Abstract
Thapsigargin is a pro-apoptotic chemical, which has been shown to be useful to study cell death of cholinergic or dopaminergic neurons, or cells, which degenerate in Alzheime’s disease or Parkinso’s disease, respectively. The aim of the present work was to study the effects of thapsigargin in the well established organotypic brain co-slice model composed of the basal nucleus of Meynert (nBM), ventral mesencephalon (vMes), dorsal striatum (dStr) and parietal cortex (Ctx). Cholinergic acetyltransferase-positive neurons in the nBM and dStr and dopaminergic tyrosine hydroxylase-positive neurons in the vMes survived, when cultured for 4 weeks with nerve growth factor and glial cell line-derived neurotrophic factor. Nerve fibers of cholinergic nBM neurons grew into the cortex and dopaminergic nerve fibers sprouted into dopamine D2 receptor-positive dStr. The whole co-slice contained a dense laminin-positive capillary network. Treatment of co-cultures with 3 μM thapsigargin for 24 hr significantly decreased the number of cholinergic neurons and dopaminergic neurons. This cell death displayed apoptotic DAPI-positive malformed nuclei and enhanced TUNEL-positive cells. Thapsigargin selectively stimulated the laminin-positive capillary growth between the nBM and Ctx. In conclusion, the induced cell death of cholinergic and dopaminergic neurons may be accompanied by enhanced angiogenic activity.
Keywords: Angiogenesis, vascularization, in vitro model, neuronal cell death
INTRODUCTION
Neurodegeneration in the central nervous system is a common characteristic of several brain disorders. Alzheime’s disease (AD) is a chronic progressive disease, accompanied by cell death of cholinergic neurons of the basal nucleus of Meynert (nBM) and septum. A lack of acetylcholine in the cortex and hippocampus directly correlates with cognitive decline and memory dysfunction (Davies and Maloney, 1976; Whitehouse et al., 1982; Wilcock et al., 1982; Winkler et al., 1998). In Parkinso’s disease (PD) neurons of the ventral mesencephalon (vMes) (esp. of the substantia nigra pars compacta (SNc)) degenerate and a lack of dopamine in the dorsal striatum (dStr) causes motor dysfunction in humans (Ganten et al., 2004; Olanow et al., 1999). The causes for cell death of cholinergic and dopaminergic neurons in AD or PD, respectively are not known. Several cell death inducing stimuli are discussed, such as oxidative stress (Zhu et al., 2004; Markesbery et al., 1997; Jenner, 2003; Zhang et al., 2000), glutamate-induced cell death (Blandini et al., 1996; Masliah et al., 1996), toxic compounds (Olanow et al., 1999; Dauer et al., 2003), proinflammatory cytokines (Mrak et al., 1995; Vila et al., 2001) or pro-apoptotic stimuli (Honig et al., 2000; Takadera et al., 2007). Thapsigargin is one of these cell death inducing stimuli, which have been discussed to be involved in the onset of AD and PD. As an inhibitor of the Ca2+-ATPase in the sarcoplasmic (SR) and endoplasmic reticula (ER), thapsigargin is able to induce ER stress, which leads to accumulation of unfolded proteins and to apoptosis of neurons (Takadera et al., 2007; Lindholm et al., 2006; Chin et al., 2007; Ferri and Kroemer, 2001). Some studies additionally indicate an influence of thapsigargin on the vascular system (Shukla et al., 1997, 2001; Birket et al., 1999; George et al., 1997).
The aim of the present study was to explore the effects of thapsigargin on cholinergic and dopaminergic neurons and vascularization in an organotypic brain slice model. The organotypic brain slice model has been originally developed by Crain et al. (1982) using spinal cord-dorsal root ganglia, was modified by Gähwiler and colleagues (Gähwiler et al., 1997) and further improved by Stoppini and colleagues (Stoppini et al., 1991). In slices individual cells are in close contact and do not lose density dependent regulatory mechanisms, three dimensional architecture as well as tissue specific transport and diffusion probabilities. Organotypic brain slices are well established in our research group and we have extensively studied the survival of cholinergic neurons in the presence of NGF (Weis et al., 2001) or of dopaminergic neurons in the presence of GDNF (Schatz et al., 1999). In addition the combination of two closely related brain areas is well established, such as e.g. septum - hippocampus (Gähwiler and Hefti, 1984; Keller et al., 1983), or vMes - dStr (Schatz et al., 1999; Franke et al., 2003) or nBM - cortex (Humpel and Weis, 2002).
In the present study we aim to culture four brain slices together to study interactions between brain areas, their axonal growth as well as angiogenesis due to thapsigargin treatment.
METHODS
Organotypic Brain Slice Cultures
Organotypic brain slice cultures were established as described by us in detail (Weis et al., 2001; Humpel and Weis, 2002; Schatz et al., 2000). Briefly, the nBM, vMes, parietal cortex and dorsal striatum of postnatal day 7-9 (P7-9) Sprague Dawley rats was dissected under aseptic conditions, 400 μm slices were cut with a tissue chopper (McIlwain, USA), and the 4 slices were placed on a 30 mm diameter Millicell-CM 0.4 μm membrane insert (Millipore, Austria), where they become attached to the membrane after 2 weeks of incubation. It is important to note, that the ipsilateral as well as contralateral brain areas were dissected at the same time, cut on the tissue chopper and all slices pooled in medium. Slices were cultured in 6-well plates (Greiner) at 37°C and 5% CO2 with 1.2 ml/well of the following culture medium: 50% MEM/HEPES (Gibco), 25% heat inactivated horse serum (Gibco/Lifetech, Austria), 25% Hank’ solution (Gibco), 2 mM NaHCO3 (Merck, Austria), 6.5 mg/ml glucose (Merck, Germany), 2 mM glutamine (Merck, Germany), pH 7.2. Medium was changed twice a week. Brain slice cultures were incubated with or without 10 ng/ml nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF) for 4 weeks. To analyze the cell death of neurons, four weeks old cultures were incubated for 3 days without growth factors, treated afterwards with or without 3 μM thapsigargin in medium for 24 hr and then incubated for further 3 days in medium without growth factors. At the end of the experiment, slices were fixed for 3 hr at 4°C in 4% paraformaldehyde/10 mM phosphate buffered saline (PBS) and then stored at 4°C in PBS until use. All experiments conformed to Austrian guidelines on the ethical use of animals and all efforts were made to minimize the number of animals used and their suffering.
Immunohistochemistry
Immunohistochemistry was performed similarly as previously described (Weis et al., 2001). All incubations were performed free floating for 2 days including 0.1% Triton, such that the antibodies can penetrate from both sides of the slices, which allows good penetration of the antibody into the brain slices. Slices were washed 30 min with 0.1% Triton/PBS (T-PBS) at room temperature and pre-treated 20 min with 20% methanol/1% H2O2/PBS (only for 3,3′-diaminobenzidine labeling). After thorough rinsing, the slices were blocked with 20% horse serum/0.2% BSA/T-PBS and then incubated for 2 days at 4°C with primary antibodies against choline acetyltransferase (ChAT, 1:750, Chemicon), tyrosine hydroxylase (TH, 1:500, Chemicon), dopamine D2 receptor (D2R, 1:250, Santa Cruz), glial fibrillary acidic protein (GFAP, 1:500, Chemicon) or laminin (1:500, Sigma). Then the slices were washed again with PBS and incubated with secondary biotinylated anti-goat (ChAT), anti-chicken (GFAP), anti-rabbit (laminin, TH), or anti-mouse (D2R) antibodies (1:200, Vector Lab., USA) for 1 hr at room temperature. Following further washing steps with PBS, slices were incubated in an avidin-biotin complex solution (ABC-Elite Vectastain reagent Vector Lab.) for 1 hr. After being washed with 50 mM Tris-buffered saline (TBS), the signal was detected by using 0.5 mg/ml 3,3′-diaminobenzidine (DAB) including 0.003% H2O2 as a substrate in Tris-buffered saline. The slices were mounted on glass slides, air dried and coverslipped with Entellan (Merck, Germany). Unspecific staining was defined by omitting the primary antibody. For fluorescence immunohistochemistry the methanol pre-treatment was omitted and as secondary antibodies Alexa-488 (Invitrogen, 1:400) was used. To label nuclei, slices were incubated with 4,6-diamidino-2-phenylindole (DAPI, 1:10000; Sigma) for 30 min. Certain sections were stained with cresyl violet for 10 min and rinsed afterwards for further 5 min in a.d. Immunolabeling was visualized with a Leica DMIRB fluorescence inverse microscope equipped with an Apple computer.
Acetylcholinesterase Histochemistry
Acetylcholinesterase (AChE) histochemistry was performed as described by us (Humpel & Weis, 2002). The slices were fixed with 4% paraformaldehyde for 3 hr at 4°C. After being washed with 100 mM maleate buffer (pH 6.0) slices were incubated in a fresh solution of 0.036 mM acetylthiocholine iodide (Sigma), 0.005 mM K3Fe(CN)6(III) (Merck), 0.03 mM CuSO4 (Merck), 0.05 mM sodium citrate (Merck), 19.77 mM ethopropazine (Aldrich) in 100 mM maleate buffer (pH 6.0) for 1.5 hr at room temperature. Thereupon, slices were rinsed in PBS buffer (pH 7.4) and Tris buffer (pH 7.6). Subsequently, slices were developed in a 0.04% DAB/0.3% nickel ammonium sulfate/0.003% H2O2/Tris pH 7.6 solution.
DNA Nick-End Labeling (TUNEL Staining)
The TUNEL reaction was performed as described earlier (Schatz et al., 1999). Fresh co-slices were carefully transferred to glass slides, frozen on a CO2 snow, and stored at −20°C until use. Co-slices were then air dried, and incubated with 150 U/ml terminal transferase (Roche) and 10 nmol/ml biotinylated 16-dUTP (Roche) in terminal transferase buffer (Roche) at 37°C for 2 hr. Afterwards, co-slices were fixed with 4% paraformaldehyd/10 mM PBS for 30 min and then washed in PBS. Co-slices were incubated with ABC reagent (Vectastain) for 30 min at room temperature, then washed in 50 mM Tris buffer pH 7.6 (3×10 min) and the staining was performed in Tris pH 7.6 with 0.5 mg/ml DAB as a substrate, including 0.003% H2O2 and 0.4% NiCl2. Sections were extensively washed in a.d., air dried, and covered with Entellan.
Analysis, Quantification and Statistics
The number of microscopically detectable immunoreactive neurons was counted in the whole slice visualized under a 20× objective. The areas were identified by the respective immunohistochemial stainings. The crossings of laminin-positive capillaries, between the respective brain areas were counted in the whole slice under the microscope. The number of crossings were normalized to crossings per cm. Cell counting was performed for DAPI-positive malformed and TUNEL-positive nuclei on a Leica DMIRB fluorescence inverse microscope equipped with an Apple computer and Improvision Openlab Darkroom software. Cell counting was performed on a random field (260 μm × 190 μm) per slice in the vMes and nBM. Multistatistical analysis was obtained by one way ANOVA, followed by Fisher PLSD posthoc test by comparing controls against the respective treatments, where p<0.05 represents statistical significance.
RESULTS
Cholinergic and Dopaminergic Neurons in Co-Cultures
Cholinergic neurons in co-slices strongly stained by immunohistochemistry for ChAT (Fig. 1A). Approximately 120 neurons per slice of ChAT-positive neurons (Table 1) were found in the nBM slice (Fig. 1A), and approximately 50 neurons per slice (Table 1) were found in the dStr slice (Fig. 1A), when the co-slice was incubated for 4 weeks with NGF and GDNF. No ChAT-positive neurons were found in the cortex (Fig. 1A) or vMes (Fig. 1A). Cholinergic nerve fibers grew from the nBM to the cortex slice (Fig. 2D), as shown by a strong staining of acetylcholinesterase-positive nerve fibers. Dopaminergic neurons in co-cultures were stained by immunohistochemistry for tyrosine hydroxylase (Fig. 1B). In a co-slice incubated for 4 weeks with NGF and GDNF approximately 80 TH-positive neurons per slice (Table 1) were found exclusively in the vMes (Fig. 1B). When slices were incubated without growth factors the number of cholinergic and dopaminergic neurons was significantly reduced (data not shown). Co-slices composed of four brain regions grew together and formed a large slice after the cultivation of four weeks. At the borders between the brain areas a weaker stained layer of cresyl violet was seen (Fig. 2A). GFAP-staining was low over the entire co-slice, whereas the connective regions between the brain slices displayed a decreased staining for GFAP (Fig. 2B). Immunohistochemistry for D2R revealed several strongly stained cells in the dStr (Fig. 2E). TH-positive nerve fibers (Fig. 2F) from the vMes grew into the dStr as identified by D2R staining in co-labelled sections (Fig. 2G).
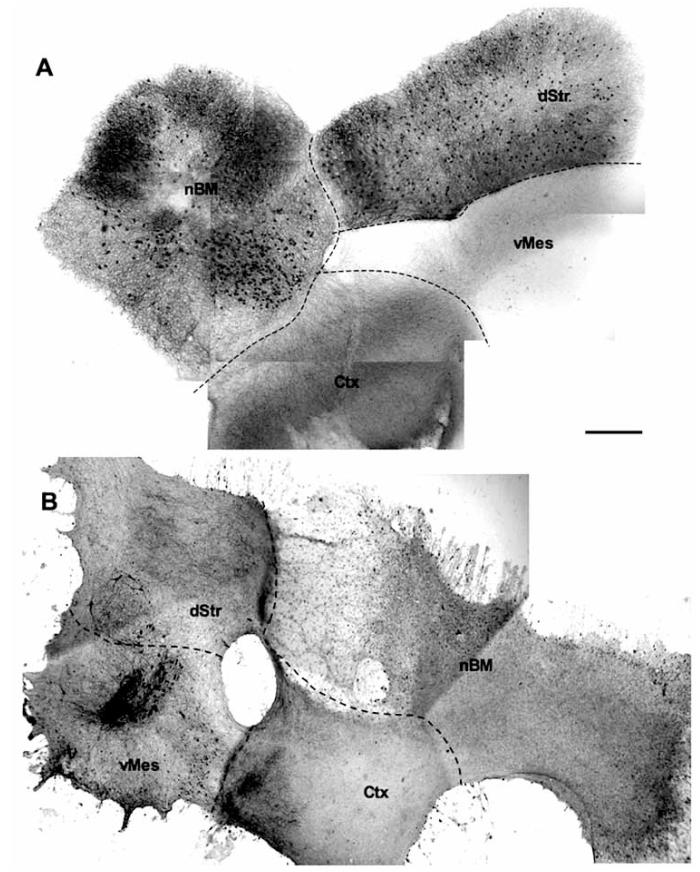
Fig. (1).
Immunohistochemistry for cholinergic (A) or dopaminergic (B) neurons in vitro. Co-slices composed of four brain regions, the basal nucleus of Meynert (nBM), dorsal striatum (dStr), ventral mesencephalon (vMes) and cortex (Ctx) were incubated for 4 weeks with nerve growth factor and glial cell line-derived neurotrophic factor and then stained for choline acetyltransferase (ChAT) (A) or tyrosine hydroxylase (TH) (B). The different brain areas are indicated by dashed lines. Scale bar in A = 550 m (A, B).
Table 1. Effects of Thapsigargin on Neuronal Cell Death.
| Region | Control | Thapsigargin | |
|---|---|---|---|
| ChAT | nBM | 124 ± 15 (7) - | 60 ± 11 (5) ** |
| dStr | 54 ± 12 (7) - | 18 ± 5 (5) ** | |
| TH | vMes | 81±18 (6) - | 18 ± 5 (5) *** |
| DAPI | nBM | 9 ± 1 (7) - | 30 ± 2 (5) *** |
| vMes | 12 ± 2 (7) - | 49 ± 3 (5) *** | |
| TUNEL | nBM | 45 ± 5 (4) - | 392 ± 44 (3) *** |
| vMes | 53 ± 7 (4) - | 313 ± 16 (4) *** |
A 4 week old co-culture brain slice, composed of the basal nucleus of Meynert (nBM), the dorsal striatum (dStr), the cortex (Ctx) and the ventral mesencephalon (vMes), was incubated with 3 μM thapsigargin for 24 hr and then for further 3 days in without. Subsequently, the slices were fixed, stained for choline acetyltransferase (ChAT) or tyrosine hydroxylase (TH) or DAPI or DNA fragments were labelled in situ using the TUNEL assay. The number of ChAT/TH or DAPI-positive nuclei with apoptotic morphology or TUNEL-positive nuclei were counted on a random field (260 μm × 190 μm) per slice are given as mean ± S.E.M.; the values in parenthesis give the number of analyzed slices. Statistical analysis was performed by one way ANOVA with a Fisher PLSD posthoc test (*** p<0.001; ** p<0.01).
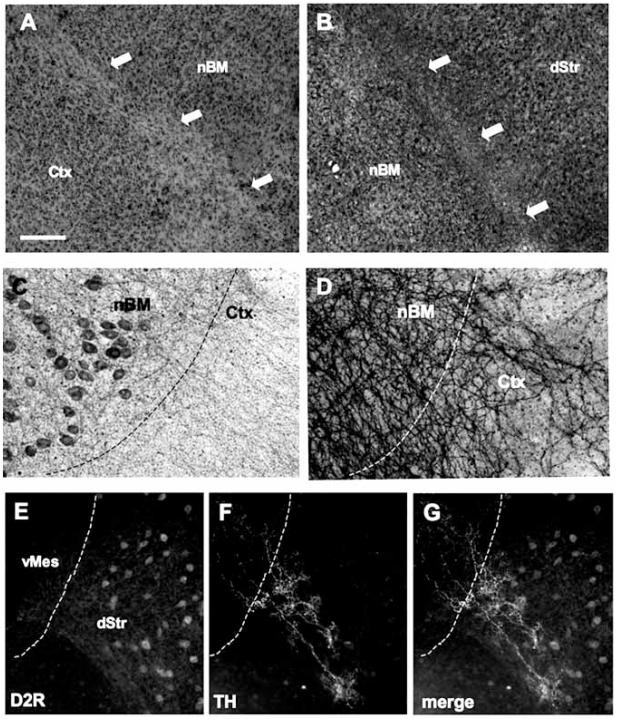
Fig. (2).
Borders between the brain areas in co-cultures. Ventral mesencephalon (vMes) Fig. (A) shows a weaker band of cresyl violet staining between the basal nucelus of Meynert (nBM) and cortex (Ctx) (arrows). Fig. (B) displays weaker glial-fibrillary acidic protein (GFAP)-like immunoreactivity between nBM and dorsal striatum (dStr) (arrows). Fig. (C), choline acetyltransferase (ChAT)-positive neurons in the nBM give rise to strong acetylcholinesterase-positive nerve fibers which cross the border between the nBM and Ctx (D). In the dStr dopamine D2 receptor-(D2R) neurons are expressed (E), and TH-positive nerve fibers (F) grow into the dStr (G, merged). The different brain areas are indicated by dashed lines. Scale bar in A = 250 μm (A, B), 100 μm (C-D), 200 μm (E-G).
Laminin-Positive Capillary Network
When co-slices composed of four brain regions were cultured for 4 weeks with growth factors, a very dense laminin-positive capillary network was seen over all 4 brain areas (Fig. 3). The laminin-positive capillaries grew within the brain areas and most of the new connections were found between the nBM and cortex (Fig. 3B). In some slices the brain capillaries were broken (Fig. 3B) or did not pass the border between the brain areas (Fig. 3C). The laminin-positive capillaries crossed the borders between cortex and nBM (Table 2) and to a lesser extent between vMes and cortex or dStr (Table 2) or nBM and dStr (Table 2), while no crossings were found between dStr and cortex (Table 2).
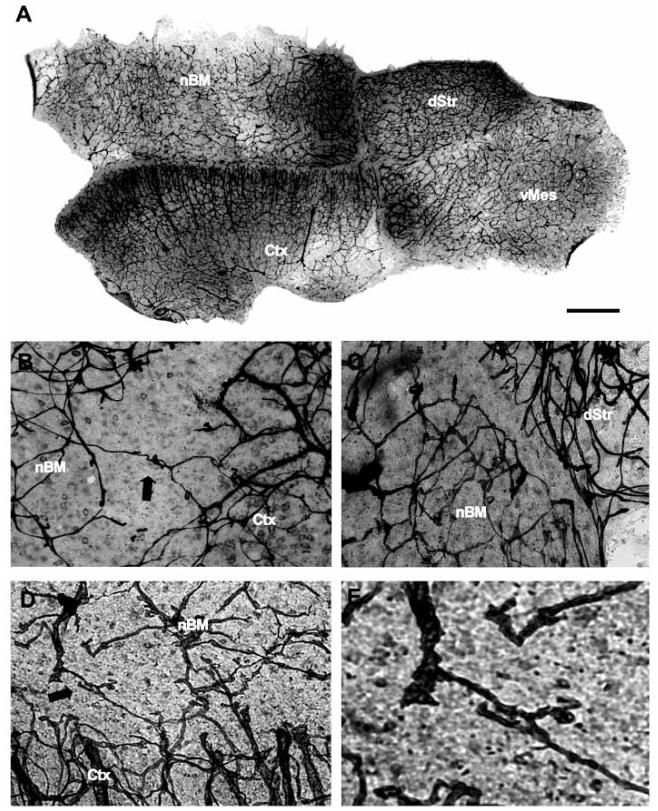
Fig. (3).
Laminin-positive capillaries in a co-slice. Co-slices composed of four brain regions, the basal nucleus of Meynert (nBM), dorsal striatum (dStr), ventral mesencephalon (vMes) and cortex (Ctx) were incubated for 4 weeks with nerve growth factor and glial cell line-derived neurotrophic factor and then stained for laminin (A-E). Higher magnifications of the borders between two brain regions are shown in Fig. (B-E). Note that laminin-positive capillaries cross the border between nBM and Ctx (B, arrow), or are damaged (B). A laminin-negative area between the nBM and dStr is displayed in (C). Co-cultures after thapsigargin treatment displayed an enhanced crossing of laminin-positive capillaries between the nBM and Ctx (D, E, arrow). Fig. (E) showed a higher magnification of vascular growth in Fig. (D). Scale bar in A = 900 μm (A), 300 μm (B-D), 100 μm (E).
Table 2. Effects of Thapsigargin on Re-Growth of Crossing of Laminin-Positive Capillaries.
| Region | Control | Thapsigargin |
|---|---|---|
| Ctx-nBM | 19 ± 3 (11) - | 38 ± 4 (21) ** |
| vMes-Ctx | 6 ± 2 (11) - | 6 ± 2 (9) ns |
| vMes-dStr | 10 ± 4 (11) - | 7 ± 3 (9) ns |
| nBM-dStr | 5 ± 2 (11) - | 2 ± 1 (9) ns |
| dStr-Ctx | n.c. (11) | n.c. (6) |
A 4 week old co-culture brain slice, composed of the basal nucleus of Meynert (nBM), the dorsal striatum (dStr), the cortex (Ctx) and the ventral mesencephalon (vMes), was incubated with 3 μM thapsigargin and then further 3 days without. Then slices were fixed and stained for laminin immunohistochemistry. The number of capillary crossings per cm between the different brain regions was counted under the microscope. Values are given as mean ± S.E.M.; values in parenthesis give the number of analyzed slices. Statistical analysis was performed by one way ANOVA with a Fisher PLSD posthoc test (**p<0.01; ns, not significant; n.c., no crossings).
Effects of Thapsigargin in Co-Cultures
The number of cholinergic and dopaminergic neurons in co-cultures composed of four brain regions was significantly decreased after the incubation with 3 μM thapsigargin (Fig. 4; Table 1). Thapsigargin had a significant toxic effect on cholinergic neurons in the nBM and dStr and on the dopaminergic neurons in the vMes (Table 1). The remaining cholinergic and dopaminergic neurons (Fig. 4B) in co-cultures after thapsigargin treatment displayed a shrunken and smaller shape compared to untreated co-slices (Fig. 4A). Furthermore, the treatment of co-cultures with thapsigargin enhanced the number of DAPI-positive malformed nuclei in the vMes and nBM (Table 1). Healthy nuclei appeared round, well-formed with an intensive staining (data not shown). Whereas, malformed nuclei emerged in spindle, enlarged or granular form, with a weaker DAPI-labeling (Fig. 4D, indicated by arrows). The incubation of co-cultures with thapsigargin (Fig. 4C) revealed a significantly increased number of TUNEL-positive nuclei (Table 1), compared to untreated co-slices (Table 1). Co-localization studies of TH-/ChAT-immunoreactivity and DAPI staining displayed that DAPI-positive malformed nuclei were not related to TH (Fig. 4D) or ChAT-positive immunoreactive neurons (data not shown). Treatment of co-cultures with thapsigargin exhibited a significantly enhanced laminin-positive capillary network between cortex and nBM (Table 2; Fig. 3D, E). Capillaries at the border between nBM and cortex displayed enhanced processes (Fig. 3D) after thapsigargin treatment compared to capillaries in untreated co-slices (Fig. 3B, C). However, the angiogenic effect of thapsigargin emerged exclusively between nBM and cortex, but not between the borders of the other brain regions (Table 2).
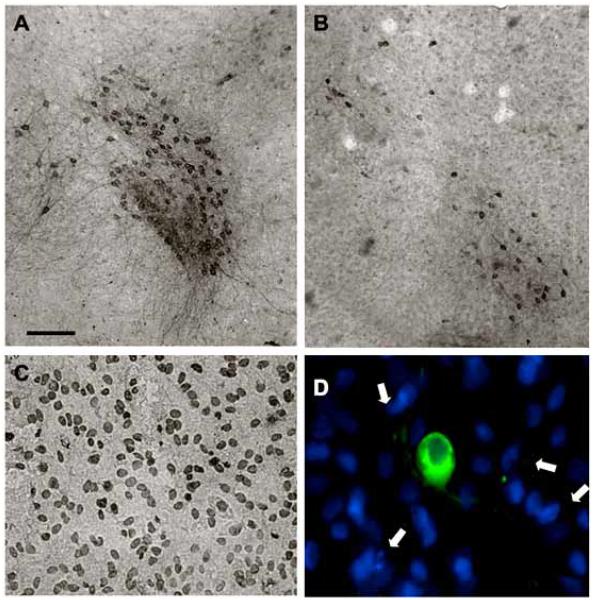
Fig. (4).
Effects of thapsigargin on cell death. Co-cultures were treated overnight without (control, A) or with 3 μM thapsigargin (B-D). After 3 days, slices were immunohistochemically stained against tyrosine hydroxylase (A, B), which stains dopaminergic neurons in the vMes. In situ labelling of nuclear DNA fragmentation exhibited an enhanced TUNEL-positive staining in vMes slices after thapsigargin (C). Co-localization experiments showed malformed DAPI-positive nuclei (blue, arrows), which did not co-localize with TH-positive neurons (green, Alexa-488) after thapsigargin treatment (D). Scale bar in A = 250 μm (A, B), 100 μm (C), 50 μm (D).
DISCUSSION
The present study demonstrates, that the pro-apoptotic chemical thapsigargin exhibited angiogenic activity between functional related brain areas and induced cell death of cholinergic and dopaminergic neurons in organotypic brain co-cultures.
Organotypic Brain Slice Model
The organotypic brain slice model is able to maintain the survival of different cell types, the cytoarchitecture of the tissue, the connections between cells and neuronal properties. The basic cellular and connective organization of the donor brain regions are well preserved, thus the slice cultures provide an easily accessible experimental model for studies of toxic, degenerative and plastic regenerative or developmental changes in the brain (Zimmer et al., 2000). In slices, the individual cells are arranged in close contact and do not lose density-dependent regulatory mechanisms, three-dimensional architecture as well as tissue-specific transport and diffusion probabilities. Organotypic brain slices were first introduced by Gähwiler and colleagues (Gähwiler and Hefti, 1984, 1997) as roller tube cultures. The technique was modified (Stoppini et al., 1991; Buchs et al., 1993) and is meanwhile used by several research groups (Ostergaard et al., 1990 and 1993; Gähwiler et al., 1990; Robertson et al., 1997). The culturing of slices on membranes is well established in our research group (Humpel et al., 1995; Schatz et al., 1999; Weis et al., 2001; Humpel and Weis, 2002). Furthermore, we have shown in previous studies, that the addition of growth factors into the culture medium prevents the neurons from cell death (Weis et al., 2001; Humpel und Weis, 2002; Schatz et al., 1999). This is necessary, because slices do not produce enough endogenous growth factors to support the survival of the cholinergic or dopaminergic neurons. It is well established, that NGF prevents the loss of cholinergic neurons in vitro and in vivo (Hagg et al., 1990; Hefti et al., 1985; Kew et al., 1996; Svendsen et al., 1994; Van der Zee et al., 1996; Connor et al., 2001). In fact we have previously shown that slices contain less than 3 pg NGF/mg tissue, which is insufficient to support the cholinergic phenotype (Weis et al., 2001). Whereas, dopaminergic neurons are protected and stimulated very potently by GDNF (Airaksinen et al., 2002; Henderson et al., 1994; Arenas et al., 1995; Gill et al., 2003), which have been additionally shown in previous studies in organotypic brain slices by Schatz et al. (1999). Moreover, co-cultures of the nBM together with the cortex (Humpel and Weis, 2002) and the vMes together with the dStr (Schatz et al., 1999) provide an excellent system to study interaction between two brain regions. In the present study, we used a co-culture system composed of four brain regions, the nBM, vMes, dStr and cortex. The slices for these co-cultures derive from early postnatal day 7-9 rat brains and neurons at this developmental stage are very resistant for explantation, the cytoarchitecture is already established and the tissue has the property to flatten. This flattening to about 100-200 m is also an internal mean for a good preparation and dissection, whereas it is not completely clear why the slices become thinner. Our data show, that the four different brain areas (nBM, Ctx, vMes, dStr) grew together and formed a single large organotypic slice. The astroglial GFAP-staining was moderate to low in all slices, indicating that no reactive gliosis occurred, being in line with previous experiments in our group (Schmidt-Kastner et al., 2002).
Cholinergic and Dopaminergic Neurons in Co-Cultures
Cell death of cholinergic and dopaminergic neurons are the central hallmark in AD and PD. Our brain slice model allows to study survival and neurodegeneration of the cholinergic and dopaminergic neurons as reported in several recent papers by us (Humpel and Weis, 2002; Zassler et al., 2003, 2005; Weis et al., 2001; Humpel et al., 1995; Schatz et al., 1999). The enzyme ChAT can be used as a key enzyme to label cholinergic neurons and the number of ChAT-positive neurons strongly correlate with the survival rate. We have previously shown, that cholinergic neurons survive in co-slices of the nBM and cortex and cholinergic nerve fibers grew into the cortex slice (Humpel and Weis, 2002). Our present study shows that cholinergic ChAT-positive nBM neurons survive in a co-slice composed of four brain areas. In addition, also a high number of smaller ChAT-positive interneurons survived in the dStr, while in the cortex and vMes no ChAT-positive neurons were seen. Similarly to ChAT, we have used the key enzyme TH to label dopaminergic neurons, which is well established (Schatz et al., 1999). Likewise to cholinergic neurons, dopaminergic neurons grew into the functional target, the dStr. Again, these nerve fibers can be regarded as new innervation from the ventral mesencephalon, because all nerve fibers in the axotomized dorsal striatum degenerate. Our present model shows further survival of TH-positive dopaminergic neurons exclusively into the dStr, D2 receptor staining in striatum and vMes and selective ingrowth of TH-positive fibers.
Effects of Thapsigargin on Cholinergic and Dopaminergic Cell Death
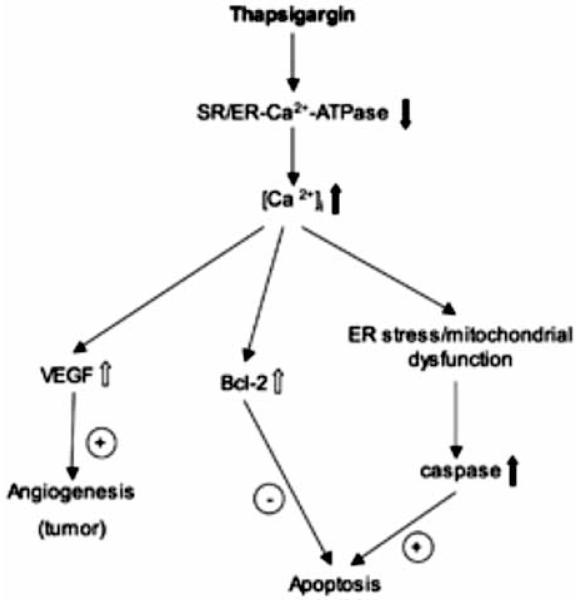
Thapsigargin is an effective inhibitor of the Ca2+-ATPase in the sarcoplasmic and endoplasmic reticulum and activates plasma membrane calcium channels (see scheme Fig. 5), resulting in enhanced influx of calcium into the cell (Takadera et al., 2007; Thastrup et al., 1990; Treimann et al., 1998; Chin et al., 2007; Ferri and Kroemer, 200; Dahmer, 2005; Deniaud et al., 2008). It is well known that thapsigargin has a dual effect on cells: it can induce apoptosis but also inhibit apoptosis (see Fig. 5), dependent on the cellular co-factors. (1) Thapsigargin exhibits apoptotic cell death in various cell types, e.g. cortical cells, hypothalamic cell lines or muscle cells (Takadera et al., 2007; Thastrup et al., 1990; Treimann et al., 1998; Wei et al., 1998; Mattson et al., 1998; Linford et al., 2002). Two major pathways have been shown to be responsible for this apoptotic cell death: the extrinsic receptor-mediated and the intrinsic signaling pathways (Chin et al., 2007). Recent studies have shown, that thapsigargin treatment stimulates the proteolytic processing of caspase-2, -3 and -7, which are involved in the intrinsic ER-stress mediated apoptosis (Dahmer, 2005; Deniaud et al., 2008; Kass and Orrenius, 1999; Chin et al., 2007; Ferri and Kroemer, 2001). (2) On the other hand it has been shown that thapsigargin can also inhibit apoptosis via activation of the bcl-2 cascade. Chin and colleagues (2007) demonstrated, that Ca2+-sensitive mitogen-activated protein kinase (MAPK) phosphorylation was induced by thapsigargin and acted as a downstream effector of phosphatidylinositol 3-kinase (PI 3-kinase), which is involved in the inhibition of apoptosis (Downward, 2004). Nevertheless, it is suggested that ER stress and mitochondrial dysfunction leads to an activation of Bcl-2 or other additional anti-apoptotic substances (Ferrari et al., 2002; Giacomello et al., 2007).
Fig. (5).
Proposed mechanism and effect of thapsigargin. Thapsigargin inhibits the calcium-ATPase of the sarcoplasmic (SR)/endoplasmic reticulum (ER), resulting in enhanced intracellular calcium levels. This enhanced Ca2+ can either inhibit apoptosis by activation of anti-apoptotic substances of e.g. the Bcl-2 class or can induce apoptosis by activation of the caspase cascade after ER stress and/or mitochondrial dysfunction. Alternatively, the enhanced production of vascular endothelial growth factor (VEGF) may result in angiogenesis and possibly tumor formation.
Little is known about the effects of thapsigargin in inducing cell death of cholinergic and dopaminergic CNS neurons. Recent studies indicate, that ER stress in conjugation with abnormal protein degradation can contribute to the pathophysiology of PD, but also AD (Lindholm et al., 2006). Our data show a significant decline of ChAT-positive nBM neurons, TH-positive vMes neurons and ChAT-positive interneurons in the dStr after thapsigargin treatment. The data suggest, that the inhibition of the Ca2+-ATPase in the sarcoplasmic and endoplasmic reticula is a vulnerable target for cholinergic as well as dopaminergic neurons in the brain. The increased TUNEL-positive staining in the nBM and vMes in our co-cultures indicates that apoptotic cell death occurred after thapsigargin application. In parallel, many DAPI-positive malformed nuclei were seen after treatment with thapsigargin. The number of TUNEL- and malformed DAPI-positive cells was many times higher than the number of cholinergic and dopaminergic neurons. In fact, co-localization studies previously showed that dopaminergic neurons did not co-localize with TUNEL-positive nuclei after 6-OHDA lesions (Schatz et al. 1999). The present study is in line with our previous data, showing that DAPI-positive malformed nuclei were not related to dopaminergic or cholinergic neurons. The data may indicate that astroglia undergo apoptotic cell death, which may result in neurodegeneration of dopaminergic and cholinergic neurons. It needs to be pointed out, if thapsigargin can induce DAPI- or TUNEL-positive staining in purified astroglial cell culture. In addition, more advanced methods need to be performed, such as caspase assays, to detect the responsible mechanisms, which are involved in the neurodegeneration of cholinergic and dopaminergic neurons due to thapsigargin treament.
Angiogenic Activity of Thapsigargin in Brain Slices
Brain capillaries constitute the blood-brain barrier and innervate all areas of the brain. We have previously shown, that organotypic brain slices contain a strong network of laminin- or RECA-1 positive brain capillaries (Moser et al., 2004, 2003). Laminin is a well-established basement membrane marker and stains excellently the vascular structures of the brain (Eriksdotter-Nilsson et al., 1986; Jucker et al., 1996; Sixt et al., 2001). Our present study is in full agreement with our previous ones and shows a strong network of laminin-positive brain capillaries all over the four brain slices. Furthermore we show, that brain capillaries grow from one side to another and connect the brain slices. Such a growth between two brain slices was most prominent between the nBM and cortex and to a lesser extent between vMes and dStr. Thus it is suggested, that the co-slice model represents a useful in vitro system, where capillary re-growth is specifically observed between two functionally related brain areas, such as the nBM - cortical and meso-striatal system. On the other hand, no connections were observed between the dorsal striatum and cortex. This region-specific effect is of importance and points to a positive influence of the neocortex to the nBM, suggesting that the nBM cholinergic input to the neocortex provides a vascular rather than a neuronal modulation, being in line with Kurosawa et al. (1989).
Thus, we demonstrated for the first time, that thapsigargin possesses pro-angiogenic activity in the brain by increasing the capillary growth between the nBM and cortex. It is known that thapsigargin has anti-angiogenic activity in vascular smooth muscle cells (VSMC) (Shukla et al., 2001; Birket et al., 1999; George et al., 1997; Shukla et al., 1997), but it is fully unknown how thapsigargin may exhibit this angiogenic activity, specifically on nBM - Ctx capillaries. It seems possible, that thapsigargin-induced Ca2+ release stimulates vascular endothelial growth factor (VEGF) production (Josko et al., 2000) and subsequently angiogenesis (Fig. 5). In fact, it is well established that thapsigargin increases the expression and release of VEGF (Koyama et al., 2008; Marjon et al., 2004), which is one of the most potent angiogenic factors and exerts its effect by binding to its specific receptors Flt-1 and Flk-1 in endothelial cells (Yancopoulos et al., 2000). Both VEGF receptors Flk-1 and Flt-1 are expressed in the cortex (Yang et al., 2003). Furthermore, recent studies demonstrated that VEGF and GDNF use the same signaling pathway in ureteric bud cells (Tufro et al., 2007). Such a direct interaction between VEGF and GDNF has not been demonstrated in the brain, however, it cannot be excluded that GDNF may stimulate VEGF in the brain too. Alternatively, it seems possible that the neurodegeneration of the neurons and/or the apoptotic cell death of e.g. glial cells may secrete chemokines or cytokines, which stimulate capillary growth. And finally, a putative anti-apoptotic effect of thapsigargin on brain capillary endothelial cells may contribute to the angiogenetic responses. This angiogenic sprouting may play a role in tumor growth and metastasis (Liekens et al., 2001), because a direct correlation between the tumor suppressor p53 and cells with a dysfunctional ER has been postulated recently (Pluquet et al., 2005). Further studies will be necessary to elucidate the cellular mechanisms of thaspigargin in the brain, especially of VEGF and p53 involvement.
In summary we demonstrated a pro-angiogenic effect of thapsigargin between functional related brain areas in an in vitro co-culture system composed of four brain regions. Furthermore we have shown, that dopaminergic as well as cholinergic neurons degenerate after ER-induced calcium stress. It can be emphasized, that the dysregulation of the ER may play a role in initiation of early PD or AD and possibly the vascular pathology in that neurodegenerative disease.
ACKNOWLEDGEMENTS
This study was supported by the Austrian Science Funds (P19122-B05). We thank Ursula Kirzenberger-Winkler for excellent technical assistance.
REFERENCES
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Neuroscience. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Arenas E, Trupp M, Akerud P, Ibanez CF. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron. 1995;15:1465–1473. doi: 10.1016/0896-6273(95)90024-1. [DOI] [PubMed] [Google Scholar]
- Birkett SD, Jeremy JY, Watts SM, Shukla N, Angelini GD, McArdle CA. Inhibition of intracellular Ca2+ mobilisation by low antiproliferative concentrations of thapsigargin in human vascular smooth-muscle cells. J Cardiovasc Pharmacol. 1999;33:204–211. doi: 10.1097/00005344-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Blandini F, Porter RHP, Greenamyre JT. Glutamate and Parkinso’s disease. Mol Neurobiol. 1996;12:73–94. doi: 10.1007/BF02740748. [DOI] [PubMed] [Google Scholar]
- Buchs PA, Stoppini L, Muller D. Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Brain Res Dev. 1993;71:81–91. doi: 10.1016/0165-3806(93)90108-m. [DOI] [PubMed] [Google Scholar]
- Connor JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci USA. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Crain B, Peterson ER. Development of cross-tolerance to 5-hydroxytryptamine in organotypic cultures of mouse spinal cord-ganglia during chronic exposure to morphine. Life Sci. 1982;31:241–247. doi: 10.1016/0024-3205(82)90584-7. [DOI] [PubMed] [Google Scholar]
- Dahmer MK. Caspases-2,-3, and -7 are involved in thapsigargin-induced apoptosis of SH-SY5Y neuroblastoma cells. J Neurosci Res. 2005;80:576–583. doi: 10.1002/jnr.20471. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinso’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheime’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Sem Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Eriksdotter-Nilsson M, Björklund H, Olson L. Laminin immunohistochemistry: a simple method to visualize and quantitate vascular structures in the mammalian brain. J Neurosci Methods. 1986;17:275–286. doi: 10.1016/0165-0270(86)90128-7. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pinton P, Szabadkai G, Chami M, Campanella M, Pozzan T, Rizzuto R. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. Cell Calcium. 2002;32:413–420. doi: 10.1016/s0143416002002014. [DOI] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nature Cell Biol. 2001;3:255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Franke H, Schelhorn N, Illes P. Dopaminergic neurons develop axonal projections to their target areas in organotypic co-cultures of the ventral mesencephalon and the striatum/prefrontal cortex. Neurochem Int. 2003;42:431–439. doi: 10.1016/s0197-0186(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Ganten D, Ruckpaul K. Molekularmedizinische Grundlagen von alters-spezifischen Erkrankungen. Springer Verlag; 2004. pp. 200–227. [Google Scholar]
- Gähwiler BH, Hefti F. Guidance of acetylcholinesterase-containing fibers by target tissue in co-cultured brain slices. Neuroscience. 1984;40:235–243. doi: 10.1016/0306-4522(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Tietschin L, Knöpfel T, Enz A. Continuous presence of nerve growth factor is required for maintenance of cholinergic septal neurons in organotypic slice cultures. Neuroscience. 1990;36:27–31. doi: 10.1016/0306-4522(90)90348-8. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- George SJ, Johnson JL, Angelini GD, Jeremy JY. Short-term exposure to thapsigargin inhibits neointima formation in human saphenous vein. Arterioscler. Thromb Vasc Biol. 1997;17:2500–2506. doi: 10.1161/01.atv.17.11.2500. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca 2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendson CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Hagg T, Vahlsing HL, Mathorpe M, Varon S. Nerve growth factor infusion into the denervated adult rat hippocampal formation promotes its cholinergic reinnervation. J Neurosci. 1990;10:3087–3092. doi: 10.1523/JNEUROSCI.10-09-03087.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Hartikka J, Eckstein F, Gnahn H, Heumann R, Schwab M. Nerve growth factor increases choline acetyltransferase but not survival or fiber outgrowth of cultured fetal septal cholinergic neurons. Neuroscience. 1985;14:55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Honig LS, Rosenberg RN. Apoptosis and neurologic disease. Am J Med. 2000;108:317–330. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Humpel C, Johansson M, Marksteiner J, Saria A, Strömberg I. Mesencephalic grafts increase preprotachykinin-A mRNA expression in striatal grafts in an in oculo co-graft model. Regul Peptides. 1995;56:9–17. doi: 10.1016/0167-0115(95)00122-r. [DOI] [PubMed] [Google Scholar]
- Humpel C, Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices. J Neural Transm. 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinso’s disease. Ann Neurol. 2003;53:26–38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Josko J, Gwozdz B, Jedrzejowsko-Szypulka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6:1047–1052. [PubMed] [Google Scholar]
- Jucker M, Tian M, Norton DD, Sherman C, Kusiak JW. Laminin α2 is a component of brain capillary basement membrane: reduced expression in dystrophic dy mice. Neuroscience. 1996;71:1153–1161. doi: 10.1016/0306-4522(95)00496-3. [DOI] [PubMed] [Google Scholar]
- Kass GEN, Orrenius S. Calcium signaling and cytotoxity. Environ Health Perspect. 1999;107:25–35. doi: 10.1289/ehp.99107s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Rimvall K, Waser PG. Choline acetyltransferase in organotypic cultures of rat septum and hippocampus. Neurosci Lett. 1983;42:273–278. doi: 10.1016/0304-3940(83)90274-4. [DOI] [PubMed] [Google Scholar]
- Kew JNC, Smith DW, Sofroniew MV. Nerve growth factor withdrawal induces the apoptotic death of developing septal cholinergic neurons in vitro: protection by cyclic AMP analogue and high potassium. Neuroscience. 1996;70:329–339. doi: 10.1016/0306-4522(95)00365-7. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Matszuzaki S, Gomi F, Yamada K, Katayama T, Sato K, Kumada T, Fukuda A, Matsuda S, Tano Y, Tohyama M. Induction of amyloid ß accumulation by ER calcium disruption and resultant upregulation of angiogenic factors in ARPE19 cells. Invest Ophthalmol Vis Sci. 2008;49:2376–2383. doi: 10.1167/iovs.07-1067. [DOI] [PubMed] [Google Scholar]
- Kurosawa M, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases acetylcholine release in the cerebral cortex in rats. Neurosci Lett. 1989;98:45–50. doi: 10.1016/0304-3940(89)90371-6. [DOI] [PubMed] [Google Scholar]
- Liekens S, de Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Dorsa DM. 17β-estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;76:1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheime’s disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheime’s disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat. Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Marjon PL, Bobrovnikova-Marjon EV, Abcouwer SF. Expression of the pro-angiogenic factors vascular endothelial growth fator and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol Cancer. 2004;3:4. doi: 10.1186/1476-4598-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser KV, Schmidt-Kastner R, Hinterhuber H, Humpel Brain capillaries and cholinergic neurons persist in organotypic brain slices in the absence of blood flow. Eur J Neurosci. 2003;18:85–94. doi: 10.1046/j.1460-9568.2003.02728.x. [DOI] [PubMed] [Google Scholar]
- Moser KV, Reindl M, Blasig I, Humpel C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res. 2004;1017:53–60. doi: 10.1016/j.brainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheime’s disease: review and pathogenic implications. Hum Pathol. 1995;26:816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinso’s disease. Ann Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Ostergaard K, Schou JP, Zimmer J. Rat ventral mesencephalon grown as organotypic slice cultures and co-cultured, hippocampus, and cerebellum. Exp Brain Res. 1990;82:547–565. doi: 10.1007/BF00228796. [DOI] [PubMed] [Google Scholar]
- Ostergaard K. Organotypic slice cultures of rat striatum, I. A histochemical and immunohistochemical study of acetylcholinesterase, choline acetyltransferase, glutamate decarboxylase and GABA. Neuroscience. 1993;53:679–693. doi: 10.1016/0306-4522(93)90616-n. [DOI] [PubMed] [Google Scholar]
- Pluquet O, Qu LK, Baltzis D, Koromilas AE. Endoplasmic reticulum stress accelerates p53 degradation by the cooperative actions of Hdm2 and glycogen synthase kinase 3beta. Mol Cell Biol. 2005;25:9392–9405. doi: 10.1128/MCB.25.21.9392-9405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RT, Baratta J, Kageyama GH, Ha DA, Yu J. Specificity of attachment and neurite outgrowth of dissociated basal forebrain cholinergic neurons seeded on to organotypic slice cultures of forebrain. Neuroscience. 1997;80:741–752. doi: 10.1016/s0306-4522(97)00067-5. [DOI] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Saria A, Humpel C. Dopamine neurons in a simple GDNF-treated meso-striatal organotypic co-culture model. Exp Brain Res. 1999;127:270–278. doi: 10.1007/s002210050796. [DOI] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Schuligoi R, Humpel C, Saria A. 3,4-methylen-edioxymetamphetamine (Ecstasy) induces c-fos-like protein and mRNA in rat organotypic dorsal striatal slices. Synapse. 2000;36:75–83. doi: 10.1002/(SICI)1098-2396(200004)36:1<75::AID-SYN8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Humpel C. Nestin expression persists in astrocytes of organotypic slice cultures from rat cortex. Int J Dev Neurosci. 2002;20:29–38. doi: 10.1016/s0736-5748(02)00003-5. [DOI] [PubMed] [Google Scholar]
- Shukla N, Jeremy JY, Nicholl P, Krijgsman B, Stansby G, Hamilton G. Short-term exposure to low concentrations of thapsigargin inhibits replication of cultured human vascular smooth muscle cells. Br J Surg. 1997;84:325–330. [PubMed] [Google Scholar]
- Shukla N, Freeman N, Gadsdon P, Angelini GD, Jeremy JY. Thapsigargin inhibits angiogensis in the rat isolated aorta: studies on the role of intracellular calcium pools. Cardiovasc Res. 2001;49:681–689. doi: 10.1016/s0008-6363(00)00269-8. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Vendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decesive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelities. J Cell Biol. 2001;153:933–945. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buch PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Kew JN, Staley K, Sofroniew MV. Death of developing septal cholinergic neurons following NGF withdrawal in vitro: protection by protein synthesis inhibition. J Neurosci. 1994;14:75–87. doi: 10.1523/JNEUROSCI.14-01-00075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takadera T, Fujibayashi M, Kaniyu H, Sakota N, Ohyashiki T. Caspase-dependent apoptosis induced by thapsigargin was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Neurochem Res. 2007;32:1336–1342. doi: 10.1007/s11064-007-9310-4. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promotor, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treimann M, Caspersen C, Christensen CB. A tool coming of age: thapsigargin as an inhibitor of sarcoendoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- Tufro A, Teichman J, Banu N, Villegas G. Crosstalk between VEGF-A/VEGFR2 and GDNF/RET signaling pathways. Biochem Biophys Res Commun. 2007;358:410–416. doi: 10.1016/j.bbrc.2007.04.146. [DOI] [PubMed] [Google Scholar]
- Van der Zee CEEM, Ross GM, Riopelle RJ, Hagg T. Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mice. Science. 1996;110:641–651. doi: 10.1126/science.274.5293.1729. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guégen C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S. The role of glial cells in Parkinso’s disease. Curr Opin Neurol. 2001;14:483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Wei H, Wei W, Bredesen DE, Perry DC. Bcl-2 protects against apoptosis in neuronal cell line caused by thapsigargin-induced depletion of intracellular calcium stores. J Neurochem. 1998;70:2305–2314. doi: 10.1046/j.1471-4159.1998.70062305.x. [DOI] [PubMed] [Google Scholar]
- Weis C, Marksteiner J, Humpel C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience. 2001;102:129–138. doi: 10.1016/s0306-4522(00)00452-8. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheime’s disease and senile dementia: loss of neurons in basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Esiri MM, Bowen DM, Smith CC. Alzheime’s disease-correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci. 1982;57:407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]
- Winkler J, Thal LJ, Gage FH, Fisher LJ. Cholinergic strategies for Alzheime’s disease. J Mol Med. 1998;76:555–67. doi: 10.1007/s001090050250. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptores in neonatal and adult rat brains. Anat Rec. 2003;274A:851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- Zassler B, Weis C, Humpel C. Tumor necrosis factor-alpha triggers cell death of sensitized potassium chloride-stimulated cholinergic neurons. Mol Brain Res. 2003;113:78–85. doi: 10.1016/s0169-328x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Zassler B, Dechant G, Humpel C. Urea enhances the nerve growth factor-induced neuroprotective effect on cholinergic neurons in organotypic rat brain slices. Neuroscience. 2005;130:317–323. doi: 10.1016/j.neuroscience.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkino’s disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Lee H, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheime’s disease. Brain Res. 2000;1000:32–39. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Kristensen BW, Jakobsen B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19:7–21. doi: 10.1007/s007260070029. [DOI] [PubMed] [Google Scholar]