Abstract
Background
The purpose of this study is to comparatively analyze outcomes of heart transplant patients bridged to transplantation with Heartware (HW-VAD) versus Heartmate II (HMII-VAD) left ventricular assist devices.
Methods and Results
The United Network for Organ Sharing Database was reviewed to identify first-time heart transplant recipients who were bridged-to-transplantation with either HW-VAD (n=141) or HMII-VAD (n=1824) from January 2009 through July 2012. HW-VAD recipients had a higher proportion of female patients (27.0% vs. 18.9%, p=0.019), a lower body surface area (2.01 ± 0.25 vs. 2.06 ± 0.25, p=0.035), and a trend toward a higher peak percentage of panel reactive antibody against Human Leukocyte Class I antigens (40.4% ± 32.8 vs. 33.0% ± 30.4, p=0.070). Pre-transplantation recipient cardiac index (2.33 ± 0.66 vs. 2.33 ± 0.68 L/min/m2), serum creatinine (1.21 ± 0.43 vs. 1.26 ± 0.57 mg/dL), and total bilirubin (1.34 ± 3.45 vs. 1.06 ± 1.84 mg/dL) were comparable between the two groups (p>0.05 for all comparisons). Post-transplantation, there were no significant differences in freedom from rejection or freedom from cardiac allograft vasculopathy. Post-transplant graft survival rates were similar between the HW-VAD group and the HMII-VAD group at one, two and three years (88.4% vs. 87.8%, 79.9% vs. 83.8%, and 77.4% vs. 79.9%, respectively, p=0.843).
Conclusions
These findings suggest similar hemodynamic unloading, pre-transplant end-organ function, and post-transplant outcomes in patients bridged to transplantation with both the Heartware LVAD and Heartmate II LVAD.
Keywords: Circulatory support devices, Transplantation – heart, Outcomes
Introduction
The last two decades have witnessed a remarkable progress in mechanical circulatory support systems for patients with advanced heart failure (HF), resulting in improved functional capacity, quality of life and survival in this population [1–3]. Recent advances in device technology led to the development of continuous-flow pumps, which have become standard of care for HF patients both as bridge-to-transplantation (BTT) and destination therapy (DT) [4–6]. The Heartmate II left ventricular assist device (Thoratec Corporation, Pleasanton, CA) is among the most widely used support systems worldwide, and remains the only continuous-flow device currently approved for both the BTT and DT indications in the U.S Although outcomes have significantly improved with this pump, Heartmate II (HMII-VAD) implantation is still associated with early and late device-related complications including infections, gastrointestinal bleeding, and thrombotic events. [7–9].
More recently, Heartware (HeartWare Inc.) ventricular assist device (HW-VAD) - a third generation continuous-flow pump - was introduced into clinical use [10–11]. HW-VAD is a miniaturized centrifugal flow pump which is directly implanted into the failing left ventricle and situated in the intra-pericardial space, eliminating the need for the abdominal incision that is routinely performed in HMII-VAD implantation [10]. Recently published ADVANCE trial demonstrated non-inferior post-implantation outcomes in patients undergoing HW-VAD implantation compared to contemporary control group from INTERMACS registry, leading to the approval of this device by the US Food and Drug Administration (FDA) for BTT [12]. However, no study to date has directly compared post-transplant outcomes of patients bridged with HW-VAD or HMII-VAD support. Moreover, data regarding differences in hemodynamic unloading patterns, end-organ function as well as antigen sensitization levels between the recipients of two device types is either limited or lacking. In an effort to address these questions, we performed a comparative analysis of patients bridged with HW-VAD or HMII-VAD using multicenter United Network for Organ Sharing (UNOS) database, given the relatively small number of Heartware implants at any individual center.
Material and Methods
Data Collection
Standard analysis and research files were obtained from UNOS in October 2012, which included data from all heart transplant recipients and donors in the United States reported to the Organ Procurement and Transplantation Network between October 1, 1987 and July 31, 2012. Given the de-identified nature of the dataset, exemption status was granted by the Institutional Review Board at our center.
Study Population
The study-included patients aged 18 and older who underwent cardiac transplantation between January 1, 2009 and July 31, 2012. Patients who underwent previous heart transplantation or simultaneous transplantation were excluded from the analysis. LVAD type was identified using LVAD data points represented in the standard UNOS transplant recipient registration forms. Patients who were transplanted following either HW-VAD or HMII-VAD implantation were included in the final analysis. Mean post-transplant follow-up time for the dataset was 439 days.
Examined Variables and Outcome Measures
The UNOS transplant data registry contains more than 400 pre-transplant and post-operative data points. Recipient baseline characteristics examined were age, gender, ethnicity, height, weight, body mass index (BMI), HF etiology, presence of diabetes, serum creatinine and total bilirubin, panel reactive antibodies, and ABO mismatch level. Body surface area was calculated using the Mosteller formula. Pre-transplant hemodynamic values examined were cardiac output, cardiac index, pulmonary artery pressures (systolic, diastolic, and mean), pulmonary capillary wedge pressure, transpulmonary gradient, and pulmonary vascular resistance. Donor baseline characteristics examined were age, gender, ethnicity, height, weight, BMI, presence of diabetes, serum creatinine, total bilirubin, and ischemic time.
Early clinical outcomes analyzed were need for dialysis, pacemaker implantation, and stroke prior to discharge. Hospital length of stays and 30-day mortality rates were also calculated for each group. Long-term outcome variables examined were post-transplant graft survival, freedom from cardiac allograft vasculopathy (CAV), and freedom from hospitalization for allograft rejection.
Statistical Analysis
Continuous data was reported as mean and standard deviation, and was compared using independent student t-tests. Categorical variables were represented as percentages and compared using chi-squared or Fisher’s exact tests where appropriate. Kaplan-Meier survival analysis was performed to compare graft survival, freedom from CAV, and freedom from hospitalization for rejection. Log-rank tests were utilized when comparing groups.
Propensity scores were created for comparing Heartmate II and Heartware LVADs as BTT devices. Recipient and donor baseline variables were used to create score, except for peak class I and class II PRA percentages, which were omitted due to missing data. A Greedy matching algorithm was used to match Heartware and Heartmate II. Standardized differences were computed before and after matching to evaluate covariate balance. For patient survival and the outcomes rejection and CAV, Cox proportional hazards models were created and the hazard ratio (HR) was used to compare groups before and after matching. For the matched analysis, a shared frailty modeling technique was used. For the binary outcomes of post-transplant dialysis, pacemaker implantation, post-transplant stroke, and 30-day mortality, logistic regression models were built. The odds ratio (OR) was used to compare Heartware to Heartmate II. A linear regression model was built for length of stay and the beta coefficient was used to estimate the difference between groups. The logistic and linear regression models were created using Generalized Estimating Equations (GEE) methods to account for matching.
All tests were conducted at the alpha=.05 level. All analyses were conducted in SAS v9.3 (SAS Institute, Inc., Cary, NC).
Results
Study Population
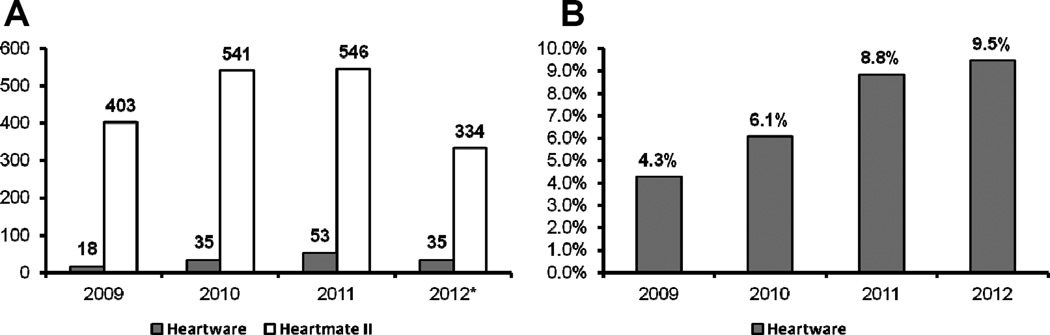
The study population was comprised of 1965 heart transplant recipients: 141 (7.2%) bridged with HW-VAD and 1824 (92.8%) bridged with HMII-VAD from 2009 through July 2012 (Figure 1A). The number of patients bridged with HW-VAD and HMII-VAD steadily increased during the study period, consistent with increased use of mechanical circulatory support systems in the advanced heart failure population. The percentage of patients bridged with HW-VAD increased from 4.3% in 2009 to 9.5% in 2012 (Figure 1B).
Figure 1. Device Implants by Transplant Year.
A) Number of Heartware and Heartmate II LVAD implants by transplant year. * Data until 6/2012. B) Percentage of Heartware LVAD implants by transplant year
Baseline Characteristics
Recipient clinical characteristics were well matched between the two device groups (Table 1). However, there was a higher proportion of female patients in the HW-VAD group compared to the HMII-VAD group (27.0% vs. 18.9%, p=0.019). Consequently, mean body weight and mean BSA were also found to be significantly lower in HW-VAD recipients (Table 1). In addition, there was a trend toward significance in peak Class I PRA levels, which were found to be higher in HW-VAD recipients (40.4% vs. 33.0%, p=0.070). However there were no significant differences between the two groups in the most recent Class I PRA or Class II PRA levels.
Table 1.
Recipient Characteristics at Transplantation
| Heartware LVAD (n= 141) |
Heartmate II LVAD (n= 1824) |
p value | |
|---|---|---|---|
| Age | |||
| 18–39 | 18 (12.8%) | 280 (15.4%) | 0.477 |
| 40–59 | 67 (47.5%) | 904 (49.6%) | |
| ≥ 60 | 56 (39.7%) | 640 (35.0%) | |
| Age, yrs | 53.8 ± 11.4 | 52.7 ± 12.3 | 0.320 |
| Gender | |||
| Female | 38 (27.0%) | 344 (18.9%) | 0.019 |
| Male | 103 (75.1%) | 1480 (81.1%) | |
| Race | |||
| White | 99 (70.2%) | 1238 (67.8%) | 0.928 |
| Black | 28 (19.9%) | 406 (22.3%) | |
| Hispanic | 9 (6.4%) | 113 (6.2%) | |
| Other | 5 (3.5%) | 67 (3.7%) | |
| Weight, kg | 84.4 ± 17.9 | 87.6 ± 18.1 | 0.044 |
| Height, cm | 174.3 ± 10.0 | 175.6 ± 9.4 | 0.119 |
| BMI, kg/m2 | 27.7 ± 5.0 | 28.3 ± 5.1 | 0.139 |
| Calculated BSA, m2 | 2.01 ± 0.25 | 2.06 ± 0.25 | 0.035 |
| Etiology of Heart Failure | |||
| Ischemic | 55 (39.0%) | 752 (41.2%) | 0.609 |
| Non-ischemic | 86 (61.0%) | 1072 (58.8%) | |
| Diabetes | 39 (27.7%) | 555 (30.4%) | 0.491 |
| Serum Creatinine, mg/dL | 1.21 ± 0.43 | 1.26 ± 0.57 | 0.255 |
| Total Bilirubin, mg/dL | 1.34 ± 3.45 | 1.06 ± 1.84 | 0.354 |
| Panel Reactive Antibody (%) | |||
| Class I (most-recent) | 10.3 ± 22.0 | 8.8 ± 20.6 | 0.427 |
| Class I (peak) | 40.4 ± 32.8 | 33.0 ± 30.4 | 0.070 |
| Class II (most-recent) | 5.41 ± 16.7 | 5.15 ± 15.9 | 0.851 |
| Class II (peak) | 32.5 ± 29.3 | 31.9 ± 29.7 | 0.912 |
| ABO Mismatch Level | 16 (11.4%) | 223 (12.2%) | 0.759 |
Donor clinical characteristics were also well matched between the two groups (Table 2). There were no significant differences in donor age, gender, bilirubin level, and ischemic time. However, donor creatinine level was significantly lower in HW-VAD group (1.19 vs. 1.35, p=0.045).
Table 2.
Donor Characteristics
| Heartware LVAD (n= 141) |
Heartmate II LVAD (n= 1824) |
p value | |
|---|---|---|---|
| Age | |||
| <18 | 8 (5.7%) | 107 (5.9%) | 0.309 |
| 18–39 | 106 (75.2%) | 1265 (69.4%) | |
| ≤ 40 | 27 (19.1%) | 452 (24.8%) | |
| Age, yrs | 30.2 ± 10.5 | 31.3 ± 10.9 | 0.275 |
| Gender | |||
| Female | 39 (27.7%) | 429 (23.5%) | 0.266 |
| Male | 102 (72.3%) | 1395 (76.5%) | |
| Ethnicity | |||
| White | 91 (64.5%) | 1211 (66.4%) | 0.938 |
| Black | 25 (17.7%) | 311 (17.1%) | |
| Hispanic | 22 (15.6%) | 256 (14.0%) | |
| Other | 3 (2.1%) | 46 (2.5%) | |
| Weight, kg | 83.3 ± 20.8 | 84.7 ± 18.2 | 0.425 |
| Height, cm | 174.8 ± 9.9 | 175.9 ± 9.2 | 0.199 |
| BMI, kg/m2 | 27.2 ± 6.5 | 27.4 ± 5.4 | 0.811 |
| Diabetes | 1(0.7%) | 53 (2.9%) | 0.177 |
| Serum Creatinine, mg/dL | 1.19 ± 0.83 | 1.35 ± 1.26 | 0.045 |
| Total Bilirubin, mg/dL | 0.95 ± 0.91 | 1.09 ± 1.97 | 0.121 |
| Ischemic Time, hours | 3.32 ± 1.22 | 3.26 ± 1.07 | 0.590 |
Hemodynamic Profiles
Pre-transplant recipient hemodynamic profile data is summarized in Table 3. Overall, there were no statistically significant differences in cardiac output, cardiac index, PA pressures, PCWP, trans-pulmonary gradient, or PVR between the two groups prior to cardiac transplantation.
Table 3.
Recipient Hemodynamic Profile
| Heartware LVAD (n= 141) |
Heartmate II LVAD (n= 1824) |
p value | |
|---|---|---|---|
| Cardiac Output, (L/min) | 4.69 ± 1.39 | 4.78 ± 1.50 | 0.506 |
| Cardiac Index, (L/min/m2) | 2.33 ± 0.66 | 2.33 ± 0.68 | 0.914 |
| Pulmonary Artery Pressure | |||
| Systolic, mmHg | 39.6 ± 12.2 | 39.8 ± 14.6 | 0.883 |
| Diastolic, mmHg | 19.7 ± 7.7 | 19.2 ± 8.9 | 0.442 |
| Mean, mmHg | 27.2 ± 8.7 | 27.1 ± 10.4 | 0.838 |
| PCWP, (mmHg) | 17.7 ± 8.3 | 17.2 ± 9.3 | 0.626 |
| TPG, (mmHg) | 9.8 ± 4.9 | 9.8 ± 5.1 | 0.912 |
| PVR, (Woods Units) | 2.31 ± 1.36 | 2.29 ± 1.81 | 0.862 |
Post-Transplant Outcomes
Early post-transplant complications including pacemaker implantation, postoperative dialysis, and post-operative stroke were comparable between the groups (Table 4). 30-day mortality rates and hospital length of stays in these patients were also similar. Only a small number of patients (n=7) required re-transplantation during the follow-up period.
Table 4.
Post-transplant Outcomes
| Heartware LVAD (n= 141) |
Heartmate II LVAD (n= 1824) |
p value | |
|---|---|---|---|
| Early Complications | |||
| Post-transplant Dialysis | 17 (12.1%) | 174 (9.6%) | 0.345 |
| Pacemaker Implantation | 7 (5.1%) | 77 (4.3%) | 0.645 |
| Post-transplant Stroke | 6 (4.3%) | 48 (2.7%) | 0.254 |
| 30-day Mortality | 3 (2.1%) | 83 (4.6%) | 0.280 |
| Hospital length of stay (days) | 21.0 ± 17.3 | 20.3 ± 21.5 | 0.663 |
| Re-transplantation | 0 (0.00%) | 7 (0.03%) | >0.999 |
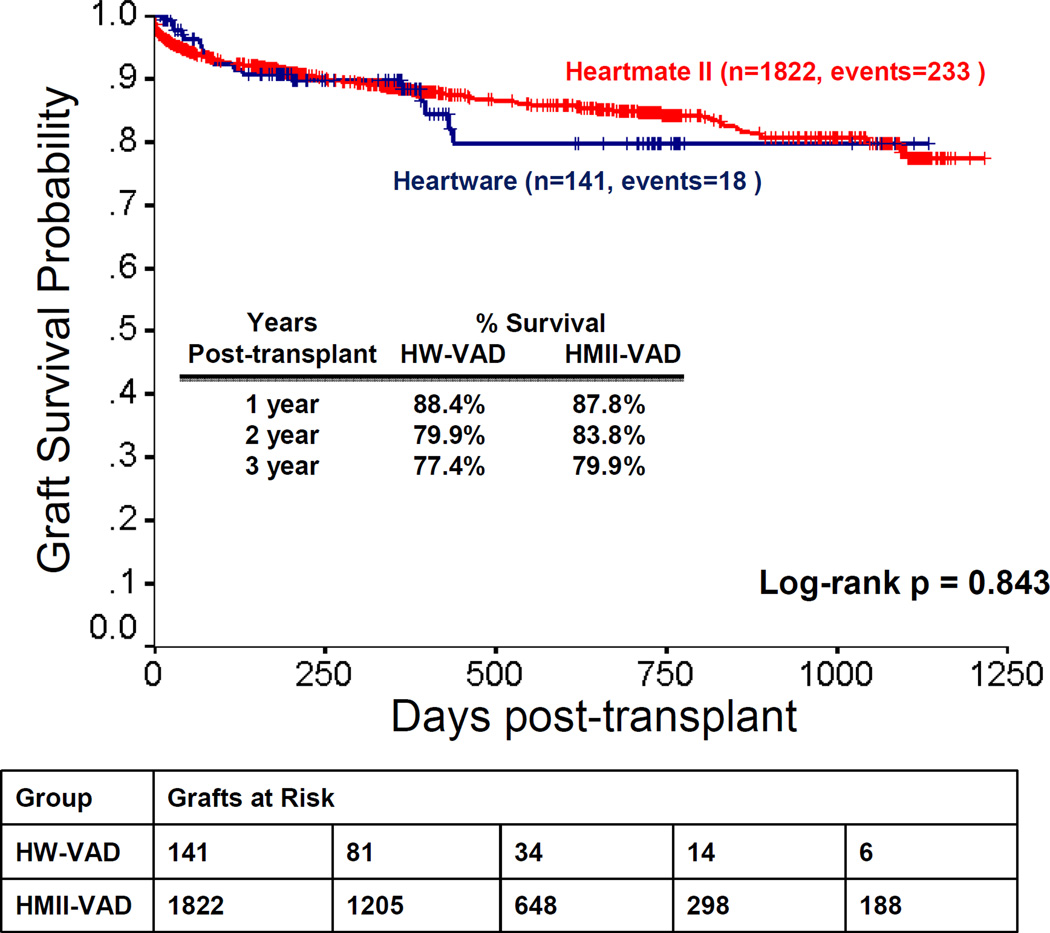
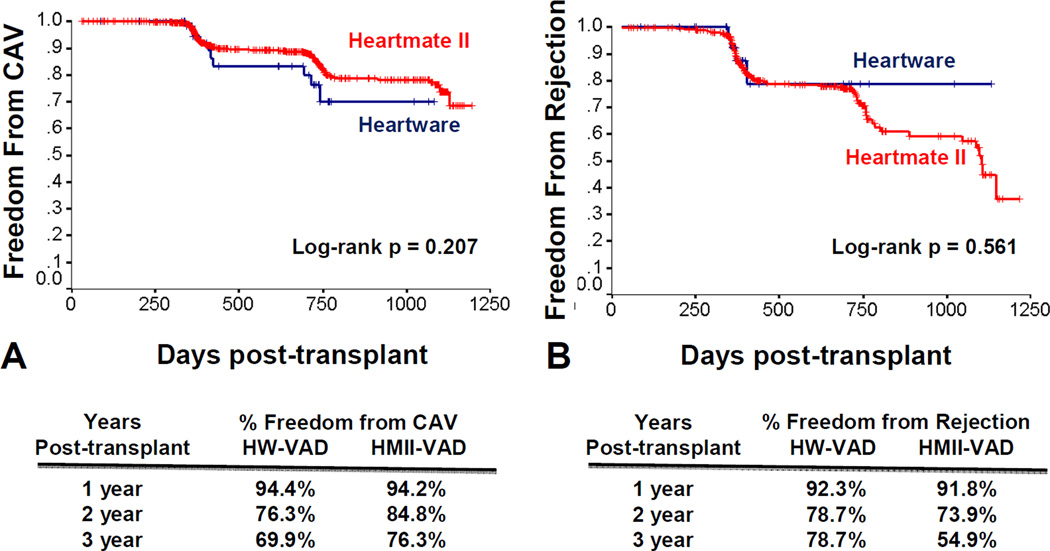
The data showed no significant difference in post-transplant graft survival between patients who were bridged with HW-VAD and those who were bridged with HMII-VAD. Graft survival rates of HW-VAD recipients at 1, 2 and 3 years were 88.4%, 79.9%, and 77.4% compared to 87.8%, 83.8%, and 79.9% in HMII-VAD recipients, respectively (p=0.843) (Figure 2).. Freedom from CAV and freedom from hospitalization for rejection were comparable between HW-VAD and HMII-VAD recipients (log-rank pNS).
Figure 2. Post-transplant Graft Survival.
Propensity Matching Analysis
Table 5 demonstrates outcome results for propensity matched HW-VAD and HMII-VAD BTT cohorts (n=124 patients each arm). As shown in the table, no significant differences were found between the groups before or after propensity matching with regards to post-transplant patient survival, freedom from rejection, freedom from CAV, post-transplant dialysis, post-transplant pacemaker implantation, post-transplant stroke, early mortality, and hospital length of stay (all p>0.05).
Table 5.
Post-transplant Outcomes Before and After Propensity Matching
| Unadjusted | Propensity Matching | |||
|---|---|---|---|---|
| HR* (95% CI) | p-value | HR* (95% CI) | p-value | |
| Patient Survival | 1.100 (0.681, 1.778) |
0.697 | 0.833 (0.417, 1.664) |
0.598 |
| Rejection | 0.745 (0.275, 2.022) |
0.564 | 0.821 (0.220, 3.064) |
0.726 |
| CAV | 1.512 (0.792, 2.886) |
0.210 | 1.557 (0.580, 4.176) |
0.344 |
| OR* (95% CI) | p-value | OR* (95% CI) | p-value | |
|
Post-transplant dialysis |
1.291 (0.759, 2.194) |
0.345 | 0.920 (0.460, 1.842) |
0.815 |
|
Pacemaker implantation |
1.205 (0.545, 2.666) |
0.645 | 2.437 (0.599, 9.907) |
0.716 |
|
Post-transplant stroke |
1.648 (0.692, 3.920) |
0.254 | 2.052 (0.580, 7.254) |
0.265 |
| 30-day mortality | 0.456 (0.142, 1.460) |
0.280 | 0.274 (0.055, 1.374) |
0.116 |
| Beta* (95% CI) | p-value | Beta* (95% CI) | p-value | |
|
Length of stay (days) |
0.684 (−2.406, 3.774) |
0.663 | −2.366 (−8.719, 3.987) |
0.465 |
For Heartware (Heartmate II is referral group)
Comment
The clinical safety and efficacy of HW-VAD therapy were initially assessed in a number of single-arm prospective studies carried out in European and Australian centers, which demonstrated excellent post-VAD survival and quality of life following HW-VAD support [13–14]. These findings provided the rationale for the ADVANCE trial, which was a multicenter, prospective study comparing the clinical efficacy of HW-VAD therapy against a contemporaneous control group obtained from the INTERMACS registry. The primary outcome of ADVANCE trial was defined as survival on the originally implanted device, transplantation, or explantation of the device for ventricular recovery at 180 days. Despite the lack of a true control group, the ADVANCE trial demonstrated non-inferiority in the primary outcome in patients who had received HW-VAD compared to control group [12]. Moreover, HW-VAD therapy was associated with a favorable adverse effect profile, and with improvements in functional capacity and quality of life. Based on these findings, HW-VAD therapy was recently approved by the FDA for bridge-to-transplantation in patients with end-stage heart failure.
The current study extends the findings of the ADVANCE trial and supports the use of HW-VAD as bridge to transplantation, and provides valuable insight into outcomes of patients undergoing HW-VAD implantation with regard to their baseline characteristics, hemodynamic profile, and post-transplant outcomes, which were not reported in the ADVANCE trial. Our data suggests that the proportion of females bridged to transplantation was significantly higher in HW-VAD recipients compared to HMII-VAD. Mean body weight and body surface area were also statistically lower in patients bridged with HW-VAD, which could be explained by the higher proportion of females in this group. Although there were no differences in gender distribution between HW-VAD and the control group in the ADVANCE trial, the smaller size of the Heartware device compared to other commercially available LVADs may allow for implantation of this device in patients with lower body surface areas. Indeed, HW-VAD was successfully implanted in a pediatric population for dilated cardiomyopathy and congenital heart disease, and thus offers an attractive alternative to paracorporeal support systems in this patient group [15–16].
The degree of hemodynamic unloading with Heartware versus Heartmate II device support in patients with advanced heart failure has been previously investigated in a number of clinical studies. Haft and colleagues reported a 26% increase in mean cardiac index, a 41% decrease in mean PA pressure, and a 52% decrease pulmonary capillary wedge pressure after three months of device support in 18 patients who underwent Heartmate II implantation [17]. Similarly, McDiarmid and colleagues reported a 35% increase in cardiac index, a 68% decrease in mean PA pressure, and a 50% decrease in PCWP after nearly one year of device support in 30 patients who underwent Heartware implantation [18]. Pauwaa and colleagues comparatively analyzed post-implantation hemodynamics in 15 patients supported with either Heartware (n=6) or Heartmate II (n=9) devices, and did not demonstrate any significant differences between the two support systems [19]. Our analysis in a larger cohort of individuals supported with continuous-flow devices also suggests that the degree of hemodynamic unloading is comparable in Heartware and Heartmate II recipients.
End-organ function on ventricular assist device support is crucial for long-term outcomes and potential candidacy for cardiac transplantation. Early preclinical studies have demonstrated excellent end-organ function in both ovine and bovine models supported by Heartware devices, up to 90 days post-transplantation [20–21]. In parallel with the preclinical data, early clinical studies have also shown improvements in end-organ function of patients supported by the Heartware device [13–14]. Similarly, improvements in renal and hepatic function were evident in patients undergoing Heartmate II implantation for bridge-to-transplantation [4]. Time-dependent changes in renal or liver function following LVAD implantation were not reported in the ADVANCE trial. However, our findings suggest that Heartware and Heartmate II devices lead to comparable improvements in end-organ function.
The presence of circulating anti-HLA antibodies in cardiac transplant candidates remains a major obstacle for donor selection and may limit the long-term success of heart transplantation due to increased rates of rejection and post-transplant mortality [22]. Previous studies have shown that patients bridged to transplantation with mechanical circulatory support have a higher prevalence of allosensitization compared to patients that are bridged with medical therapy, and that the prevalence of sensitization in LVAD recipients is correlated with the duration of support [23–24]. Studies have also demonstrated lower rates of allosensitization in patients who received a Heartmate II continuous-flow device compared to patients who received a Heartmate I pulsatile-flow device [24–25]. However, the effect of Heartware device implantation on allosensitization of transplant eligible patients remains unknown. The current analysis suggests comparable levels of Class I and Class II panel reactive antibodies in patients supported with Heartware and Heartmate II LVADs, although we found a trend toward higher peak Class I PRA% in Heartware recipients. Although this finding could be explained in part by the presence of a higher proportion of female recipients in the Heartware group, further studies are warranted to investigate the effects of Heartware implantation on recipient allosensitization., Freedom from allograft rejection and cardiac allograft vasculopathy were found to be comparable between Heartware and Heartmate II recipients suggesting that the observed difference in peak Class I PRA levels in the current study did not lead to an increase in clinically significant acute or chronic rejection episodes in Heartware recipients.
Our study showed no difference in post-transplant graft survival between HW-VAD and HMII-VAD recipients. These findings are consistent with previous analyses from the ISHLT and UNOS registries that demonstrated comparable post-transplantation survival in patients bridged with either pulsatile or continuous-flow devices. These observations suggest that the support device type may not have an effect on post-transplant mortality in patients requiring mechanical circulatory support prior to transplantation [26–28].
There were several limitations of the current study. The number of patients bridged to transplantation with the Heartware device was relatively small compared to patients bridged to transplantation with the Heartmate II during the study period. Important clinical data points, such as post-LVAD outcomes and duration of mechanical support, were not available in the UNOS dataset. Moreover, long-term post-transplant complication data was missing for a significant proportion of patients. Given the recent approval of the Heartware device by the FDA, the great majority of Heartware patients analyzed in this study likely represent clinical trial patients which may introduce selection bias. Although the UNOS reporting system provides consistent definitions, we cannot rule out variations in data entry from center to center.
In conclusion, our study extends the findings of the ADVANCE trial, and provides valuable long-term data in patients who received continuous-flow device support as a bridge to transplantation. Mechanical unloading with the Heartware device leads to comparable levels of allosensitization, hemodynamic profiles, and end-organ function prior to transplantation compared to the Heartmate II device. Post-transplantation outcomes including freedom from rejection, cardiac allograft vasculopathy, and post-transplant survival are also similar between the two device types. Our findings support use of both the Heartware and Heartmate II devices in transplant eligible patients.
Figure 3. Freedom from Cardiac Allograft Vasculopathy and Hospitalization for Rejection.
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract 234-2005-38011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This research has been supported by research funds from the NIH (T32HL007081) (Dr. Topkara).
Abbreviations and Acronyms
- BMI
Body mass index
- BSA
Body surface area
- BTT
Bridge to transplantation
- CAV
Cardiac allograft vasculopathy
- DT
Destination therapy
- FDA
Food and Drug Administration
- HLA
Human Leukocyte Antigen
- HW-VAD
Heartware Ventricular Assist Device
- HMII-VAD
Heartmate II Ventricular Assist Device
- LVAD
Left ventricular assist device
- PCWP
Pulmonary Capillary Wedge Pressure
- PRA
Panel Reactive Antibody
- PVR
Pulmonary Vascular Resistance
- TPG
Transpulmonary gradient
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frazier OH, Rose EA, Macmanus Q, et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann Thorac Surg. 1992;53:1080–1090. doi: 10.1016/0003-4975(92)90393-i. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Topkara VK, Kondareddy S, Malik F, et al. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg. 2010;90:1270–1277. doi: 10.1016/j.athoracsur.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 8.Morgan JA, Paone G, Nemeh HW, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012;31:715–718. doi: 10.1016/j.healun.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Moazami N, Milano CA, John R, et al. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg. 2013;95:500–505. doi: 10.1016/j.athoracsur.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Larose JA, Tamez D, Ashenuga M, Reyes C. Design concepts and principle of operation of the Heartware ventricular assist system. ASAIO J. 2010;56:285–289. doi: 10.1097/MAT.0b013e3181dfbab5. [DOI] [PubMed] [Google Scholar]
- 11.Wood C, Maiorana A, Larbalestier R, Lovett M, Green G, O'Driscoll G. First successful bridge to myocardial recovery with a Heartware HW-VAD. J Heart Lung Transplant. 2008;27:695–697. doi: 10.1016/j.healun.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 13.Wieselthaler GM, O'Driscoll G, Jansz P, et al. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant. 2010;29:1218–1225. doi: 10.1016/j.healun.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Strueber M, O'Driscoll G, Jansz P, et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–1382. doi: 10.1016/j.jacc.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 15.Miera O, Potapov EV, Redlin M, et al. First experiences with the Heartware ventricular assist system in children. Ann Thorac Surg. 2011;91:1256–1260. doi: 10.1016/j.athoracsur.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 16.D'Alessandro D, Forest SJ, Lamour J, Hsu D, Weinstein S, Goldstein D. First reported use of the Heartware HW-VAD in the US as bridge to transplant in an adolescent. Pediatr Transplant. 2012;16:E356–E359. doi: 10.1111/j.1399-3046.2012.01718.x. [DOI] [PubMed] [Google Scholar]
- 17.Haft J, Armstrong W, Dyke DB, et al. Hemodynamic and exercise performance with pulsatile and continuous-flow left ventricular assist devices. Circulation. 2007;116:I8–I15. doi: 10.1161/CIRCULATIONAHA.106.677898. [DOI] [PubMed] [Google Scholar]
- 18.McDiarmid A, Gordon B, Wrightson N, et al. Hemodynamic, Echocardiographic, and Exercise-Related Effects of the Heartware Left Ventricular Assist Device in Advanced Heart Failure. Congest Heart Fail. 2013;19:11–15. doi: 10.1111/j.1751-7133.2012.00302.x. [DOI] [PubMed] [Google Scholar]
- 19.Pauwaa S, Bhat G, Tatooles AJ, et al. How effective are continuous flow left ventricular assist devices in lowering high pulmonary artery pressures in heart transplant candidates? Cardiol J. 2012;19:153–158. doi: 10.5603/cj.2012.0027. [DOI] [PubMed] [Google Scholar]
- 20.Tuzun E, Roberts K, Cohn WE, et al. In vivo evaluation of the Heartware centrifugal ventricular assist device. Tex Heart Inst J. 2007;34:406–411. [PMC free article] [PubMed] [Google Scholar]
- 21.Slaughter MS, Sobieski MA2nd, Tamez D, et al. Heartware miniature axial-flow ventricular assist device: design and initial feasibility test. Tex Heart Inst J. 2009;36:12–16. [PMC free article] [PubMed] [Google Scholar]
- 22.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84:1556–1562. doi: 10.1016/j.athoracsur.2007.05.095. [DOI] [PubMed] [Google Scholar]
- 23.Joyce DL, Southard RE, Torre-Amione G, Noon GP, Land GA, Loebe M. Impact of left ventricular assist device (LVAD)-mediated humoral sensitization on post-transplant outcomes. J Heart Lung Transplant. 2005;24:2054–2059. doi: 10.1016/j.healun.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Drakos SG, Kfoury AG, Kotter JR, et al. Prior human leukocyte antigen-allosensitization and left ventricular assist device type affect degree of post-implantation human leukocyte antigen-allosensitization. J Heart Lung Transplant. 2009;28:838–842. doi: 10.1016/j.healun.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnaoutakis GJ, George TJ, Kilic A, et al. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: update in a modern cohort. J Thorac Cardiovasc Surg. 2011;142:1236–1245. doi: 10.1016/j.jtcvs.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong KN, Iribarne A, Yang J, et al. Do post-transplant outcomes differ in heart transplant recipients bridged with continuous and pulsatile flow left ventricular assist devices? Ann Thorac Surg. 2011;91:1899–1906. doi: 10.1016/j.athoracsur.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Ventura PA, Alharethi R, Budge D, et al. Differential impact on post-transplant outcomes between pulsatile- and continuous-flow left ventricular assist devices. Clin Transplant. 2011;25:E390–E395. doi: 10.1111/j.1399-0012.2011.01433.x. [DOI] [PubMed] [Google Scholar]
- 28.Nativi JN, Drakos SG, Kucheryavaya AY, et al. Changing outcomes in patients bridged to heart transplantation with continuous-versus pulsatile-flow ventricular assist devices: an analysis of the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2011;30:854–861. doi: 10.1016/j.healun.2011.03.019. [DOI] [PubMed] [Google Scholar]