Abstract
Mammalian target of rapamycin (mTOR) plays a pivotal role in many aspects of cellular proliferation and recent evidence suggests that an altered mTOR signaling pathway plays a central role in the pathogenesis of aging, tumor progression, neuropsychiatric and major depressive disorder. Availability of a mTOR-specific PET tracer will facilitate monitoring early response to treatment with mTOR inhibitors that are under clinical development. Towards this we have developed the radiosynthesis of [18F]1-(4-(4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo [3,4-d] pyrimidin-6-yl)phenyl)-3-(2-fluoroethyl)urea [18F]ATPFU ([18F]1) as an mTOR PET ligand. Synthesis of reference 1 and the precursor for radiolabeling, 4-(4-8-oxa-3-azabicyclo[3.2.1]- octan-3yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-6yl)aniline (10), were achieved from beta-chloroaldehyde 3 in 4 and 5 steps respectively with an overall yield of 25-28%. [18F]Fluoroethylamine was prepared by heating N-[2-(toluene-4-sulfonyloxy)ethyl]phthalimide with [18F]fluoride ion in acetonitrile. [18F]1 was obtained by slow distillation under Ar of [18F]FCH2CH2NH2 into amine 10 that was pre-treated with triphosgene at 0-5 °C. The total time required for the 2-step radiosynthesis including semi-preparative HPLC purification was 90 minutes and the overall radiochemical yield of [18F]1 for the process was 15 ± 5% based on [18F]fluoride ion (decay corrected). At the end of synthesis (EOS), the specific activity was 37-74 GBq/μfmol (N= 6).
Keywords: mTOR, Radiotracer, PET, Fluorine-18
Introduction
Mammalian target of rapamycin (mTOR) is an evolutionarily conserved 289kDa serine/threonine protein kinase and a member of the phosphoinositide-3-kinase (PI3K) family that integrates signals from multiple pathways, including nutrients, growth factors, hormones, and stresses to regulate cellular functions such as translation, transcription, protein turnover, cell growth, differentiation, cell survival, metabolism, energy balance, and stress response.1-3 Thus, mTOR functions as a molecular sensor, which regulates protein synthesis, enhancing mRNA translation of genes involved in the regulation of cell proliferation and survival, working as part of two distinct multimeric complexes known as mTORC1 and mTORC2. mTOR pathway plays a central role in controlling protein homeostasis and negatively regulates autophagy. Recent evidence suggests that an altered mTOR signaling pathway plays a central role in aging4, tumors including glioblastoma5 Alzheimer's disease (AD)6,7, and epileptogenesis. 8,9 A 50% reduction in mTOR expression is reported in the prefrontal cortex of major depressive disorder (MDD) subjects compared to controls.10,11 Recently, Tang et al demonstrated that mTOR accumulates in tangle-bearing neurons at early stages in AD brains and mediates the synthesis and aggregation of tau.12 Pharmacological reduction of mTOR signaling with rapamycin alleviates tau pathology and the associated behavioral deficits in mouse model overexpressing mutant human tau.13 Thus, mTOR inhibitors are currently proposed as alternate candidates for the treatment of AD, epilepsy and depression. Several mTOR inhibitors are also currently in Phase II/ III clinical trials for cancer treatment through mono-targeted or combination therapy.14 Availability of an mTOR-specific PET tracer would facilitate monitoring early response to treatment with mTOR inhibitors that are under clinical development for human cancers and CNS disorders. Although GSK2126458, a potent mTOR inhibitor, was recently radiolabeled, subnanomolar affinity against PI3K subtypes and protein kinase B (Akt) makes it unsuitable for specific in vivo evaluation of mTOR.15
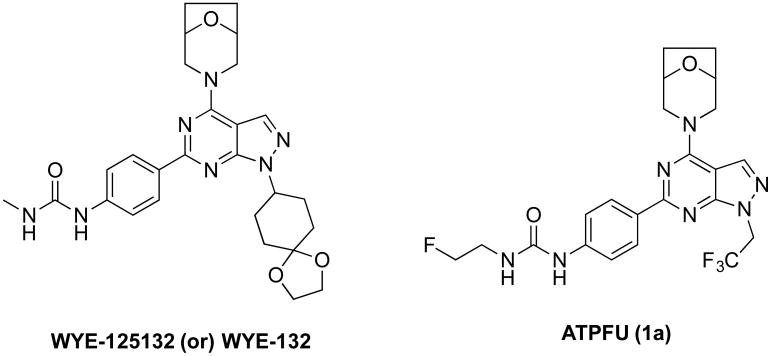
Pyrazolopyrimidines and pyridopyrimidines substituted with morpholine moiety that have examples of nanomolar affinity mTOR inhibitors with high selectivity over PI3K subtypes, favourable lipophilicity and easy access to radiolabeling were selected as candidates for developing mTOR PET ligands. Among these, WYE-125132 is a highly potent, ATP-competitive, and specific inhibitor of mTOR that inhibited mTORC1 and mTORC2 in diverse cancer models in vitro and in vivo (Figure 1).18 In the SAR studies, the presence of fluoroalkyl group connected to the amino group of urea linkage as in compound 1-(4-(4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo-[3,4-d]pyrimidin-6-yl)phenyl)-3-(2-fluoroethyl)urea (ATPFU or 1) was found to be equally potent as WYE-125132 towards mTOR.16,17 ATPFU is a highly potent, and specific inhibitor of mTOR that inhibited mTORC1 and mTORC2 in vitro (IC50 = 0.23 nM). ATPFU has no significant binding to P13KR (IC50 = 2746 nM) and a favorable ClogP (2.03) for facile blood brain barrier (BBB) penetration.17 Herein we describe the radiosynthesis of [18F]ATPFU as a potential mTOR PET ligand.
Figure 1.
The structures of highly specific pyrazolopyrimidine mTOR inhibitor WYE-132 and the proposed mTOR PET tracer ATPFU
Experimental section
General Methods
All commercial reagents and solvents were used without further purification unless otherwise specified. The pyrazolopyrimidine 5 was prepared from barbituric acid (2) in 3 steps based on a literature procedure.18 The boronate esters 8a19 and 8b19 were prepared from commercially available 4-aminophenylboronic acid pinacol ester (6) on treatment with triphosgene followed by the corresponding 2-fluoro (or) 2-hydroxy ethylamine. High-resolution mass spectra were acquired under fast atom bombardment (FAB1) mode using a tandem mass spectrometer (JKS-HX, 11UHF/HX110 HF). The 1H NMR spectra of all the compounds were recorded on a 400 MHz spectrometer (Bruker, PPX-400). The spectra were recorded in CDCl3 or DMSO-d6 and chemical shift (δ) data for the proton resonances were reported in parts per million (ppm) relative to internal standard TMS. Thin-layer chromatography was performed using Silica Gel 60 F254 plates (EM Science) and visualized by UV light. Flash column chromatography was carried out using silica gel 60 (Fisher Scientific, 230–400 mesh). Analytical HPLC was performed using a reverse phase column (4.6 × 250 mm, 5 μm, Phenomenex Prodigy ODS(3)) and eluted with mobile phase; 50:50 acetonitrile: 0.1 M ammonium formate buffer. Semipreparative HPLC was performed using a reverse phase column (Phenomenex Prodigy ODS-Prep 10 × 250 mm, 10 μm)and eluted with 40:60:0.5 acetonitrile: 0.1 M ammonium formate solution: acetic acid buffer. For detection of radiolabeled compounds, γ-ray detector (Bioscan Flow-Count fitted with a NaI detector) was used in series with the UV absorbance (Waters Model 2487 set at 254 nm). The chemical identity of the radiotracer was established by co-injecting with a sample of nonradioactive standard. The specific activity was determined based on a standard 5 point mass curve of cold ligand using analytical HPLC. The F-18 isotope for radiosynthesis (25-50 mCi in 0.5 mL of O-18 water) was supplied by IBA Molecular, North America. Data acquisition for both the analytical and preparative systems was accomplished using a USB data acquisition starter kit (DATAQ DI-158U) equipped with high speed acquisition software (WinDaq). The experimental partition coefficient (logD at pH 7.4) of the radiotracer was determined by partitioning between1-octanol and freshly prepared PBS buffer (pH 7.4) and measuring the radioactivity in both phases with a Gamma Counter (Packard Instruments) using the modified method of Wilson et. al.20
Chemistry and radiochemistry
1-(2-Fluoroethyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)urea (1a). (106 mg, 0.345 mmol), (1R,5S)-3-(6-chloro-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)-8-oxa-3-azabicyclo[3.2.1]octane (100 mg, 0.288 mmol), and sodium carbonate (61.0 mg, 0.575 mmol) were combined in acetonitrile: water (1:1, volume: 2 mL) in a 10-mL round bottom flask to give a colorless suspension. The mixture was purged under Ar. Pd(PPh3)4 (25 mg, 0.022 mmol) was added and the reaction mixture was heated overnight at 90−95 °C. After cooling to rt, the reaction mixture was quenched with water and repeatedly extracted with EtOAc, washed with brine, dried over MgSO4, concentrated under vacuum and column chromatographed (gradient from 25% EtOAC to 80% EtOAc in hexane) to yield the desired product 1a as a colorless solid (91 mg, 64%); 1H NMR (400 MHz, CDCl3) δ 8.38−8.16 (m, 3H), 7.90 (d, J = 2.2 Hz, 1H), 7.54 −7.34 (m, 2H), 6.22 (s, 1H), 4.96 (q, J = 8.4 Hz, 2H), 4.51 (dd, J = 5.4, 3.4 Hz, 3H), 4.39 (t, J = 4.8 Hz, 1H), 3.51−3.36 (m, 4H), 2.55−2.48 (m, 2H), 1.95 (dd, J = 7.9, 4.2 Hz, 2H), 1.81 (d, J = 5.8 Hz, 2H); HRMS Calcd for C22H24F4N7O2 (MH+): 494.1928; Found: 494.1917.
1-(4-(4-(8-Oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-6-yl)phenyl)-3-(2-hydroxyethyl)urea (1b). In a 10-mL round bottom flask, was 1-(2-hydroxyethyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)urea (114 mg, 0.374 mmol), (1R,5S)-3-(6-chloro-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)-8-oxa-3-azabicyclo[3.2.1]octane (100 mg, 0.288 mmol), and sodium carbonate (61.0 mg, 0.575 mmol) combined in CH3CN: water (1:1 ratio, 4 mL) to give a colorless solution. The reaction mixture was purged under Ar. Pd(PPh3)4 (25 mg, 0.022 mmol) was then added in one portion. The reaction mixture was heated overnight at 90−95 °C. After cooling to rt, the reaction mixture was quenched with water and repeatedly extracted with EtOAc, washed with brine, dried over MgSO4, concentrated under vacuum and column chromatographed (3% MeOH in EtOAc) to yield the desired product 1b as a colorless solid (82 mg, 0.167 mmol) in 58% yield. RP-HPLC: Phenomenex 4.6 x 250 mm, ODS-3, 5μm column, mobile phase: 40:60 CH3CN: 0.1 M ammonium formate solution, flow rate: 2 mL/min, tR = 5.6 min. 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.49 −8.25 (m, 3H), 7.62 − 7.41 (m, 2H), 6.27 (t, J = 5.6 Hz, 1H), 5.29 (q, J = 9.1 Hz, 2H), 4.69 − 4.46 (m, 3H), 3.47 (t, J = 5.7 Hz, 3H), 3.43 − 3.24 (m, 2H), 3.19 (q, J = 5.6 Hz, 2H), 1.96 − 1.86 (m, 2H), 1.79 (d, J = 7.0 Hz, 2H); HRMS Calcd for C22H25F3N7O3 (MH+): 492.1971; Found: 492.1966.
2-(3-(4-(4-(8-Oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-6-yl)phenyl)ureido)ethyl 4-methylbenzenesulfonate (1c). In a 10-mL round bottom flask was 1-(4-(4-((1R,5S)-8-oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-6-yl)phenyl)-3-(2-hydroxyethyl)urea (10mg, 0.02 mmol) in CH2Cl2 (2 mL) to give a colorless suspension. Triethylamine (9 μL, 0.061 mmol) was added followed by p-toluenesulfonic anhydride (9.96 mg, 0.031 mmol). A clear colorless solution was formed. The reaction mixture was stirred at rt for 30 min. The reaction mixture was directly column chromatographed (CH2Cl2: methanol 92:8 to 96:4) on a neutral alumina column under Ar to give the desired product 1c as a light yellow solid (10 mg, 78%). RP-HPLC: Phenomenex 4.6 x 250 mm, ODS-3, 5μm column, mobile phase: 45:55 CH3CN: 0.1 M ammonium formate solution , flow rate: 2 mL/min, tR = 16 min. 1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 8.2 Hz, 2H), 8.01 (d, J = 6.0 Hz, 1H), 7.81 (d, J = 7.9 Hz, 2H), 7.43 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.3 Hz, 2H), 7.01 (s, 1H), 5.53 (t, J = 6.0 Hz, 1H), 5.06 (q, J = 8.2 Hz, 2H), 4.62 (d, J = 5.4 Hz, 3H), 4.16 (dd, J = 9.4, 5.5 Hz, 3H), 3.56 (dd, J = 10.3, 5.2 Hz, 4H), 2.39 (s, 3H), 2.06-2.09 (m, 2H), 1.89 (d, J = 7.1 Hz, 2H).
4-(4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-6-yl)aniline (10). 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (82 mg, 0.374 mmol), (1R,5S)-3-(6-chloro-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)-8-oxa-3-azabicyclo[3.2.1]-octane (100 mg, 0.288 mmol), and sodium carbonate (61.0 mg, 0.575 mmol) were combined in CH3CN : water (1:1 ratio, 4 mL) in a 10-mL round bottom flask to give a colorless suspension. The mixture was purged under Ar. Pd(PPh3)4 (25mg, 0.022 mmol) was then added in one portion. The reaction mixture was heated overnight at 90−95 °C. After cooling to rt, the reaction mixture was quenched with water and repeatedly extracted with EtOAc, washed with brine, dried over MgSO4, passed through a celite pad and concentrated under vacuum and column chromatographed (hexane: EtOAc 20:80) to give the desired product 10 as a brown solid (75 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 8.41-8.28 (m, 2H), 7.98 (d, J = 2.6 Hz, 1H), 6.84-6.71 (m, 2H), 5.05 (q, J = 8.5 Hz, 2H), 4.61 (d, J = 4.5 Hz, 2H), 3.93 (brs, 2H), 3.58 (brs, 2H), 2.06 (dd, J = 8.5, 4.3 Hz, 2H), 1.90 (d, J = 7.3 Hz, 2H).
[18F]1-(2-Fluoroethyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)urea ([18F]1a). An aqueous solution of [18F]fluoride ion (25-50 mCi in 0.5 mL of O-18 water) in a 5-mL V-vial was treated with 200 μL of 15:1 acetonitrile: water containing kryptofix K222 (36 mg) and potassium carbonate (2 mg). The mixture was azeotropically heated and dried at 95 °C under a stream of argon by the repeated addition of acetonitrile (3 × 0.5 mL).22 A solution of 30 mg of N-[2-(toluene-4-sulfonyloxy)ethyl]phthalimide in 1 mL of anhydrous acetonitrile was then added to the reaction vial, sealed, and heated for 10 min at 100 °C.23 The reaction mixture was then evaporated under vacuum and hydrazine monohydrate (100 μL, 99+%, 64% NH2NH2 in water) was added for deprotection of the phthalimide. The resultant free [18F]fluoroethylamine was distilled under Ar at 75 °C into a second V-vial containing a solution of arylamine 10 (2 mg) in CH2Cl2 pretreated with triphosgene (1.4 mg) and triethylamine (20 μL) at 0 °C. A slow argon flow (<10 psi) is maintained for a steady transfer of activity into the second V-vial. After 15-20 min, the CH2Cl2 was removed under Ar at rt and the precipitate was dissolved in acetonitrile (0.5 mL), and then injected onto a semipreparative HPLC column (Phenomenex, Prodigy ODS-Prep 10 × 250 mm, 10 μm; and eluted with 40:60:0.5 acetonitrile: 0.1 M ammonium formate solution: acetic acid; pH= 5.5-6.0; flow rate: 10 mL/min). The product fraction of [18F]1a eluted at 11-12 min was collected based on the γ-detector, diluted with 100 mL of deionized water and passed through a classic C-18 Sep-Pak® cartridge, washed with 10 mL of deionized water and eluted with 1 mL of absolute ethanol. Reconstitution of the product in 1 mL of ethanol and 9 mL of 0.9% sodium chloride solution for injection, USP afforded [18F]ATPFU. A portion of the ethanol solution was injected into an analytical HPLC (Phenomenex, Prodigy ODS(3) 4.6 × 250 mm, 5 μm; mobile phase; 50:50 acetonitrile: 0.1 M ammonium formate solution, flow rate 2 mL/min, tR = 7–8 min) to determine the specific activity, chemical and radiochemical purities.
Result and discussion
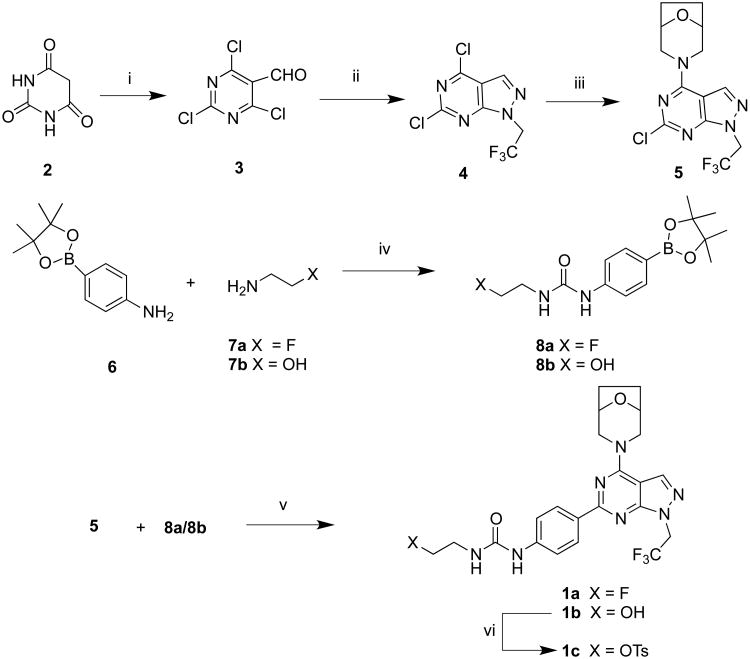
Unlabeled ATPFU was synthesized based on a previous report. In brief, the pyrazolopyrimidine skeleton was constructed from beta-chlorovinylaldehyde 3 obtained by the Vilsmeier formylation of pyrimidine-2,4,6(1H,3H,5H)trione (2) in 72% yield.21 The aldehyde 3 was reacted with trifluoroethyl hydrazine at -78 °C followed by treatment with bridged morpholine (1R,5S)8-oxa-3-azabicyclo[3.2.1]octane to provide 5 in 60% yield. Compound 5 was then coupled with phenylboronate 8a to give reference standard 1a in 64% yield (Scheme 1).
Scheme 1.
Synthesis of ATPFU, hydroxyl and tosyl analogues. i) DMF/POCl3, rt, 72%; ii) CF3CH2NHNH2, EtOH, Et3N, -78 °C, 64%; iii) (1R,5S)8-oxa-3-azabicyclo[3.2.1]octane hydrochloride, Et3N, rt, 60%; iv) triphosgene, CH2Cl2, rt; 84%; v) Na2CO3, acetonitrile, Pd(PPh3)4, 90 °C, 64%; vi) TsCl, CH2Cl2, Et3N, rt, 78%.
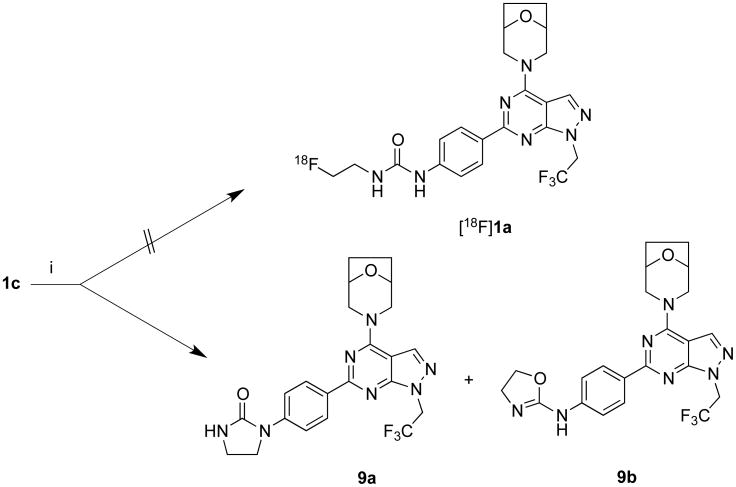
Our initial strategy was to perform a tosyl to [18F]fluoro nucleophilic displacement reaction to achieve [18F]ATPFU. Towards this, the hydroxyl derivative 1b was synthesized by coupling of compound 5 with boronic ester 8b in 58% yield. The compound 1b upon reaction with TsCl yielded tosyl-precursor 1c in 78% yield (Scheme 1). The radiosynthesis of [18F]ATPFU was attempted with tosyl-ATPFU (1c) with [18F]KF and [18F]TBAF under a variety of conditions. However, the tosyl precursor 1c did not undergo radiofluorination, instead, imidazolidin-2-one 9a and 4,5-dihydrooxazol-2-amine 9b were obtained as the only products under experimental conditions (Scheme 2). The formation of 9a and 9b can be attributed to the competing intramolecular nucleophilic reaction of amide or carbonyl group with carbanion derived from 1c.
Scheme 2.
Reactions of 1c with [18F]fluoride ion. i) [18F]TBAF (or) [18F]KF/K222, heat.
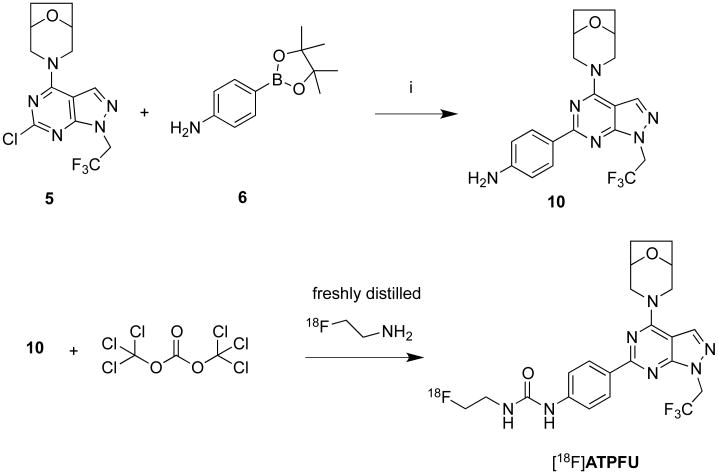
Subsequently we decided to construct [18F]ATPFU from 4-(4-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazolo[3,4-d]pyrimidin-6-yl)aniline (10) by a sequential carbonylation followed by tethering with fluoroethyl group (Scheme 3). Compound 10, the precursor for radiosynthesis, was prepared in 64% yield by the Suzuki coupling of pyrazolopyrimidine 5 with 4-aminophenylboronic acid pinacol ester (6). [18F]Fluoroethylamine was prepared from N-[2-(toluene-4-sulfonyloxy)ethyl]phthalimide and [18F]fluoride ion and purified by distillation.22, 23 The radiosynthesis of [18F]1a was achieved by freshly distilling [18F]fluoroethylamine to a solution of 10 containing triphosgene at 0−5 °C (Scheme 3, Figure 2). The crude product was purified via semipreparative HPLC, followed by solid phase extraction with C18 Sep-Pak®. [18F]1a was obtained in 90 minutes in 15 ± 5 % yield at end of synthesis (EOS) in high radiochemical purity (>95%) and specific activity (37−74 GBq/μmol). The stability of radioproduct in 10% ethanol-saline solution was determined by HPLC injections over a course of 6 hours. [18F]1a was determined to be stable under these conditions based on the absence of any additional radiopeaks in HPLC. The logD at pH 7.4 of [18F]1 was measured as 1.9, indicating that the radioligand has adequate lipophilicity for passive brain entry.20
Scheme 3.
Radiosynthesis of [18F]ATPFU. i) Na2CO3, acetonitrile, Pd(PPh3)4, 90°C, 65%.
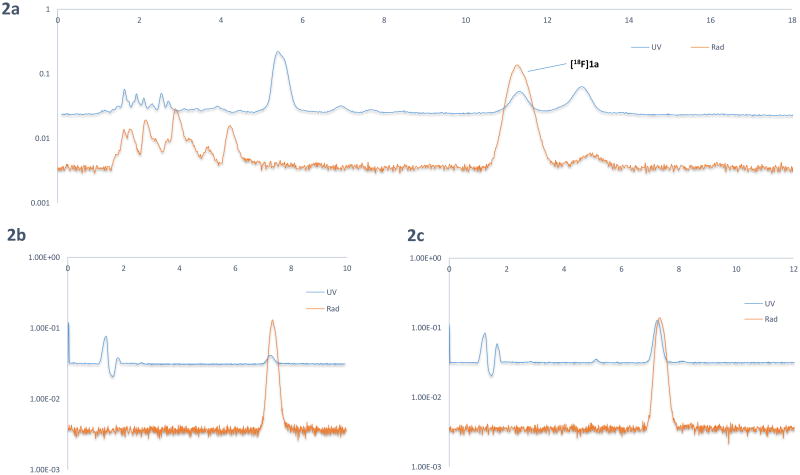
Figure 2.
HPLC chromatograms of [18F]ATPFU; 2A. Semipreparative HPLC; 2B. Analytical QC; 2C. Co-injection.
Conclusions
In conclusion, we have successfully developed a method for the radiosynthesis of [18F]ATPFU, a highly selective mTOR ligand. The synthesis of standard and radiolabeling precursor were achieved from c in 5 and 4 steps respectively. Conventional 18F-labeling with tosyl precursor did not yield radioproduct under thermal and radiochemical conditions as a result of the intramolecular cyclization to yield imidazolidone 9a and (or) dihydrooxazlo-2-amine 9b. The radiosynthesis of [18F]ATPFU was achieved by reacting freshly distilled [18F]fluoroethylamine to a solution of amine derivative 10 and triphosgene in dichloromethane at 0-5 °C. The total time required for the radiosynthesis was 90 minutes. [18F]ATPFU was obtained in 15 ± 5% yield from [18F] fluoride ion (decay corrected) with excellent radiochemical purity and specific activity. Further studied will be aimed at determined by the in vitro and in vivo specificity of [18F]ATPFU in normal and altered pathology.
Acknowledgments
This work was funded by NIMH grant MH062185.
Footnotes
Conflict of interest: The authors did not report any conflict of interest.
References
- 1.Garelick MG, Kennedy BK. Exp Gerontol. 2011;46:155. doi: 10.1016/j.exger.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pignataro G, Capone D, Polichetti G, Vinciguerra A, Gentile A, Di Renzo G, Annunziato L. Curr Opin Pharmacol. 2011;11:378. doi: 10.1016/j.coph.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Maiese K, Chong ZZ, Shang YC, Wang S. Expert Opin Ther Targets. 2012;16:1203. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdaguer E, Junyent F, Folch J, Beas-Zarate C, Auladell C, Pallas M, Camins A. Expert Opin Drug Discov. 2012;7:217. doi: 10.1517/17460441.2012.660144. [DOI] [PubMed] [Google Scholar]
- 5.Perry J, Okamoto M, Guiou M, Shirai K, Errett A, Chakravarti A. Neurol Res Int. 2012;2012:428565. doi: 10.1155/2012/428565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddo S. Front Biosci (Schol Ed) 2012;4:941. doi: 10.2741/s310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pei JJ, Hugon J. J Cell Mol Med. 2008;12:2525. doi: 10.1111/j.1582-4934.2008.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryther RC, Wong M. Curr Neurol Neurosci Rep. 2012;12:410. doi: 10.1007/s11910-012-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong M, Crino PB. In: mTOR and Epileptogenesis in Developmental Brain Malformations. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies; Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 10.Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:240. doi: 10.1016/j.pnpbp.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, Branca RM, Lehtio J, Guan Z, Filipcik P, Xu S, Winblad B, Pei JJ. J Biol Chem. 2013;288:15556. doi: 10.1074/jbc.M112.435123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, Oddo S. Aging Cell. 2013;12:370. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faivre S, Kroemer G, Raymond E. Nat Rev Drug Discov. 2006;5:671. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Gao M, Miller KD, Sledge GW, Zheng QH. Bioorg Med Chem Lett. 2012;22:1569. doi: 10.1016/j.bmcl.2011.12.136. [DOI] [PubMed] [Google Scholar]
- 16.Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, Zhang WG, Mahoney R, Gaydos C, Tardio L, Kim SK, Conant R, Curran K, Kaplan J, Verheijen J, Ayral-Kaloustian S, Mansour TS, Abraham RT, Zask A, Gibbons JJ. Cancer Res. 2010;70:621. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 17.Zask A, Kaplan J, Verheijen JC, Richard DJ, Curran K, Brooijmans N, Bennett EM, Toral-Barza L, Hollander I, Ayral-Kaloustian S, Yu K. J Med Chem. 2009;52:7942. doi: 10.1021/jm901415x. [DOI] [PubMed] [Google Scholar]
- 18.Curran KJ, Verheijen JC, Kaplan J, Richard DJ, Toral-Barza L, Hollander I, Lucas J, Ayral-Kaloustian S, Yu K, Zask A. Bioorg Med Chem Lett. 2010;20:1440. doi: 10.1016/j.bmcl.2009.12.086. [DOI] [PubMed] [Google Scholar]
- 19.Lynch R, Cansfield AD, Niblock HS, Hardy DP, Taylor J. WO2011107585A1. 2011 [Google Scholar]
- 20.Wilson AA, Jin L, Garcia A, DaSilva JN, Houle S. Appl Radiat Isot. 2001;54:203. doi: 10.1016/s0969-8043(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 21.Borzsonyi G, Beingessner RL, Yamazaki T, Cho JY, Myles AJ, Malac M, Egerton R, Kawasaki M, Ishizuka K, Kovalenko A, Fenniri H. J Am Chem Soc. 2010;132:15136. doi: 10.1021/ja105028w. [DOI] [PubMed] [Google Scholar]
- 22.Tewson TJ. Nucl Med Biol. 1997;24:755. doi: 10.1016/s0969-8051(97)00135-2. [DOI] [PubMed] [Google Scholar]
- 23.Antunes IF, Haisma HJ, Elsinga PH, Dierckx RA, de Vries EFJ. Bioconjug Chem. 2010;21:911. doi: 10.1021/bc9004602. [DOI] [PubMed] [Google Scholar]