,E,F).
Table 2. Effects of Homocysteine on Monocyte Transmigration through a BCEC Monolayer.
| Control | Hcy (24 hr) | Hcy (6 day) | ||
|---|---|---|---|---|
| Adhesion | 90 min | 700±46 (21) - | 583±34 (11) ns | 600±76 (9) ns |
| 18 hr | 709±65 (11) - | 504±37 (11) ns | 534±168 (6) ns | |
| Migration | 90 min | 12442±757 (21) - | 10440±1758 (11) ns | 17579±1879 (9)* |
| 18 hr | 14234±2790 (11) - | 10754±1278 (11) ns | 18777±4942 (6) ns |
Brain capillary endothelial cells (BCEC) were plated on 3 μm membrane inserts and grown for 14 days yielding a fully confluent BCEC-monolayer. The BCEC-monolayer was then cultured in medium in the presence or absence (control) of 20 μM homocysteine (Hcy) for 24 hours or 6 days. Approx. 100,000 primary monocytes were added to the apical side of the membrane and incubated for 90 min or 18 hr at 37°C. Following incubation, monocytes that adhered to or migrated through the BCEC-monolayer were counted. Values are given as mean ± SEM cells per well. Values in parenthesis indicate the number of experiments. Statistical analysis was performed by one-way ANOVA with Fisher’s LSD postdoc test.
p < 0.05
ns not significant
Effects of Homocysteine on ICAM-1 Expression on BCEC
Western blot analysis of BCEC showed that primary BCEC expressed ICAM-1 as visualized by a 90 kDa band (Fig. 4A). A weak band was also observed in primary monocytes (Fig. 4A). Quantitative analysis demonstrated that ICAM-1 expression (as determined by correction to intracellular actin staining) was significantly enhanced by 20 μM Hcy treatment (Fig. 4B). Western blot analysis was also performed with two different VCAM-1 and PECAM-1 antibodies to evaluate their expression on BCECs, however, no bands were detectable (data not shown).
Fig. (4).
(A) Western Blot analysis of ICAM-1 expression in brain capillary endothelial cells (BCEC). BCEC were cultured for 6 days in the presence or absence (control) of 20 μM homocysteine in BCEC medium. Following incubation, cell extracts were analyzed for ICAM-1 expression. Actin Western Blot analysis was also performed to normalize protein expression. (B) Quantification of ICAM-1 expression in BCEC. Values are given as mean±SEM in arbitrary units. Statistical analysis was performed by one-way ANOVA with Fisher’s LSD posthoc test (*p < 0.05).
DISCUSSION
Our present study shows that chronic 20 μM Hcy has little to no effect on BCEC survival as observed by AO, PI, and nitrite content in our BCEC-monolayer model. However, LDH activity released by BCEC into the supernatant was significantly enhanced in Hcy-treated BCECs. Our data also show that levels of inflammatory markers MIP3α and RANTES are significantly reduced in cells treated with Hcy. TNFα levels, on the other hand, are significantly enhanced by 20 μM Hcy treatment. In addition, 20 μM Hcy enhances monocyte transmigration across BCECs in a time-dependent manner. Finally, this study demonstrates that 20 μM Hcy increases intercellular adhesion molecule-1 (ICAM-1) expression in BCECs.
Homocysteine and BBB Damage
The BBB is composed of brain capillary endothelial cells joined together by tight junctions and is encased by astrocytes. This cell physiology serves a unique filter system for the brain and plays an important role in proper synaptic transmission, neuronal remodelling, angiogenesis, and neurogenesis [10]. Considerable evidence has demonstrated a relationship between elevated Hcy and endothelial damage and dysfunction [11,28-32]. Mild HHcy impairs vasoreactivity, vasodilation and blood flow in transgenic mice [33-35] and in humans [36,37]. In this investigation, we used AO, PI, nitrite content, and LDH activity to evaluate the effects of Hcy on BCEC-monolayer viability and survival. Our results indicate that chronic 20 μM Hcy treatment does not affect the survival of BCEC and does not induce BBB damage, as seen by unchanged AO and PI staining. Previous investigations suggest Hcy-mediated endothelial dysfunction is associated with decreased nitric oxide (NO) production [35,38-40] and enhanced formation of reactive oxygen species [41,42]. Thus, BCEC nitrite levels were also evaluated to determine whether Hcy treatment effects NO production and cell oxidative stress. Our results show no significant change in nitrite content in Hcy-treated cells compared to controls. However, Hcy did significantly elevate LDH release possibly indicating plasma membrane damage. It seems likely that LDH release may be more sensitive at detecting cellular deregulation. Hydrogen peroxide was used as a positive control. This treatment markedly induced BCEC-monolayer damage, as seen by decreased AO staining and enhanced PI staining, nitrite content, and LDH release. Taken together, our data shows that mild Hcy treatment for 6 days does not induce breakdown of the BCEC-monolayer. This acquired knowledge is of great significance and will help in further studies that seek to understand the functional transmigration of monocytes through the BCEC-monolayer.
Adhesion and Transmigration of Rat Monocytes
Monocytes circulate throughout the bloodstream eventually giving rise to macrophages and microglia. Upon pathological stimulation monocytes can transmigrate across the BBB and enter the brain. In order to study the in vitro effects of Hcy on the adherence and transmigration of monocytes across the BBB, we used a simple artificial rat BCEC monolayer, as described previously in detail [22,23]. The BCEC monolayer model consists of a fully confluent BCEC monolayer expressing BBB-specific proteins grown on a 3 μm pore membrane insert. Previously, we have demonstrated that primary rat monocytes adhere to the apical side of the BCEC and transmigrate across the BCEC to the basolateral side, where they express microglia-like markers. We have also shown that transmigrated monocytes can partly re-migrate back to the apical side of the BCEC. In addition, we have shown that monocyte migration is strongly enhanced by basolateral administration of monocyte chemotactic protein-1 (MCP-1) [22]. A recent study also demonstrated that, in proof of principle, monocytes can be used as a carrier system to deliver NGF to the brain [24]. Data from this present investigation shows that prolonged 6 day Hcy exposure significantly increases the transmigration of monocytes across the BCEC 90 min after monocyte addition. However, the same Hcy treatment does not significantly enhance the transmigration of monocytes across the BCEC 18 hr after monocyte addition. The process of adhesion and migration is a very rapid event. Previous studies including our own study have reported that monocyte adhesion can be detected as early as 30 min after monocyte addition and that migration can start immediately after adhesion [18,22,23]. In our present study we measured adhesion and migration after 90 min and 18 hr, which reflects a limited time window. It is also possible that different subsets of monocytes may adhere and migrate at later time points, e.g. after activation by cytokines. Alternatively, feedback regulation to ICAM-1 may occur, resulting in downregulation or inactivation of monocyte adhesion and migration. Furthermore, it is also possible that the monocytes have re-migrated to the apical side of the monolayer [22] and thus less cells are present on the basolateral side at 18 hrs. In addition, increased transmigration was not seen when BCEC were treated for 1 day with 20 μM Hcy. While Hcy treatment increased the number of transmigrated cells; the number of adhered cells remained unchanged. However, we did observe a tendency for decreased numbers of adhered cells in cultures subject to this Hcy treatment. This could be explained by fast adhesion and migration resulting in lower populations of adhered cells. Alternatively, it is possible that the effects of Hcy are moderate and too little for efficient detection of significant changes in our system. In contrast to our findings, others have reported a significant increase in monocyte adhesion to human peripheral endothelial, aortic endothelial cells [14,15,18], and human intestinal micro-vascular endothelial cells [43]. It should also be noted that our BCEC model does not fully reflect the in vivo BBB situation and it will be necessary to establish a more complex in vitro model that more accurately reflects the BBB situation, including the addition of astrocytes to the BCEC.
Migration of Monocytes and Inflammatory Markers
Chemokines, which belong to a superfamily of structurally related small chemotactic cytokines, are implicated in leukocyte trafficking. TNFα is a proinflammatory cytokine that in combination with Hcy mediates monocyte adhesion and migration into inflammatory sites through enhanced expression of VCAM-1 and MCP-1 [43]. Further evidence has shown that HHcy elevates plasma TNFα and MCP-1 in transgenic mice [16] and enhances MCP-1 and IL-8 expression and secretion in human primary monocytes [44] and human aortic endothelial cells [13]. Our data does not show significantly changed cytokine secretion profiles of several cytokines, including MCP-1, MIP-1α, or IL-10, in Hcy-treated cells compared to controls. Since we measured the release after 6 days, it is possible that significant changes have already returned to basal levels by the time of measurement or that cytokines have already been taken up by BCEC and/or degraded. Previous investigations reported maximal MCP-1 expression within 2-4 hrs of Hcy addition on endothelial cells with subsequent decline within 24 hrs to basal levels [13]. Alternatively, it is also reasonable that our primary BCEC system markedly differs from cellular systems used by others, such as e.g. the human aortic endothelial cells. However, the results from this study support other findings that TNFα is enhanced with elevated Hcy levels. Macrophage Inflammatory Protein-3α (MIP-3α/CCL20) is a chemokine which, through the CCR6 receptor, signals lymphocytes and dendritic cells. It also promotes the adhesion of memory CD4+ T cells [45,46]. RANTES is a chemokine which, through the CCR1, CCR3, CCR5, and US28 receptors, signals monocytes, memory T cells, basophils, and eosinophils. RANTES has also been shown to induce the expression and release of other chemokines and cytokines from astrocytes. This present study demonstrates that the release of MIP3α and RANTES inflammatory markers by BCEC are suppressed with 20 μM Hcy treatment. In summary, our data show that prolonged exposure (6 day) to Hcy affects inflammatory markers in the BCEC. This may contribute to the observed enhancement of monocyte transmigration through the BBB.
Migration of Monocytes and Cell Adhesion Molecules
In addition to inflammatory markers, previous studies have demonstrated the important and central role of adhesion molecules in monocyte migration and interaction with endothelial cells [47-49]. As previously stated, HHcy can promote endothelial activation by increasing adhesion molecules expression on the epithelium [14,15]. In human and rat aortic endothelial cells, levels of VCAM-1 and E-selectin were enhanced by elevated levels of Hcy [15,50]. ICAM-1 has also been implicated in Hcy-mediated expression [18]. It has also been shown that Hcy can effectively induce T and monocyte cell adhesion through VCAM-1 [43]. One study conducted by Floris et al. [48] showed that by diminishing VCAM-1 and ICAM-1 expression, monocyte migration was also diminished, suggesting that these molecules play a major role in modulating monocyte adhesion and transmigration across the BBB during inflammation. In our model we have previously shown that Aβ-induced monocyte transmigration is blocked by adding anti-ICAM-1 and anti-PECAM-1 antibodies suggesting their role in migration across the BCEC [23]. In this study we observed a significant increase in ICAM-1 expression on the BCEC with 20 μM Hcy, but were unable to observe VCAM-1 or PECAM-1 expression on the BCEC in control or treated cells. Thus our data indicate that Hcy-induced transmigration of monocytes through the BBB may involve ICAM-1.
In conclusion, our data shows that chronic 6 day Hcy treatment enhances transmigration of primary rat monocytes through a rat BCEC-monolayer without disrupting the cell layer. Our data also provides evidence that enhanced ICAM-1 expression on the BCEC and release of various cytokines may modulate Hcy-mediated monocyte transmigration.
ACKNOWLEDGEMENTS
This study was supported by the Austrian Science Funds (P191220-B05) and the Tyrolean Science Funds. We thank Ursula Kirzenberger-Winkler for excellent technical support. L.A.H. was supported in part by a U.S. Fulbright Student Research grant, sponsored by the Austrian-American Educational Commission.
REFERENCES
- [1].Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1206–H13. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tyagi SC, Lominadze D, Roberts AM. Homocysteine in microvascular endothelial cell barrier permeability. Cell Biochem Biophys. 2005;43:1–8. doi: 10.1385/CBB:43:1:037. [DOI] [PubMed] [Google Scholar]
- [3].Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- [4].Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. PNAS. 2008;105(34):12474–79. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–55. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- [6].Hankey GJ, Eikelbloom JW. Homocysteine and vascular disease. Lancet. 1999;354(9176):407–13. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- [7].Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- [8].Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol. 2010;610:285–308. doi: 10.1007/978-1-60327-029-8_17. [DOI] [PubMed] [Google Scholar]
- [10].Marlatt MW, Lucassen PJ, Perry G, Smith MA, Zhu X. Alzheimer’s disease: cerebrovascular dysfunction, oxidative stress, and advanced clinical therapies. J Alzheimers Dis. 2008;15(2):199–210. doi: 10.3233/jad-2008-15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kamath AF, Chauhan AK, Kisucka J, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107(2):591–3. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lehmann M, Regland B, Blennow K, Gottfries CG. Vitamin B12-B6-folate treatment improves blood-brain barrier function in patients with hyperhomocysteinemia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2003;16(3):145–50. doi: 10.1159/000071002. [DOI] [PubMed] [Google Scholar]
- [13].Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103(22):2717–23. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- [14].Silverman MD, Tumuluri RJ, Davis M, Lopez G, Rosenbaum JT, Lelkes PI. Homocysteine upregulates vascular cell adhesion molecule-1 expression in cultured human aortic endothelial cells and enhances monocyte adhesion. Arterioscler Thromb Vasc Biol. 2002;22(4):587–92. doi: 10.1161/01.atv.0000014221.30108.08. [DOI] [PubMed] [Google Scholar]
- [15].Wang G, Woo CW, Sung FL, Siow YL, O K. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscler Thromb Vasc Biol. 2002;22(11):1777–83. doi: 10.1161/01.atv.0000035404.18281.37. [DOI] [PubMed] [Google Scholar]
- [16].Zhang D, Jiang X, Fang P, et al. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120(19):1893–902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9(11):1941–58. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- [18].Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol. 2006;26(3):508–13. doi: 10.1161/01.ATV.0000201039.21705.dc. [DOI] [PubMed] [Google Scholar]
- [19].Dudman NP, Temple SE, Guo XW, Fu W, Perry MA. Homocysteine enhances neutrophil-endothelial interactions in both cultured human cells and rats In vivo. Circ Res. 1999;84(4):409–16. doi: 10.1161/01.res.84.4.409. [DOI] [PubMed] [Google Scholar]
- [20].Pruefer D, Scalia R, Lefer AM. Homocysteine provokes leukocyte-endothelium interaction by downregulation of nitric oxide. Gen Pharmacol. 1999;33(6):487–98. doi: 10.1016/s0306-3623(99)00045-2. [DOI] [PubMed] [Google Scholar]
- [21].Zassler B, Humpel C. Transplantation of NGF secreting primary monocytes counteracts NMDA-induced cell death of rat cholinergic neurons in vivo. Exp Neurol. 2006;198(2):391–400. doi: 10.1016/j.expneurol.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [22].Moser KV, Humpel C. Primary rat monocytes migrate through a BCEC-monolayer and express microglia-markers at the basolateral side. Brain Res Bull. 2007;74(5):336–43. doi: 10.1016/j.brainresbull.2007.07.004. [DOI] [PubMed] [Google Scholar]
- [23].Humpel C. Basolateral aggregated rat amyloid beta(1-42) potentiates transmigration of primary rat monocytes through a rat blood-brain barrier. Curr Neurovasc Res. 2008;5(3):185–92. doi: 10.2174/156720208785425701. [DOI] [PubMed] [Google Scholar]
- [24].Böttger D, Ullrich C, Humpel C. Monocytes deliver bioactive nerve growth factor through a brain capillary endothelial cell-monolayer in vitro and counteract degeneration of cholinergic neurons. Brain Res. 2010;1312:108–19. doi: 10.1016/j.brainres.2009.11.062. [DOI] [PubMed] [Google Scholar]
- [25].Scriba A, Luciano L, Steiniger B. High-yield purification of rat monocytes by combined density gradient and immunomagnetic separation. J Immunol Methods. 1996;189(2):203–16. doi: 10.1016/0022-1759(95)00248-0. [DOI] [PubMed] [Google Scholar]
- [26].Moser KV, Stöckl P, Humpel C. Cholinergic neurons degenerate when exposed to conditioned medium of primary rat brain capillary endothelial cells: counteraction by NGF, MK-801 and inflammation. Exp Gerontol. 2006;41(6):609–18. doi: 10.1016/j.exger.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [27].Geng YJ, Wu Q, Muszynski M, Hansson GK, Libby P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler Thromb Vasc Biol. 1996;16(1):19–27. doi: 10.1161/01.atv.16.1.19. [DOI] [PubMed] [Google Scholar]
- [28].Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke. 2004;35(2):345–7. doi: 10.1161/01.STR.0000115161.10646.67. [DOI] [PubMed] [Google Scholar]
- [29].Lentz SR, Sobey CG, Piegors DJ, et al. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98(1):24–9. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stanger O, Weger M. Interactions of homocysteine, nitric oxide, folate and radicals in the progressively damaged endothelium. Clin Chem Lab Med. 2003;41(11):1444–54. doi: 10.1515/CCLM.2003.222. [DOI] [PubMed] [Google Scholar]
- [31].Stühlinger MC, Oka RK, Graf EE, et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108(8):933–8. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- [32].Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JF, Jr, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state-impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41(11):1455–61. doi: 10.1515/CCLM.2003.223. [DOI] [PubMed] [Google Scholar]
- [33].Eberhardt RT, Forgione MA, Cap A, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106(4):483–91. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. PNAS. 2001;98(22):12503–8. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22(1):34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- [36].Holven KB, Holm T, Aukrust P, et al. Effect of folic acid treatment on endothelium-dependent vasodilation and nitric oxide-derived end products in hyperhomocysteinemic subjects. Am J Med. 2001;110(7):536–42. doi: 10.1016/s0002-9343(01)00696-9. [DOI] [PubMed] [Google Scholar]
- [37].Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997;95(5):1119–21. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- [38].Faraci FM. Hyperhomocysteinemia: a million ways to lose control. Arterioscler Thromb Vasc Biol. 2003;23(3):371–3. doi: 10.1161/01.ATV.0000063607.56590.7F. [DOI] [PubMed] [Google Scholar]
- [39].Upchurch GR, Jr, Welch GN, Fabian AJ, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272(27):17012–7. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- [40].Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol. 2000;279(4):F671–8. doi: 10.1152/ajprenal.2000.279.4.F671. [DOI] [PubMed] [Google Scholar]
- [41].Heydrick SJ, Weiss N, Thomas SR, et al. L-Homocysteine and L-homocysteine stereospecifically induce endothelial nitric oxide synthase-dependent lipid peroxidation in endothelial cells. Free Radic Biol Med. 2004;36(5):632–40. doi: 10.1016/j.freeradbiomed.2003.12.001. [DOI] [PubMed] [Google Scholar]
- [42].Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98(1):5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Danese S, Sgambato A, Papa A, et al. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol. 2005;100(4):886–95. doi: 10.1111/j.1572-0241.2005.41469.x. [DOI] [PubMed] [Google Scholar]
- [44].Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ Res. 2003;93(4):311–20. doi: 10.1161/01.RES.0000087642.01082.E4. [DOI] [PubMed] [Google Scholar]
- [45].Hromas R, Kim CH, Klemsz M, et al. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J Immunol. 1997;159(6):2554–8. [PubMed] [Google Scholar]
- [46].Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol. 1997;158(3):1033–6. [PubMed] [Google Scholar]
- [47].Beekhuizen H, van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993;54(4):363–78. [PubMed] [Google Scholar]
- [48].Floris S, Ruuls SR, Wierinckx A, et al. Interferon-β directly influences monocyte infiltration into the central nervous system. J Neuroimmunol. 2002;127(1-2):69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- [49].Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154(8):4099–112. [PubMed] [Google Scholar]
- [50].Koga T, Claycombe K, Meydani M. Homocysteine increases monocyte and T-cell adhesion to human aortic endothelial cells. Atherosclerosis. 2002;161(2):365–74. doi: 10.1016/s0021-9150(01)00670-0. [DOI] [PubMed] [Google Scholar]
- [51].da Cunha AA, Ferreira AGK, Wyse ATS. Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab Brain Dis. 2010;25:199–205. doi: 10.1007/s11011-010-9188-8. [DOI] [PubMed] [Google Scholar]