Abstract
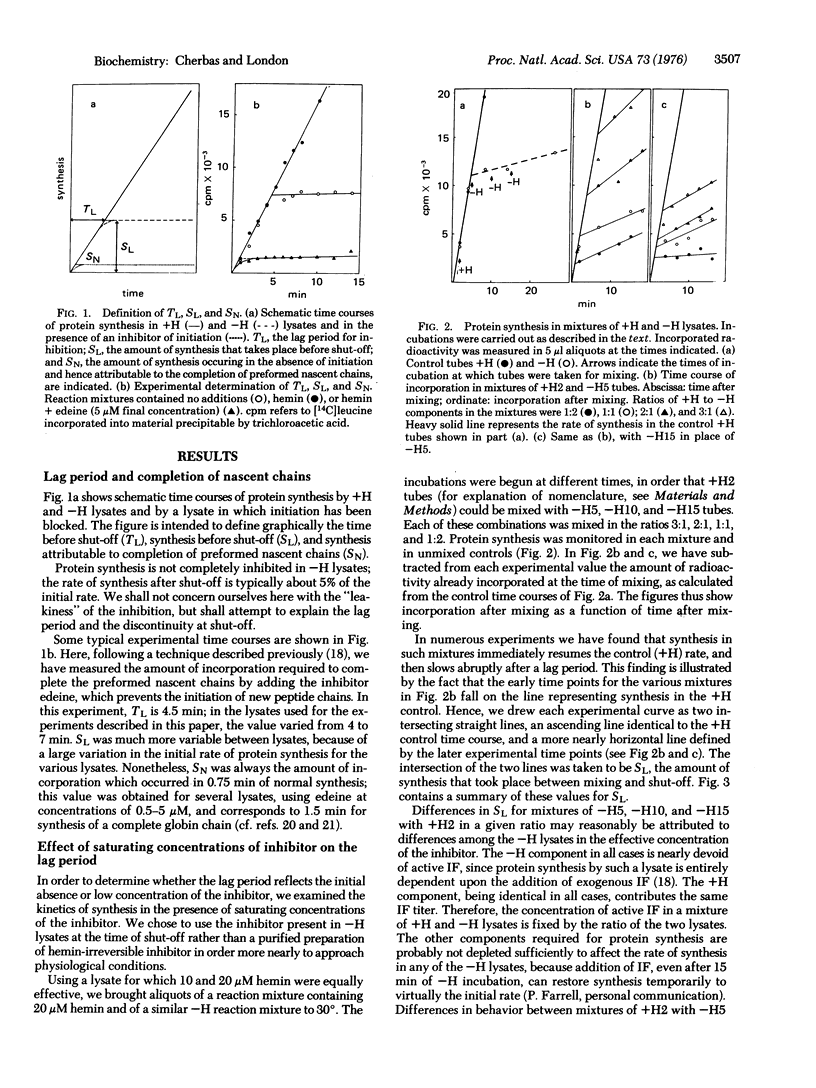
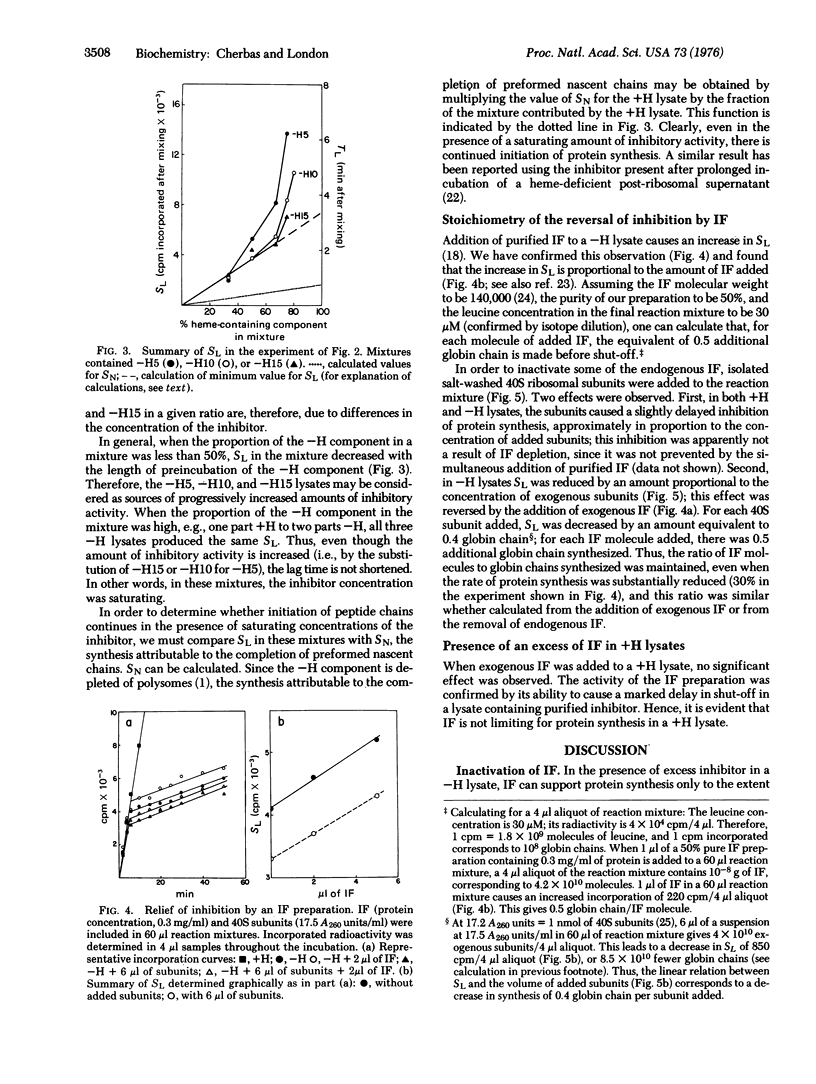
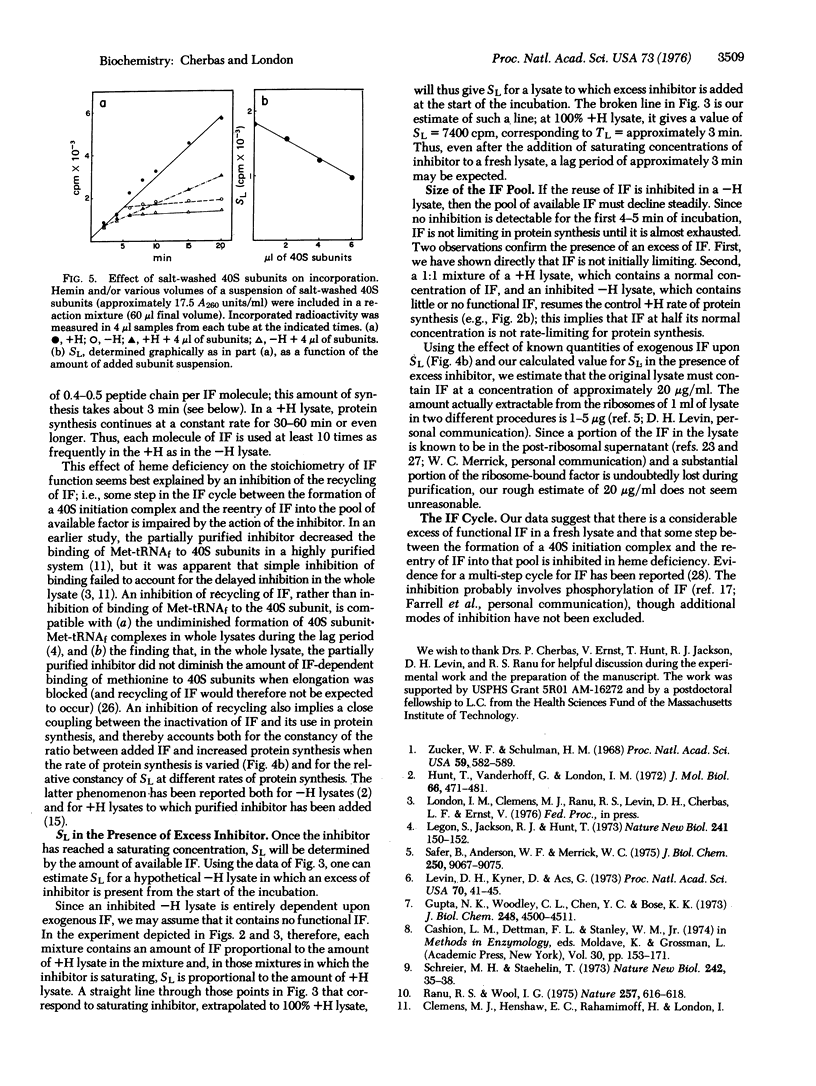
In the absence of added hemin, protein synthesis in a rabbit reticulocyte lysate declines abruptly (shuts off) after about 5 min at 30 degrees. In these studies we have examined the basis for the lag period preceding shut-off. The initiation factor that binds Met-tRNAf, previously shown to be rate-limiting in inhibited, heme-deficient lysates, is found to be used stoichiometrically in the presence of excess inhibitor. We suggest that a principal effect of the inhibitor is to impair the recycling of the Met-tRNAf-binding factor; the lag period is attributable largely to the presence of a pool of excess Met-tRNAf-binding factor, which, once used in initiation, cannot be recycled because of the action of the inhibitor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkow K., Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: effect of a translational repressor on Met-tRNAf binding to the small ribosomal subunit and its relation to the activity and alailability of an initiation factor. Biochim Biophys Acta. 1973 Oct 26;324(3):397–409. doi: 10.1016/0005-2787(73)90284-0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Fuhr J. E., London I. M., Grayzel A. I. A factor promoting the initiation of globin synthesis in a rabbit reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):129–134. doi: 10.1073/pnas.63.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Gross M. Reversal of the inhibitory action of the hemin-controlled translational repressor by a post-ribosomal supernatant factor from rabbit reticulocyte lysate. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1507–1515. doi: 10.1016/0006-291x(75)90197-7. [DOI] [PubMed] [Google Scholar]
- Gupta N. K., Woodley C. L., Chen Y. C., Bose K. K. Protein synthesis in rabbit reticulocytes. Assays, purification, and properties of different ribosomal factors and their roles in peptide chain initiation. J Biol Chem. 1973 Jun 25;248(12):4500–4511. [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- Hunt T., Hunter T., Munro A. Control of haemoglobin synthesis: rate of translation of the messenger RNA for the alpha and beta chains. J Mol Biol. 1969 Jul 14;43(1):123–133. doi: 10.1016/0022-2836(69)90083-7. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Formation of a mammalian initiation complex with reovirus messenger RNA, methionyl-tRNA F , and ribosomal subunits. Proc Natl Acad Sci U S A. 1972 May;69(5):1234–1238. doi: 10.1073/pnas.69.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Ranu R. S., Ernst V., Fifer M. A., London L. M. Association of a cyclic AMP-dependent protein kinase with a purified translational inhibitor isolated from hemin-deficient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4849–4853. doi: 10.1073/pnas.72.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Jacobsen M. Regulation of hemoglobin synthesis. Equal rates of translation and termination of - and -globin chains. J Biol Chem. 1972 Jun 10;247(11):3622–3629. [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Rabinovitz M. Evidence for an inhibitor in the control of globin synthesis by hemin in a reticulocyte lysate. Biochem Biophys Res Commun. 1969 Apr 10;35(1):79–85. doi: 10.1016/0006-291x(69)90485-9. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., Levin D. H., Delaunay J., Ernst V., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: characteristics of inhibition of protein synthesis by a translational inhibitor from heme-deficient lysates and its relationship to the initiation factor which binds Met-tRNAf. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2720–2724. doi: 10.1073/pnas.73.8.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., Wool I. G. Discrimination between eukaryotic and prokaryotic, and formylated and non-formylated, initiator tRNAs by eukaryotic initiation factor EIF-3. Nature. 1975 Oct 16;257(5527):616–618. doi: 10.1038/257616a0. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., Wool I. G. Preparation and characterization of eukaryotic initiation factor EIF-3. Formation of binary (EIF-3-Met-tRNAf) and ternary (EIF-3-Met-tRNAf-GTP) complexes. J Biol Chem. 1976 Apr 10;251(7):1926–1935. [PubMed] [Google Scholar]
- Safer B., Anderson W. F., Merrick W. C. Purification and physical properties of homogeneous initiation factor MP from rabbit reticulocytes. J Biol Chem. 1975 Dec 10;250(23):9067–9075. [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]


