Abstract
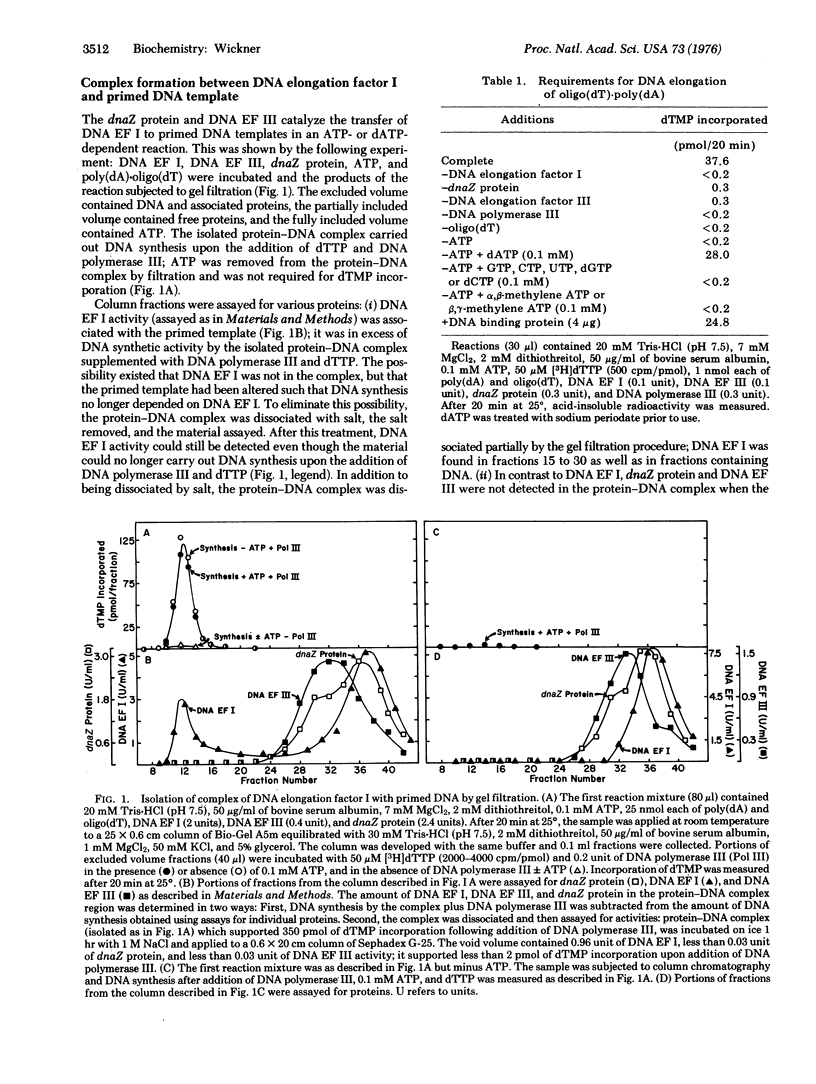
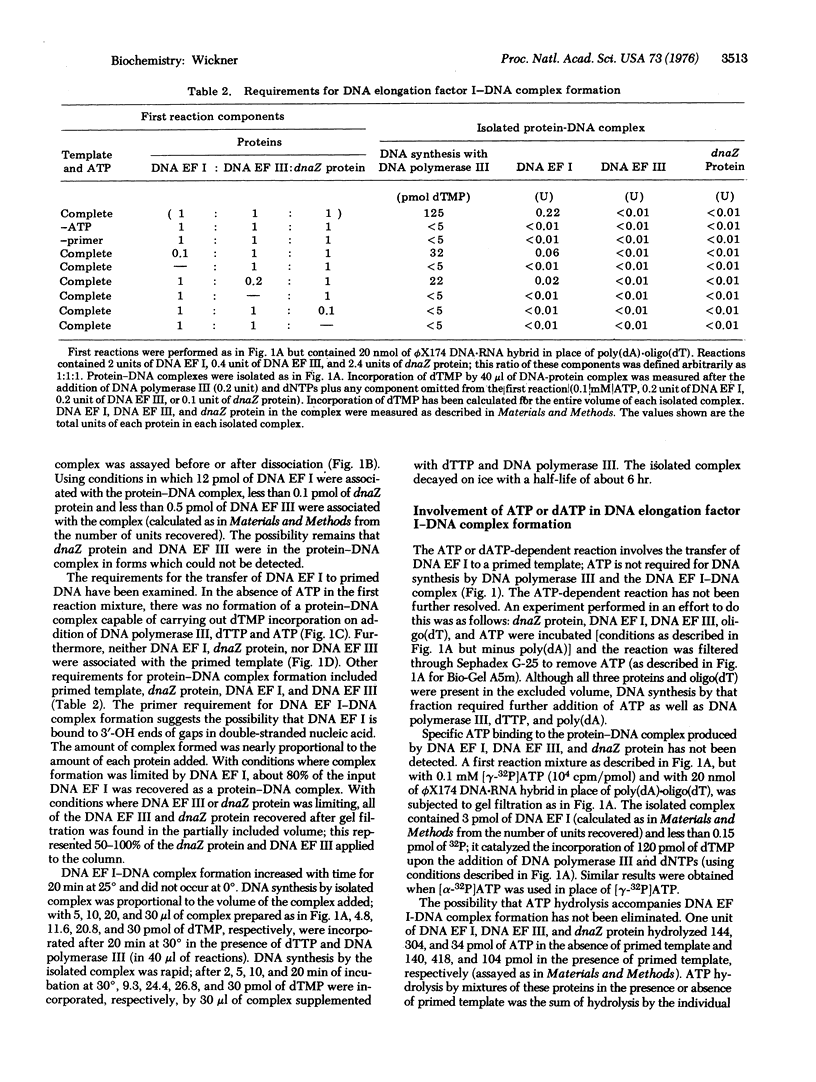
Elongation of a primed single-stranded DNA template catalyzed by E. coli DNA polymerase III (DNA nucleotidyltransferase, deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, EC 2.7.7.7) requires dnaZ protein and two other protein factors, DNA elongation factors I and III. The reaction occurs by the following mechanism: (i) dnaZ protein and DNA elongation factor III together catalyze the transfer of DNA elongation factor I to a primed DNA template. This transfer reaction requires ATP or dATP in addition to dnaZ protein, DNA elongation factors I and III, and primed template; it does not require DNA polymerase III. (ii) DNA polymerase III binds to the complex of DNA elongation factor I with primed template; it does not bind to primed template which is not complexed with DNA elongation factor I. This binding reaction proceeds in the absence of ATP or dATP as cofactor, dnaZ protein, and DNA elongation factor III and without additional DNA elongation factor I. (iii) The complex of DNA polymerase III, DNA elongation factor I, and primed template catalyzes DNA synthesis upon the addition of dNTPs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Wickner S. Involvement of two protein factors and ATP in in vitro DNA synthesis catalyzed by DNA polymerase 3 of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jan;71(1):6–10. doi: 10.1073/pnas.71.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Wickner S., Wright M. Studies on in vitro DNA synthesis. II. Isolation of a protein which stimulates deoxynucleotide incorporation catalyzed by DNA polymerase of E. coli. Biochem Biophys Res Commun. 1973 Mar 17;51(2):257–267. doi: 10.1016/0006-291x(73)91251-5. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Low R. L., Rashbaum S. A., Cozzarelli N. R. Purification and characterization of DNA polymerase III from Bacillus subtilis. J Biol Chem. 1976 Mar 10;251(5):1311–1325. [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo A. Replication of phiA and phiX174 in Escherichia coli mutants thermosensitive in DNA synthesis. Mol Gen Genet. 1975 Sep 8;139(4):285–291. doi: 10.1007/BF00267968. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Kornberg A. A holoenzyme form of deoxyribonucleic acid polymerase III. Isolation and properties. J Biol Chem. 1974 Oct 10;249(19):6244–6249. [PubMed] [Google Scholar]


