Abstract
Chinese white dolphins (Sousa chinensis) inhabiting shallow coastal waters are vulnerable to impacts from human activities in the near shore waters. This study examined the population of Chinese white dolphins occurring off the coast of Zhanjiang in the northern South China Sea. A total of 492 Chinese white dolphins were identified, 176 of which were photographed on more than one occasion. The Zhanjiang Chinese white dolphin population is isolated from populations of conspecifics along the Guangdong coast. It is composed of approximately 1485 individuals (95% CI = 1371–1629; SE = 63.8), with estimates of mean representative range and core area of 168.51 and 44.26 km2, respectively. The high site fidelity and long-term residence of Chinese white dolphins in the study area are well established. A review of all available data indicates that based on what is currently known, the Zhanjiang Chinese white dolphin population is the second largest of the species and genus in the world. However, the recent industrial boom along the Zhanjiang coast has increased concerns regarding the conservation of the Zhanjiang Chinese white dolphin population. We recommend the designation of a national nature reserve as a most urgent measure for protecting Chinese white dolphins in Zhanjiang waters.
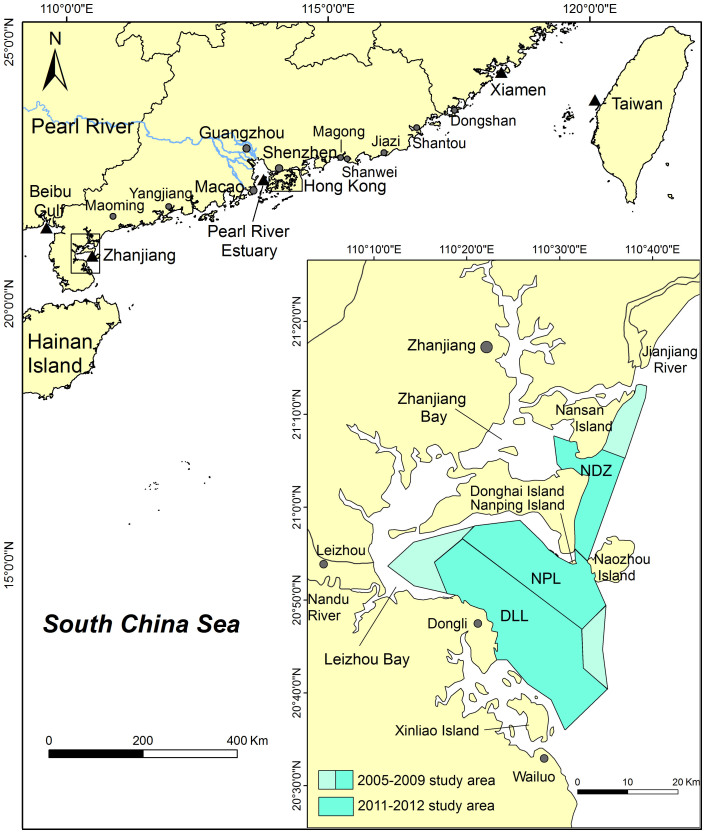
Coastal dolphins are among the most threatened species of cetaceans because of their close proximity to human activities1,2. Humpback dolphins (Sousa spp.) inhabit shallow coastal waters, where they come into particularly frequent contact with human activity. The humpback dolphins found in Chinese waters are known as Chinese white dolphins (Sousa chinensis). In recent decades, human activities along the Chinese coast have been increasing because of rapid economic growth3. As a result, the water quality along China's coast has been progressively deteriorating4,5. The Chinese white dolphin populations, historically distributed in nearshore waters south of the Yangtze River, have declined drastically and become fragmented since the 1960s due to anthropogenic factors. Sightings of Chinese white dolphins in coastal waters of Dongshan, Shantou, Jiazi, Shanwei and Magong were reported by aged fishermen in survey questionnaires in 2010 in 12 fishing ports. The result suggests that the Chinese white dolphins lived in coastal waters between Xiamen and Pearl River Estuary approximately 20–30 years ago, and the current fragmentation is secondary and the result of human impacts6. The colonies now exist in five discontinuous locales, including Xiamen, western Taiwan, Pearl River Estuary, Zhanjiang, and Beibu Gulf in the East China Sea and South China Sea7 (Fig. 1).
Figure 1. Distribution of S. chinensis in Chinese waters.
Solid triangles, recognized small populations of S. chinensis. Inset, detailed map of Leizhou Bay and Zhanjiang Bay indicating the survey areas. DLL, Dongli village area in Leizhou Bay; NPL, Nanping Island area in Leizhou Bay; NDZ, Nansan and Donghai Islands area in and adjacent to Zhanjiang Bay. Figure was produced using ArcMap in ArcGIS 9.3.
In this study, we acquired comprehensive survey data on the abundance, long-term site fidelity, and home range patterns of Chinese white dolphins found off the east coast of Zhanjiang city in the northern South China Sea. Assessing the number of animals in a population is a fundamental requirement for effective wildlife management. Site fidelity refers to the degree to which an individual or a population maintains residency in a particular region and is thus important for a basic understanding of population ecology and behavior. Knowledge regarding home ranges is essential for understanding the resources required by a species, identifying critical habitats, and revealing the overlap with anthropogenic impacts8,9. This information is fundamental for identifying conservation and research that will help the survival of this major dolphin population and drive recommendations for governmental agencies.
Results
Survey effort and photo-identification
Across the 7-year (2005–2009, 2011–2012) study, 374 boat-based surveys were conducted, resulting in a total of 2310.14 survey hours and 4959 dolphin sightings. The study revealed an identical sighting rate of 0.27 (the number of groups/h of search effort10) for two of the three principal areas: Nanping Island area in Leizhou Bay (NPL) and Dongli village area in Leizhou Bay (DLL). The sighting rate for the Nansan and the Donghai Islands area in and adjacent to Zhanjiang Bay (NDZ), 0.22, was slightly lower.
A total of 611 groups of Chinese white dolphins were sighted. The group size ranged from 1 to 35 individuals with a mean of 8.12 ± 5.85 and a median of 7 individuals. Most solitary individuals were adult or elderly animals. Age classes were determined for all 4959 sighted dolphins. Most of the sighted dolphins were adult (n = 1787, 36%) and subadult (n = 2032, 41%). The 992 juvenile dolphins made up 20% and the 149 newborn calves were 3% of the total sighted individuals. In addition to the carcass of an adult recovered in February 20087, a decayed adult carcass was found drifting in NPL on 6 August 2012.
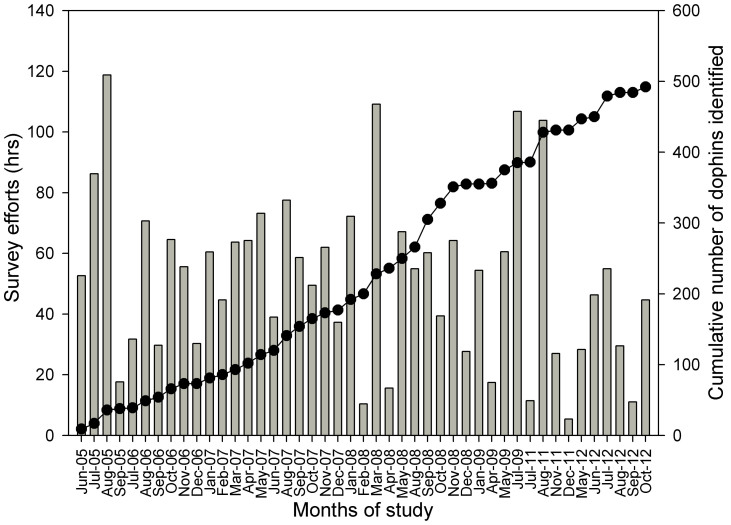
From 2005 to 2012, 492 distinct Chinese white dolphins were identified by photographs. The number of new photo-identified individuals per year fluctuated from 30 in 2009 to 178 in 2008. The cumulative number of Chinese white dolphins increased throughout the study period. The slope of the discovery curve for newly photographed dolphins showed a sharp rise (Fig. 2), suggesting that we have not yet captured all of the dolphins in Zhanjiang waters or that the population is not closed and ‘new’ animals are arriving from outside the study area.
Figure 2. Discovery curve showing the cumulative number of Chinese white dolphins (grey line) identified in relation to the hours of survey effort per month (grey bars) in Zhanjiang waters between 2005 and 2012.
Validation of open model assumptions
Utilization of an open model requires the validation of basic assumptions, the violation of which can lead to bias in parameter estimates11: (1) Capture and survival probabilities are the same for all animals (marked and unmarked) between each pair of sampling occasions (homogeneous survival). The pooled χ2 statistic (Test 2 + Test 3) indicated that the assumptions of homogeneous capture and survival probabilities were not violated (GOF Test: χ2 = 175.74, df = 26, P = 0.0122); (2) Capture results in similar risks and fates for all individuals. With photo-identification techniques, animals were not subject to stress associated with capturing, handling, or physical marking by researchers; (3) Marks are recognized properly, unique (no twins), and do not change or become lost. To identify and catalog individuals, we used only good or excellent quality photos of dolphins showing permanent marks, so marks could be recognized and identified on each sighting occasion; and (4) Samples are instantaneous, and all individuals are released after capture. Photo-identification avoided problems of dolphins being captured or retrieved from the environment. To guarantee that all the dolphins in the area were captured and that the entire region was surveyed, 6 mo was used as the sampling unit, a relative short time compared with the dolphins' lifespan (30–40 yr).
Population abundance
Of the 8 models tested, the {ϕt P. bt} model appears to be the most appropriate and the one that best describes our data and accounts for the proportion of identifiable individuals (Table 1). This model allows constant capture probability (P) and allows survival (ϕ) and probability of entry (b) to vary with time. The proportion of identifiable individuals (all marked individuals) was 0.52, and the total population was estimated at 1485 (95% CI = 1371–1629; SE = 63.8) (Table 1).
Table 1. Model choice criteria, identifiable individuals population size estimate (N) and total population size estimate (N total, corrected for the proportion of identifiable individuals) for 8 models tested in a mark-recapture analysis of individual sighting histories of Chinese white dolphins in the Zhanjiang region, using the open-population POPAN parameterization in program MARK. AIC = Akaike's Information Criterion value, No. Par = number of parameters, SE = standard error, 95% CI = 95% confidence interval, CV = coefficient of variation.
| Model choice criteria | Identifiable individuals | Total population | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | AICc | ΔAICc | AICc weight | Model Likelihood | No. Par | N | SE | 95% CI | CV | θ | N total | SE | 95% CI | CV |
| ϕt P. bt | 1640.0232 | 0.0000 | 0.99727 | 0.1000 | 18 | 772 | 33.8 | 713–847 | 0.043 | 0.52 | 1485 | 63.8 | 1371–1629 | 0.043 |
| ϕt Pt bt | 1652.4084 | 12.3852 | 0.00204 | 0.0020 | 25 | 687 | 28.9 | 638–752 | 0.042 | 0.52 | 1321 | 55.5 | 1227–1446 | 0.042 |
| ϕ. P. bt | 1654.6481 | 14.6249 | 0.00067 | 0.0007 | 10 | 768 | 32.7 | 711–840 | 0.043 | 0.52 | 1477 | 63.5 | 1367–1615 | 0.043 |
| ϕ. Pt bt | 1661.2300 | 21.2068 | 0.00002 | 0.0000 | 19 | 781 | 42.8 | 709–878 | 0.054 | 0.52 | 1502 | 81.1 | 1363–1688 | 0.060 |
| ϕt Pt b. | 52132.9909 | 50492.9677 | 0.00000 | 0.0000 | 17 | 492 | 0 | 492–492 | 0 | 0.52 | 946 | 0 | 946–946 | 0 |
| ϕt P. b. | 52235.0293 | 50595.0061 | 0.00000 | 0.0000 | 9 | 492 | 0 | 492–492 | 0 | 0.52 | 946 | 0 | 946–946 | 0 |
| ϕ. P. b. | 52247.0086 | 50606.9854 | 0.00000 | 0.0000 | 3 | 700 | 20.0 | 665–743 | 0.028 | 0.52 | 1346 | 37.7 | 1278–1428 | 0.026 |
| ϕ. Pt b. | Numerical convergence not reached | |||||||||||||
Site fidelity
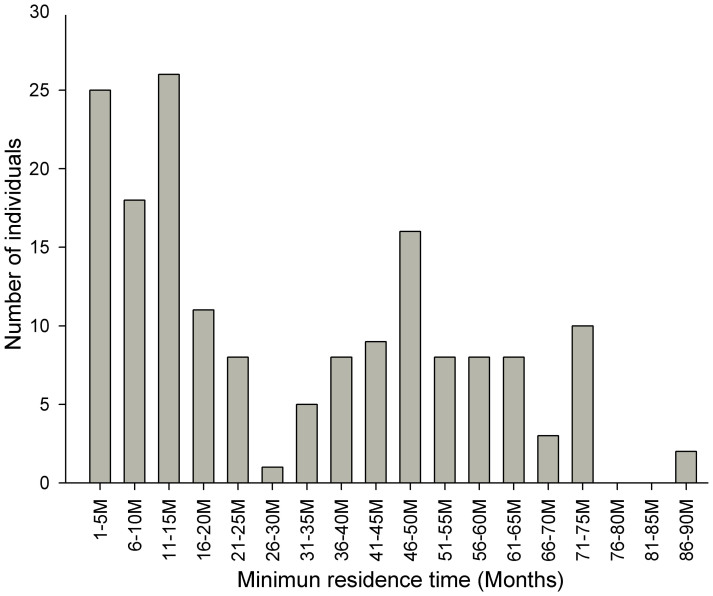
A total of 176 cataloged individuals (n = 492, 36%) showed some degree of site fidelity and were resighted from 2 to 29 times. Sixty-five individuals were seen on five or more occasions, and eighteen individuals were seen on ten or more occasions. Multiyear site fidelity was displayed by 28% (n = 136) of the identified individuals. Long-term site fidelity (up to 5 yr = 60 mo) was observed in a small number of individuals (n = 23, 5%) (Fig. 3). Individuals ZJ25 and ZJ29 were first documented in 2005 in DLL and subsequently resighted 7 and 8 times, respectively, in five different years in Leizhou Bay over a 7-year period. In addition, another 10 Chinese white dolphins were resighted between 71 and 86 months in the study area. Continued resightings of some dolphins substantiated the regular use of the study area by the animals.
Figure 3. Minimum residence time of Chinese white dolphins identified between 2005 and 2012 in Zhanjiang waters.
Representative range and core area
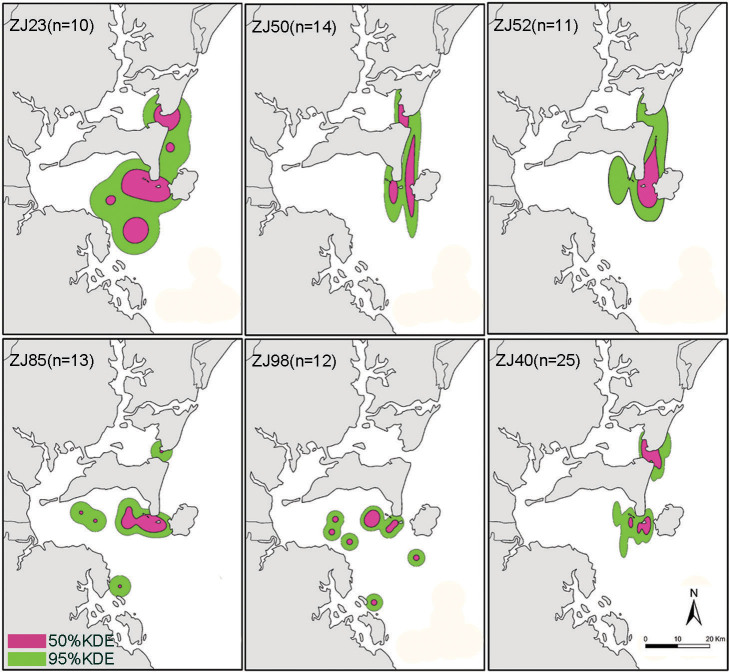
Univariate kernel density estimates of home range were calculated for the 18 individuals with 10 or more sightings. The areas of individual ranging patterns for each dolphin differed in size and shape. The mean estimates of 95% KDE (representative range) and 50% KDE (core area) were 168.51 km2 and 44.26 km2, respectively (Table 2). Kernel density plots showed a home range of habitat use of one individual in DLL, NPL and NDZ, four individuals in NPL and NDZ, and one individual in NPL and DLL. The home range was split between two areas (NPL and NDZ, or NPL and DLL) for six individuals and three areas (DLL, NPL and NDZ) for two individuals. Relatively shallow waters near Nanping Island in Leizhou Bay and Nansan Island in Zhanjiang Bay, particularly near shallow sand beaches, appeared to be most frequently used by Chinese white dolphins, and there was extensive overlap in the home ranges for all individuals. Photo-identification data showed that animals moved among these two or three areas, indicating that a single population is present in the study area. Examples of individual ranging patterns of dolphins with 10 sightings or more are shown in Figure 4. Through the individual range use analysis (Fig. 4), we observed that the dolphins are a strictly inshore coastal and estuarine species most often found in waters less than 20 m deep and within 10 km of the coast.
Table 2. Ranging patterns of 18 individual dolphins with 10 or more sightings each. DLL, Dongli village area in Leizhou Bay; NPL, Nanping Island area in Leizhou Bay; NDZ, Nansan and Donghai Islands area in and adjacent to Zhanjiang Bay.
| Dolphin ID # | No. of sightings | 95% KDE (km2) | 50% KDE (km2) | Area |
|---|---|---|---|---|
| ZJ40 | 25 | 161.84 | 39.53 | NPL, NDZ |
| ZJ50 | 14 | 218.78 | 66.94 | NPL-NDZ |
| ZJ52 | 11 | 279.58 | 67.26 | NPL-NDZ |
| ZJ60 | 20 | 181.01 | 46.99 | NPL-NDZ |
| ZJ70 | 19 | 59.06 | 12.48 | NPL, NDZ |
| ZJ81 | 15 | 115.99 | 24.80 | NPL, NDZ |
| ZJ86 | 18 | 117.62 | 30.58 | NPL, NDZ |
| ZJ88 | 11 | 137.76 | 42.98 | NPL-NDZ |
| ZJ186 | 11 | 163.76 | 36.83 | NPL, NDZ |
| ZJ63 | 10 | 403.31 | 116.63 | NPL-DLL |
| ZJ98 | 12 | 169.54 | 35.40 | NPL, DLL |
| ZJ23 | 10 | 616.18 | 179.96 | DLL-NPL-NDZ |
| ZJ43 | 29 | 59.68 | 13.93 | DLL, NPL, NDZ |
| ZJ85 | 13 | 238.74 | 54.81 | DLL, NPL, NDZ |
| ZJ44 | 17 | 15.16 | 2.74 | NDZ |
| ZJ46 | 18 | 16.72 | 2.85 | NDZ |
| ZJ78 | 10 | 16.25 | 3.29 | NDZ |
| ZJ259 | 11 | 62.23 | 18.69 | NPL |
| Mean | 168.51 | 44.26 | ||
| Range | 15.16–616.18 | 2.74–179.96 |
Figure 4. Individual ranging patterns of six identified Chinese white dolphins with 10 sightings or more from the waters of Zhanjiang using the fixed kernel home-range analysis.
Figure was produced using ArcMap in ArcGIS 9.3 and home range tools.
Discussion
Mendez et al12 proposed that the humpback dolphin genus includes at least four member species: the Atlantic humpback dolphin (Sousa teuszii), the Indo-Pacific humpback dolphin (Sousa plumbea), the Chinese white dolphin (Sousa chinensis), and a fourth yet-to-be-named Sousa species found off northern Australia. Estimates of population sizes available for selected areas around the world indicate that most humpback dolphin populations are small, with only a few hundred or dozens of individuals, except for the populations of the Pearl River Estuary and the Zhanjiang Chinese white dolphins (Table 3). Several hundreds of Sousa teuszii were found in the waters of Canal do Gêba and Bijagos Archipelago in Guinea-Bissau. The sampled population of Sousa plumbea in Algoa Bay, South Africa, yielded an estimate of 466 individuals13. The largest population reported for the Australian humpback dolphins is approximately 150 dolphins in the Great Sandy Strait Marine Park, Queensland11. Based on line-transect surveys conducted in the Pearl River Estuary between 2005 and 2008, the total population size of the PRE Chinese white dolphins was estimated to be 2555 during the wet season and 2517 during the dry season14. A total population of approximately 1485 individuals was estimated for the Zhanjiang Chinese white dolphin from the current study. This result is 5–6 times larger than the previous estimates (2005: 237, 95% CI = 189–318; 2005–2007: 268, 95% CI = 189–413)7,15. This makes the Zhanjiang Chinese white dolphin population the second largest in the genus Sousa and particularly of Sousa chinensis in the world, based on what is currently known.
Table 3. Reported population sizes of humpback dolphins in the genus Sousa around the world.
| Species | Location | Time | Population size1) | Reference |
|---|---|---|---|---|
| Sousa teuszii | Banc d'Arguin, Mauritania | 1997–2006 | <100 | 40 |
| Saloum Delta, Senegal | 1997–2006 | Low hundreds | 40 | |
| Canal do Gêba and Bijagos Archipelago, Guinea-Bissau | 1997–2006 | Several hundred | 40 | |
| Sousa plumbea | Algoa Bay, South Africa | 1991–1994 | 466 | 13 |
| Richards Bay, South Africa | 1998 | 74 | 41 | |
| Maputo Bay, Mozambique | 1995–1997 | 105 | 42 | |
| Bazaruto Bay, Mozambique | 1990s | 60 | 43 | |
| Anakao, Madagascar | 1999 | 65 | 44 | |
| South coast of Zanzibar, Tanzania | 1999–2002 | 63 | 45 | |
| Shimoni Archipelago, Kenya | 2006 | 104 | 46 | |
| Saudi–Bahrain–Qatar | 1986 | (16 groups) | 47 | |
| United Arab Emirates | 1986 | (13 groups) | 47 | |
| United Arab Emirates | 1999 | (2 groups) | 47 | |
| Arabian Sea coast of Oman | (Groups of 30 individuals or more) | 48 | ||
| Jubail, Saudi Arabia | 1991–1993 | (50 groups, 1–15 individuals) | 49 | |
| Indus Delta, Pakistan | 2005–2009 | Low hundreds | 50 | |
| Gulf of Kachchh Marine Protected Area, Gujarat, India | 2002 | 21 | 51 | |
| Goa, India | 2002 | 135 | 51 | |
| Sousa chinensis | Between the Sundarbans mangrove forest and the Swatch-of-No Ground submarine canyon, Bay of Bengal, Bangladesh | 2010–2011 | 191 | 52 |
| Khanom, Nakhon Si Thammarat, Thailand | 2008–2009 | 49 | 53 | |
| Xiamen | 2004–2008 | 76 | 54 | |
| Central west coast of Taiwan | 2002–2004 | 99 | 55 | |
| Pearl River Estuary | 2005–2008 | 2517–2555 | 14 | |
| Zhanjiang | 2005–2012 | 1485 | This study | |
| Beibu Gulf | 2003–2004 | 153 | 54 | |
| Sousa spp.2) | Moreton Bay, Queensland | 1984–1987 | 119–163 | 56 |
| Great Sandy Strait, Queensland | 2004–2007 | 150 | 11 | |
| Capricorn-Curtis coast, Queensland | 2007–2011 | Approximately 150 | 57 | |
| Cleveland Bay, Queensland | 2001–2002 | <100 | 34 | |
| North West Cape, Western Australia | 2010 | 53 identified individuals | 58 |
1)Number of groups and group size in parentheses,
2)The Australian humpback dolphins are an as-yet-unnamed species of Sousa12.
Interannual site fidelity of Chinese white dolphins to the area off the eastern coast of Zhanjiang was reported in our previous study7. If we suspect that there are emigrant individuals leaving and immigrant individuals joining the Zhanjiang Chinese white dolphin population, we must ask where the emigrant individuals are going and where the immigrant individuals are coming from.
The distribution of Chinese white dolphins in the western section of the Pearl River Estuary extends from the mouth of Modaomen to the channel between Shangchuan and Xiachuan Islands14. The Leizhou and Zhanjiang Bays are located 250 km from the western section of the Pearl River Estuary, where the PRE Chinese white dolphins live. The northernmost distribution of Chinese white dolphins in the waters of Zhanjiang is the mouth of Jianjiang River (authors' unpublished data). Maoming and Yangjiang are located between the Pearl River Estuary and Zhanjiang. Survey questionnaires supported by Ocean Park Conservation Foundation, Hong Kong, (OPCFHK Project: Initial Establishment of Southern China Marine Mammal Stranding Network) were employed along the coast of Maoming and Yangjiang in January 2003. Boat surveys and questionnaire surveys supported by the Oceanic and Fisheries Administration of Guangdong Province were undertaken in November 2012 along the Maoming coast. During these surveys, no sightings or incidental catches of Chinese white dolphin were reported by fishermen, and no Chinese white dolphins were sighted during the boat surveys. Although no attempt has been made to assess the genetic distinctiveness of the Zhanjiang Chinese white dolphin population, considering the high resighting rate of marked individuals in the study area and the absence of nearby populations of Chinese white dolphins to the north of Zhanjiang Bay and Leizhou Bay, emigration from or immigration to the PRE Chinese white dolphin population appears unlikely. The data suggest that the Zhanjiang Chinese white dolphin population is isolated from populations of conspecifics along the Guangdong coast and support the findings of this study that high site fidelity and long-term residence of Chinese white dolphins are well established in the study area.
The water quality in Leizhou Bay and the outer portions of Zhanjiang Bay is good overall; the Pearl River Estuary receives residential and industrial water discharges that are severely polluted with both sewage and industrial wastes. Most Grade IV and greater than Grade IV waters are concentrated in the Pearl River Estuary, with inorganic nitrogen and active phosphate being the main pollutants16. An ecosystem health assessment in the Pearl River Estuary shows that the ecosystem health index (EHI) over the last three decades has decreased from 0.91 to 0.50, indicating deterioration from healthy to unhealthy status17. Although a small portion of Leizhou Bay is lightly polluted by oil and inorganic nitrogen, the rest of Leizhou Bay is within Grade I or II of the National Seawater Quality Standards for China. The inner portions of Zhanjiang Bay suffer from inorganic nitrogen and reactive phosphorus pollutants and have water quality grades of IV or worse. Water quality in the outer portions of Zhanjiang Bay, where the Chinese white dolphins live, was assessed as Grade I or II per the national standards16. The coastal waters of Zhanjiang city are rich in fish, with more than 220 species18; many are prey of Chinese white dolphins. Compared with the Pearl River Estuary, where about sixty Chinese white dolphin carcasses were found between 2010 and 201219,20, only two dead adults have been recorded in the waters of Zhanjiang since 2005. A Sousa carcass was found in oyster piles near Sanhewo Fishing Port in Zhanjiang Bay in February 20087, and another carcass was found drifting in Leizhou Bay in August 2012 (the current study).
The frequent sightings of young and neonate dolphins in Zhanjiang waters are indicative of a healthy population. Thus, it appears that Leizhou Bay and the outer portions of Zhanjiang Bay are relatively healthy habitat for Chinese white dolphins. However, the Zhanjiang water conditions may soon begin to deteriorate. Construction work for two large industrial facilities on the northern coast of Donghai Island is underway. Bao Steel Zhanjiang, the country's largest steel manufacturer, is scheduled to start trial operations for the Guangdong Iron and Steel Base Project in October of next year. The petrochemical complex, a joint venture of China Petroleum and Chemical Corp. (Sinopec) and Kuwait Petroleum Corp., is scheduled to start production by 2017. The coveted steel and petrochemical deals, plus a papermaking development, are expected to generate an output of 250 billion Chinese yuan (39.1 billion U.S. dollars) by 2016. The annual throughput of goods at Zhanjiang Port is expected to surpass 300 million tons in 2016, up from the current 171 million.
These projects may increase the lethal threats to Sousa chinensis in Zhanjiang Bay, such as deteriorating water quality, loss of the capacity of the habitat to provide critical resources for dolphins, and direct mortality and injury through boat strikes. Direct evidence supporting such concerns comes from the recent extinction of the baiji (Lipotes vexillifer)21 and the rapid decline of the Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis)22 and the PRE Chinese white dolphin23, which are directly related to fast economic growth and large-scale developments. If not promptly protected, the Zhanjiang Chinese white dolphin population may follow its counterparts in the Pearl River Estuary and risk an unrecoverable collapse from inevitable habitat degradation. Therefore, the Zhanjiang population of Chinese white dolphins and its habitat deserve the highest degree of attention and protection.
In China, the Chinese white dolphin is listed as a Grade 1 National Key Protected Animal under the Wild Animal Protection Law approved in 1988. Three of the five populations of Chinese white dolphins are currently under the protection of national natural reserves. The PRE Chinese white dolphin population is protected by the Pearl River Estuary Chinese White Dolphin National Nature Reserve. The Xiamen and Beibu Gulf Chinese white dolphin populations are protected by the Xiamen Rare Species National Marine Nature Reserve and the Hepu Dugongs National Nature Reserve, respectively. Although a municipal nature reserve designation for the Chinese white dolphin population in Leizhou Bay was established by the Zhanjiang city government in 2007, the conservation effort for this dolphin population is insufficient in many aspects because the reserve does not have independent and permanent offices and full-time employees.
We have learned a profound lesson from the extinction of a marine mammal in the Yangtze River. The baiji, or Yangtze River dolphin (Lipotes vexillifer), found only in the Yangtze River, was declared “functionally extinct” in 200721. The baiji experienced a catastrophic population collapse in recent decades, due largely to various extreme anthropogenic pressures24. The first estimate of baiji abundance based on quantitative survey data (1979–81) was approximately 400 animals25. At the time, we warned that in the absence of drastic protection measures, the baiji could be extinct in half a century. The same warning was repeated with heightened urgency in subsequent publications with additional survey data26,27. Despite all of the efforts made to conserve the baiji since the early 1980s, the population declined drastically in less than 2 decades as rapid industrialization led to heavy river transportation traffic, over-fishing, and hydroelectric dam construction.
We hope that the loss of the baiji serves as a stern lesson and raises concerns regarding the safeguarding of the Chinese white dolphin population in the waters of Zhanjiang. We recommend the designation of a national nature reserve as an urgent measure to protect Chinese white dolphins in the waters of Zhanjiang. As we envision it, the national nature reserve would manage existing and future activities with higher impacts to maintain the viability of this dolphin population. This national nature reserve would also be an area with a greater degree of legal protection. Given these rights, the reserve could promote ecologically sustainable development to maintain, protect and restore key habitat features and to ensure that the ecosystem is sustained for the Chinese white dolphins.
Methods
Data were collected with approval from the Oceanic and Fishery Bureau of Zhanjiang city and from the Animal Research Ethics Committee of Nanjing Normal University (Permit No. AREC 2005-05-008). The methods were carried out in accordance with the approved guidelines.
Study area and data collection
Leizhou and Zhanjiang Bays are subtropical bays of the northern South China Sea, located on the southernmost Chinese mainland, on the eastern coast of the Leizhou Peninsula. The study area included the nearshore waters of east Leizhou Peninsula, from the Jianjiang River mouth to Wailuo Town, including Leizhou and Zhanjiang Bays (110°37′E, 21°11′N; 110°31′E, 20°36′N). The area includes approximately 1,500 km2 along the coast (Fig. 1). Leizhou Bay is located on the south end of Donghai Island; the water depth is 8–28 m. With an elongated beach, Nanping Island, approximately 7 km long and less than 1 km in width, lies near the south side of Donghai Island. Zhanjiang Bay (formerly known as Guangzhou Bay) is located on the north of Donghai Island.
Our study of the Zhanjiang population of S. chinensis was initiated in 2005. Exploratory surveys for Chinese white dolphins were conducted throughout the entire area of Leizhou Bay from June to September 200515. A follow-up project, intended to broaden our overall knowledge of the dolphins in Zhanjiang waters, began in 2006. Line-transect surveys were conducted in Leizhou Bay, Zhanjiang Bay and the mouth of Jianjiang River between July 2006 and June 20077 and between January 2008 and July 2009 (unpublished).
To ensure maximal habitat coverage of the above regions, three principal areas were selected for boat-based surveys in 2011 and 2012: the Dongli village area in Leizhou Bay (DLL), the Nanping Island area in Leizhou Bay (NPL), and the Nansan and Donghai Islands area in and adjacent to Zhanjiang Bay (NDZ) (Fig. 1). These areas were selected based on our previous line-transect surveys conducted throughout the entire region7,15.
Vessel-based surveys were conducted in permissible weather and marine conditions, with winds not exceeding 3 on the Beaufort scale, in a range of 5.5–15 km/h. Two or three researchers searched for dolphin groups (i.e., aggregations of dolphins with relatively close spatial cohesion and involved in similar behavioral activities28), with one researcher on each side of the boat and the driver observing the forward area. Upon sighting a group of dolphins, the boat slowed to idling speed. One researcher photographed the individuals in the group, and the other recorded time, position, tidal changes, depth, salinity, temperature, group size, group composition, and predominant behavior. A sighting of a ‘group’ of 10 would count as 10 ‘dolphin sightings’ and 1 ‘group sighting’. The predominant behavior was recorded as the activity displayed by the majority of the animals in the group, based on the initial observation. The dolphins photographed in the same group were considered associated. However, individual associations of humpback dolphins seemed unstable7,13; some individuals could appear in many different groups in one day. If individuals were rediscovered within the same day, the later groups containing the rediscovered individuals were excluded from analysis. Information on the boat's position was logged every 30 s with a Geographic Positioning System unit (Garmin GPSmap 60CSx, position accuracy: <15 m; GARMIN Corporation, Lenexa, Kansas, USA).
Photographs were taken with a Nikon D3 equipped with an 80 mm to 400 mm zoom lens. Laboratory photo-analysis began with the initial sorting and identification of a collection of digital images7. Initial analysis and comparison of photographs were conducted via digital images on computers. Photographs were rated for quality, and those with the highest quality rating were compared manually to cataloged photographs. The best pictures of the identified dolphins were compiled into a catalog of all identified dolphins in the study area. All animals that had not been previously identified were given a unique catalog number.
Population abundance and mark-recapture population models
The population size was estimated assuming an open-population, using photo-ID data and the mark–recapture method. The data sets from 2005 to 2012 were organized into nine events (Table 4) and were analyzed using the software program MARK v.7.129, which uses Maximum Likelihood models to estimate population parameters30. Population parameters were estimated using the open-population POPAN parameterization31, where parameter N represents population, parameter ϕ represents apparent survival rate, P is the probability of capture, and b denotes the probability of entry into the group. In model notation, the subscripts (t) and (.) represent time-dependent and constant parameters, respectively. The initial analysis was based on the fully time-dependent/Cormack–Jolly–Seber (CJS) model {ϕt Pt bt}. The first step in the analysis involves Goodness-of-Fit (GOF) tests for the CJS model, using the program RELEASE GOF30 to validate model assumptions. Taking into consideration the biology of the species and the sampling method, 8 models were further constructed. The appropriate model for inference was selected using the Akaike Information Criterion (AICc) corrected for small sample sizes32. AICc weighs the deviance (quality of fit) and the precision based on the number of estimable parameters to select a model or models that best describe the data33. Models differing by less than two units from the model with the minimum AICc (ΔAICc) provide good descriptions of the data32. When more than one model provided a good description of the data, we followed the principle of parsimony and selected the model with the lower number of parameters as the most appropriate34.
Table 4. The 2005 to 2012 data were organized into nine time periods, showing the number of new identified individuals and cumulative number of identified individuals in each period.
| Time periods | Number of new identified individuals | Cumulative number of identified individuals | Survey efforts (h) |
|---|---|---|---|
| 2005 (second half year) | 38 | 38 | 275.00 |
| 2006 (second half year) | 35 | 73 | 282.58 |
| 2007 (first half year) | 47 | 120 | 345.23 |
| 2007 (second half year) | 57 | 177 | 284.87 |
| 2008 (first half year) | 73 | 250 | 274.48 |
| 2008 (second half year) | 105 | 355 | 246.45 |
| 2009 (first half year) | 30 | 385 | 239.11 |
| 2011 (second half year) | 46 | 431 | 147.57 |
| 2012 (second half year) | 61 | 492 | 214.85 |
The mark-recapture population estimates apply only to the marked animals. Therefore, we calculated the total population size, N (total), using equation (1)
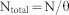 |
where θ is the proportion of marked individuals calculated within the area. The proportion of marked individuals, θ, is the ratio between the cumulative number of dolphins identified and the total number of dolphins sighted during the study. Standard errors for the total population size were derived from the variance of N, given generally by equation (2)
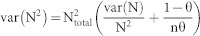 |
where n is the total number of marked individuals from which θ was calculated. Confidence intervals for Ntotal assumed the same error distribution as the mark-recapture estimates35.
Site fidelity
We used the entire 7-yr (2005–2009, 2011–2012) collection of photographs to assess site fidelity. To investigate the presence of marked animals in the study area throughout the study period, we calculated: (i) the total number of sightings for each cataloged individual from all sighting data in the study area; and (ii) the minimum residence time or the maximum month interval between captures. To maintain data independence, we used only one sighting per day for each dolphin.
Representative range and core area
We used the kernel density estimation (KDE) to estimate utilization distributions (UDs). The fixed kernel density estimator uses probability density functions to identify areas of intense use36,37. Animals with ten or more sightings were selected to calculate kernel density estimates of home range between 2005 and 2012. Using a geographic information system (GIS), global positioning system (GPS) coordinates for identified individuals were downloaded and converted to shapefiles (point layers) (ESRI, v. 9.3). Shapefiles were then analyzed for the ranging pattern using the kernel home range (KHR) analysis38. The fixed KHR with the least-squares cross-validation technique (LSCV) was used to create a 50% kernel and 95% kernel UD39. The 95% kernel was used for the overall occurrence estimations, and the 50% kernel was used for the core occurrence area estimation. Area calculations were based on a Universal Transverse Mercator (UTM) projection, zone 49 N.
Author Contributions
K.Z. conceived and coordinated the research. X.X. and G.Y. contributed to the research design. X.X., J.S. and Z.Z. conducted the surveys and collected the data. J.S. and P.L. contributed to the data analysis. K.Z. and J.S. wrote the paper.
Acknowledgments
We are exceptionally grateful to Youtong Lin, Feng Yang, and Jianan Liang of the Oceanic and Fishery Bureau of Zhanjiang city for their cooperation. Special thanks to Chao Tian, Liga Ma, Jiayong Zhang, Yanfu Qu, Yi Kong, Che Chen, Jiayi Li and Yi Hu for their assistance in field and lab work, to Kai Liu for GIS advice, and to Shiang-Lin Huang of the National Taiwan Ocean University for CJS modeling. We are grateful to Ellen Hines and reviewers for valuable comments and language corrections that greatly improved the manuscript. This research was funded by the National Natural Science Foundation of China (Grant No. 31172110), Ocean Park Conservation Foundation, Hong Kong (OPCFHK Projects 2006–07, 2007–08, 2008–09, 2011–12) and the Priority Academic Program Development of Jiangsu Higher Education Institutions, to KZ.
References
- Thompson P. M., Wilson B., Grellier K. & Hammond P. S. Combining power analysis and population viability analysis to compare traditional and precautionary approaches to conservation of coastal cetaceans. Conserv. Biol. 14, 1253–1263 (2000). [Google Scholar]
- Davidson A. D. et al. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. USA 109, 3395–3400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q. et al. Economic development and coastal ecosystem change in China. Sci. Rep. 4, 5995; 10.1038/srep05995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. Environmental problems in coastal waters of China. Mankind and the Oceans [Miyazaki, N., Adeel, Z. & Ohwada, K. (eds)] [59–67] (United Nations University Press, Tokyo, 2005). [Google Scholar]
- CIMA (China Institute for Marine Affairs). China's Ocean Development Report (2011) (Ocean Press, Beijing, 2011). [Google Scholar]
- Wang X. et al. Investigation on the distribution of Sousa chinensis in the coastal waters between Xiamen and the Pearl River Estuary. Journal of Oceanography in Taiwan Strait 31, 225–230 (2012). [Google Scholar]
- Xu X., Zhang Z., Ma L., Yang G. & Zhou K. Site fidelity and association patterns of Indo-Pacific humpback dolphins off the east coast of Zhanjiang, China. Acta Theriol. 57, 99–109 (2012). [Google Scholar]
- Ingram S. N. & Rogan E. Identifying critical areas and habitat preferences of bottlenose dolphins Tursiops truncatus. Mar. Ecol. Prog. Ser. 244, 247–255 (2002). [Google Scholar]
- Seminoff J. A., Resendiz A. & Nichols W. J. Home range of green turtles Chelonia mydas at a coastal foraging area in the Gulf of California, Mexico. Mar. Ecol. Prog. Ser. 242, 253–265 (2002). [Google Scholar]
- Merriman M. A., Markowitz T. M., Harlin-Cognato A. D. & Stockin K. A. Bottlenose dolphin (Tursiops truncatus) abundance, site fidelity, and group dynamics in the Marlborough Sounds, New Zealand. Aquatic Mammals 35, 511–522 (2009). [Google Scholar]
- Cagnazzi D. B., Harrison P., Ross G. B. & Lynch P. Abundance and site fidelity of Indo-Pacific Humpback dolphins in the Great Sandy Strait, Queensland, Australia. Mar. Mammal Sci. 27, 255–281 (2011). [Google Scholar]
- Mendez M. et al. Integrating multiple lines of evidence to better understand the evolutionary divergence of humpback dolphins along their entire distribution range: a new dolphin species in Australian waters? Mol. Ecol. 22, 5731–5961 (2013). [DOI] [PubMed] [Google Scholar]
- Karczmarski L., Winter P. E. D., Cockroft V. G. & Mclachlan A. Population analyses of Indo-Pacific humpback dolphins Sousa chinensis in Algoa Bay, Eastern Cape, South Africa. Mar. Mammal Sci. 15, 1115–1123 (1999). [Google Scholar]
- Chen T., Hung S. K., Qiu Y., Jia X. & Jefferson T. A. Distribution, abundance, and individual movements of Indo-Pacific humpback dolphins (Sousa chinensis) in the Pearl River Estuary, China. Mammalia 74, 117–125 (2010). [Google Scholar]
- Zhou K., Xu X. & Tian C. Distribution and abundance of Indo-Pacific humpback dolphins in Leizhou Bay, China. New Zealand J. Zool. 34, 35–42 (2007). [Google Scholar]
- OFAG (Oceanic and Fisheries Administration of Guangdong Province). Guangdong Marine Environment Quality Report in 2013. (2014). http://www.gdofa.gov.cn/index.php/Catagories/view/id/174869/(17/06/2014). [Google Scholar]
- Chen X. et al. Ecosystem health assessment in the Pearl River Estuary of China by considering ecosystem coordination. PLoS ONE 8, e70547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P. The addendum of coastal fishes of Zhanjiang City. Journal of Zhanjiang Fisheries College 18, 30–33. (1992). [Google Scholar]
- SOA (State Oceanic Administration of China). China Marine Environmental Quality Communique in 2010. (Ocean Press, Beijing, 2011). [Google Scholar]
- SOA (State Oceanic Administration of China). China Marine Environmental Quality Communique in 2012. (Ocean Press, Beijing, 2013). [Google Scholar]
- Turvey S. T. et al. First human-caused extinction of a cetacean species? Biol. Lett. 3, 537–540 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z. et al. The Yangtze finless porpoise: On an accelerating path to extinction? Biol. Conserv. 172, 117–123 (2014). [Google Scholar]
- Huang S. L. et al. Demography and population trends of the largest population of Indo-Pacific humpback dolphins. Biol. Conserv. 147, 234–242 (2012). [Google Scholar]
- Yang G., Bruford M. W., Wei F. & Zhou K. Conservation options for the baiji: time for realism? Conserv. Biol. 20, 620–622 (2006). [DOI] [PubMed] [Google Scholar]
- Zhou K. On the conservation of the baiji, Lipotes vexillifer. J. Nanjing Normal Coll. (Nat. Sci.) 4, 71–74 (1982). [Google Scholar]
- Zhang X. et al. The Yangtze River dolphin or baiji (Lipotes vexillifer): population status and conservation issues in the Yangtze River, China. Aquatic Conserv: Mar. Freshw. Ecosyst. 13, 51–64 (2003). [Google Scholar]
- Zhou K., Sun J., Gao A. & Würsig B. Baiji (Lipotes vexillifer) in the lower Yangtze River: movements, numbers, threats and conservation needs. Aquatic Mammals 24, 123–132 (1998). [Google Scholar]
- Parra G. J. Resource partitioning in sympatric delphinids: space use and habitat preferences of Australian snubfin and Indo-Pacific humpback dolphins. J. Anim. Ecol. 75, 826–874 (2006). [DOI] [PubMed] [Google Scholar]
- White G. C. & Burnham K. P. Program MARK: Survival estimation from populations of marked animals. Bird Study 46 Suppl. 120–138 (1999). [Google Scholar]
- Cooch, E. & White, G. eds. Program MARK: A Gentle Introduction. 11th edition (2012). http://www.phidot.org/software/mark/docs/book/.(18/11/2012). [Google Scholar]
- Schwarz C. J. & Arnason A. N. Jolly-Seber models in MARK. Program MARK: A Gentle Introduction. Fifth edition (2012). http://www.phidot.org/software/mark/docs/book/.(18/11/2012). [Google Scholar]
- Burnham K. P. & Anderson D. R. Model Selection and Inference: A Practical Information-theoretic Approach. 2nd edition. (Springer-Verlag, New York, 1998). [Google Scholar]
- Lebreton J. D., Burnham K. P., Clobert J. & Anderson D. R. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 62, 67–118 (1992). [Google Scholar]
- Parra G. J., Corkeron P. J. & Marsh H. Population sizes, site fidelity and residence patterns of Australian snubfin and Indo-Pacific humpback dolphins: Implications for conservation. Biol. Conserv. 129, 167–180 (2006). [Google Scholar]
- Wilson B., Hammond P. S. & Thompson P. M. Estimating size and assessing trends in a coastal bottlenose dolphin population. Ecol. Appl. 9, 288–300 (1999). [Google Scholar]
- Seaman D. E. & Powell R. A. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 77, 2075–2085 (1996). [Google Scholar]
- Worton B. J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168 (1989). [Google Scholar]
- Rodgers A. R., Carr A. P., Beyer H. L., Smith L. & Kie J. G. HRT: Home Range Tools for ArcGIS. Version 1.1. (2007). http://flash.lakeheadu.ca/~arodgers/hre/HRT%20Users%20Manual%20Draft%20August%2010%202011.pdf/. (20/10/2013). [Google Scholar]
- Seaman D. E. et al. Effects of sample size on kernel home range estimates. J. Wildl. Manage. 63, 739–747 (1999). [Google Scholar]
- Van Waerebeek K. & Perrin W. F. Conservation status of the Atlantic humpback dolphin, a compromised future? (Paper CMS/ScC14/Doc.6, 14th Meeting of the CMS Scientific Council, Bonn, Germany, 2007). [Google Scholar]
- Keith M., Peddemors V. M., Bester M. N. & Ferguson J. M. H. Population characteristics of Indo-pacific humpback dolphins at Richards Bay, South Africa: implications for incidental capture in shark nets. South African J. Wildl. Res. 32, 153–162 (2002). [Google Scholar]
- Guissamulo A. & Cockcroft V. Ecology and population estimates of Indo-Pacific humpback dolphins (Sousa chinensis) in Maputo Bay, Mozambique. Aquatic Mammals 30, 94–102 (2004). [Google Scholar]
- Guissamulo A. T., & Cockcroft V. G. Dolphin and dugong occurrence and distribution and fisheries inter-actions in Maputo and Bazaruto Bays, Mozambique (SC/49/SM24). (Paper presented at the 49th Meeting of the International Whaling Commission, September 1998, London, 1997). [Google Scholar]
- Razafindrakoto Y., Andrianarivelo N. & Rosenbaum H. C. Sightings, catches, and other records of Indo-Pacific humpback dolphins in the coastal waters of Madagascar. Aquatic Mammals 30, 103–110 (2004). [Google Scholar]
- Stensland E., Carlén I., Särnblad A., Bignert A. & Berggren P. Population size, distribution, and behavior of Indo-Pacific bottlenose (Tursiops aduncus) and humpback (Sousa chinensis) dolphins off the south coast of Zanzibar. Mar. Mammal Sci. 22, 667–682 (2006). [Google Scholar]
- Meyler S. V., Felix H. & Crouthers R. Abundance and distribution of Indo-Pacific humpback dolphins (Sousa chinensis) in the Shimoni Archipelago, Kenya. Western Indian Ocean J. Mar. Sci. 10, 201–209 (2011). [Google Scholar]
- Preen A. Distribution, abundance and conservation status of dugongs and dolphins in the southern and western Arabian Gulf. Biol. Conserv. 118, 205–218 (2004). [Google Scholar]
- Baldwin R. M., Collins M., Van Waerebeek K. & Minton G. The Indo-Pacific humpback dolphin of the Arabian Region: A status review. Aquatic Mammals 30, 111–124 (2004). [Google Scholar]
- Robineau D. & Fiquet P. The cetacea of the Jubail Marine Wildlife Sanctuary, Saudi Arabia. In: A Marine Wildlife Sanctuary for the Arabian Gulf. Environmental Reasearch and Conservation Following the 1991 Gulf War Oil Spill. NCWCD, Riyadh and Senckenberg Institute, Frankfurt., 438–458 (1996).
- Kiani M. S., Gore M. & Siddiqui P. J. Photo-identification mark-recapture studies on Indus delta Indo-Pacific humpback dolphins of Pakistan. (Indian Ocean Cetacean Symposium, 2009).
- Sutaria D. & Jefferson T. A. Records of Indo-Pacific humpback dolphins (Sousa chinensis, Osbeck, 1765) along the coasts of India and Sri Lanka: An overview. Aquatic Mammals. 30, 125–136 (2004). [Google Scholar]
- Smith B. D., Rosenbaum H. C. & Mansur R. M. Investigation on the population identity of Indo-Pacific humpback dolphins (Sousa chinensis) in the northern Bay of Bengal, Bangladesh and implications for population level conservation and taxonomy of the species. (Interim report to the International Whaling Commission, Wildlife Conservation Society., 2012).
- Jaroensutasinee M., Jutapruet S. & Jaroensutasinee K. Population size of Indo-Pacific humpback dolphins (Sousa chinensis) at Khanom, Thailand. Walailak J. Sci. Tech. 7, 115–126 (2010). [Google Scholar]
- Chen B. Y., Zheng D. M., Yang G., Xu X. R. & Zhou K. Y. Distribution and conservation of the Indo-Pacific humpback dolphin in China. Integr. Zool. 4, 240–247 (2009). [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Yang S. C., Hung S. K. & Jefferson T. A. Distribution, abundance and conservation status of the eastern Taiwan Strait population of Indo-Pacific humpback dolphins, Sousa chinensis. Mammalia 71, 157–165 (2007). [Google Scholar]
- Corkeron P. J., Morissette N. M., Porter L. & Marsh H. Distribution and status of humpback dolphins, Sousa chinensis in Australian waters. Asian Mar. Biol. 14, 49–59 (1997). [Google Scholar]
- Cagnazzi D., Parra G. J., Westley S. & Harrison P. L. At the heart of the industrial boom: Australian snubfin dolphins in the Capricorn Coast, Queensland, need urgent conservation action. PLoS ONE 8, e56729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Bejder L., Cagnazzi D., Parra G. J. & Allen S. The North West Cape, Western Australia: A hotspot for Indo-Pacific humpback dolphins Sousa chinensis. Pacific Conserv. Biol. 18, 240–246 (2012). [Google Scholar]