Abstract
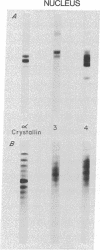
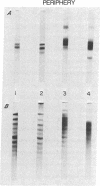
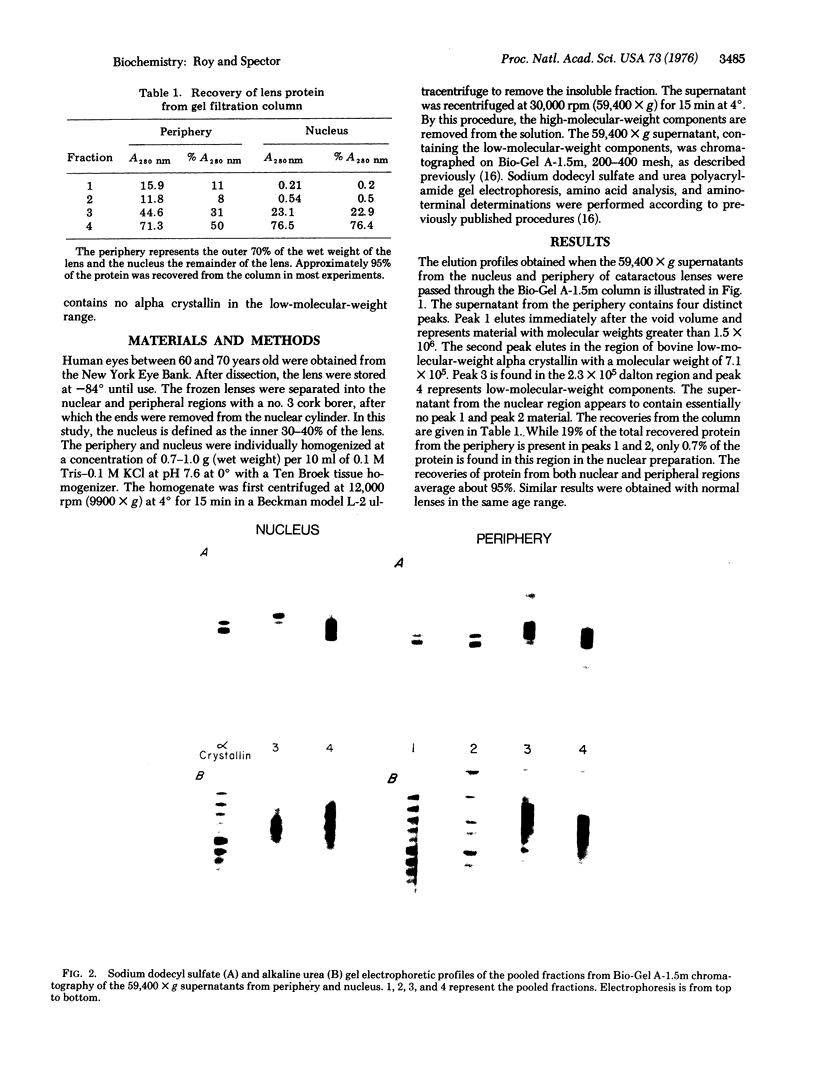
Examination of old human lenses indicates that low-molecular-weight alpha crystallin is not present in the inner 30-40%, the nucleus, of the lens. The remainder of such lenses, the periphery, contains normal levels of this protein. This finding is in marked contrast to observations in young lenses, where a large quantity of this protein is found throughout the tissue.
Full text
PDF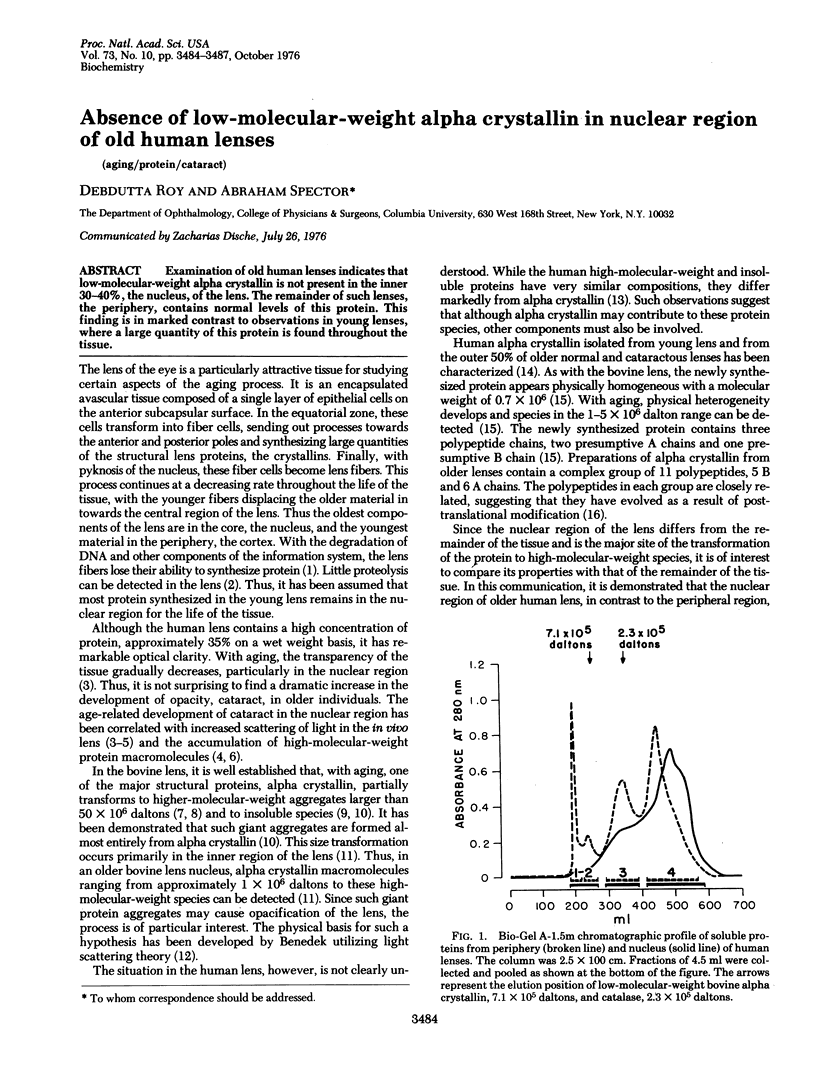


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BJORK I. Studies on gamma-crystallin from calf lens. I. Isolation by gel filtration. Exp Eye Res. 1961 Dec;1:145–154. doi: 10.1016/s0014-4835(61)80020-1. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H. The behaviour of reduced proteins from normal and cataractous lenses in highly dissociating media: cross-linked protein in cataractous lenses. Exp Eye Res. 1972 Sep;14(2):123–129. doi: 10.1016/0014-4835(72)90057-7. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H., Yates E. M., Benedek G. B. The concentration and localization of heavy molecular weight aggregates in aging normal and cataractous human lenses. Exp Eye Res. 1975 Apr;20(4):367–369. doi: 10.1016/0014-4835(75)90118-9. [DOI] [PubMed] [Google Scholar]
- Manski W., Behrens M., Martinez C. Immunochemical studies on albuminoid. Exp Eye Res. 1968 Jan;7(1):164–171. doi: 10.1016/s0014-4835(68)80041-7. [DOI] [PubMed] [Google Scholar]
- Manski W., Martinez C. Immunochemical studies on albuminoid. II. Changes associated with age. Exp Eye Res. 1971 Sep;12(2):206–211. doi: 10.1016/0014-4835(71)90092-3. [DOI] [PubMed] [Google Scholar]
- Roy D., Spector A. High molecular weight protein from human lenses. Exp Eye Res. 1976 Mar;22(3):273–279. doi: 10.1016/0014-4835(76)90055-5. [DOI] [PubMed] [Google Scholar]
- Roy D., Spector A. Human alpha-crystallin-III isolation and characterization of protein from normal infant lenses and old lens peripheries. Invest Ophthalmol. 1976 May;15(5):394–399. [PubMed] [Google Scholar]
- Roy D., Spector A. Human alpha-crystallin: characterization of the protein isolated from the periphery of cataractous lenses. Biochemistry. 1976 Mar 9;15(5):1180–1188. doi: 10.1021/bi00650a034. [DOI] [PubMed] [Google Scholar]
- Sigelman J., Trokel S. L., Spector A. Quantitative biomicroscopy of lens light back scatter. Changes in aging and opacification. Arch Ophthalmol. 1974 Nov;92(5):437–442. doi: 10.1001/archopht.1974.01010010449016. [DOI] [PubMed] [Google Scholar]
- Spector A. Aggregation of a-crystallin and its possible relationship to cataract formation. Isr J Med Sci. 1972 Aug-Sep;8(8):1577–1582. [PubMed] [Google Scholar]
- Spector A., Freund T., Li L. K., Augusteyn R. C. Age-dependent changes in the structure of alpha crystallin. Invest Ophthalmol. 1971 Sep;10(9):677–686. [PubMed] [Google Scholar]
- Spector A., Li S., Sigelman J. Age-dependent changes in the molecular size of human lens proteins and their relationship to light scatter. Invest Ophthalmol. 1974 Oct;13(10):795–798. [PubMed] [Google Scholar]
- Spector A., Roy D., Stauffer J. Isolation and characterization of an age-dependent polypeptide from human lens with non-tryptophan fluorescence. Exp Eye Res. 1975 Jul;21(1):9–24. doi: 10.1016/0014-4835(75)90053-6. [DOI] [PubMed] [Google Scholar]
- Spector A., Stauffer J., Roy D., Li L. K., Adams D. Human alpha-crystallin. I. The isolation and characterization of newly synthesized alpha-crystallin. Invest Ophthalmol. 1976 Apr;15(4):288–296. [PubMed] [Google Scholar]
- Spector A. The aging of alpha-crystallin: a review. Exp Eye Res. 1973 Jun;16(2):115–121. doi: 10.1016/0014-4835(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., van Heyningen R. Neutral proteinase activity in the human lens. Exp Eye Res. 1976 Mar;22(3):251–257. doi: 10.1016/0014-4835(76)90052-x. [DOI] [PubMed] [Google Scholar]
- Wannemacher C. F., Spector A. Protein synthesis in the core of calf lens. Exp Eye Res. 1968 Oct;7(4):623–625. doi: 10.1016/s0014-4835(68)80018-1. [DOI] [PubMed] [Google Scholar]