Abstract
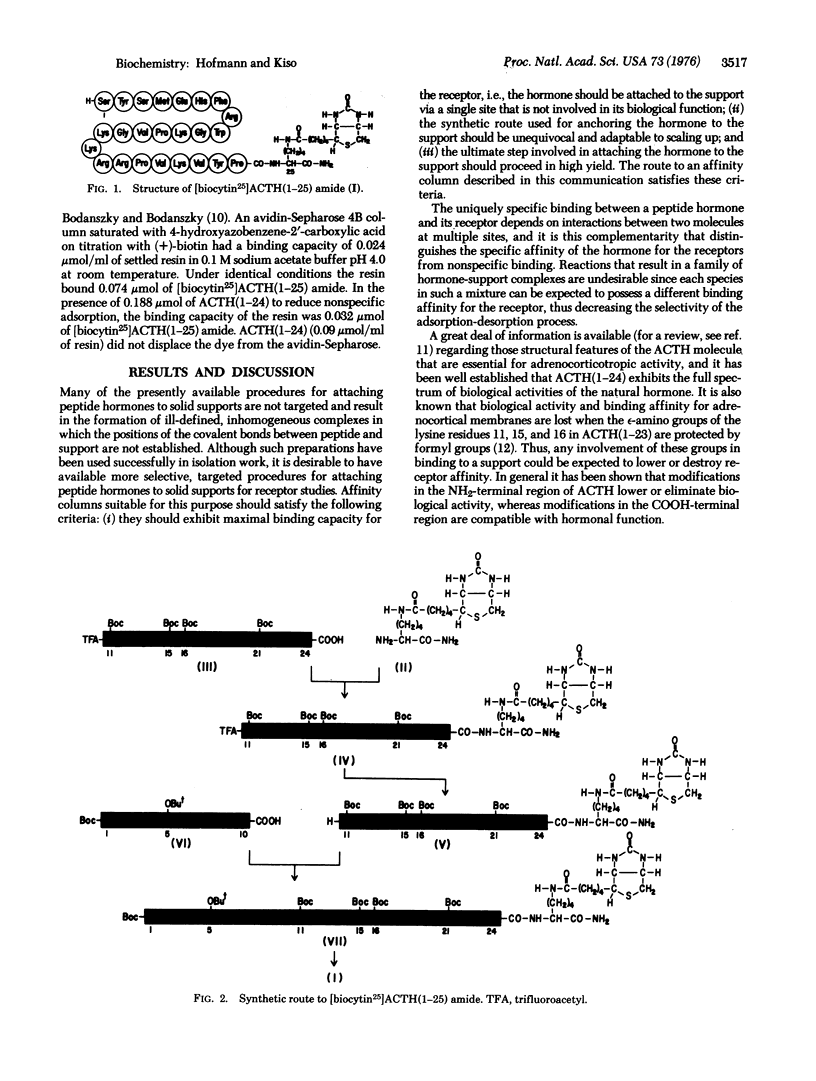
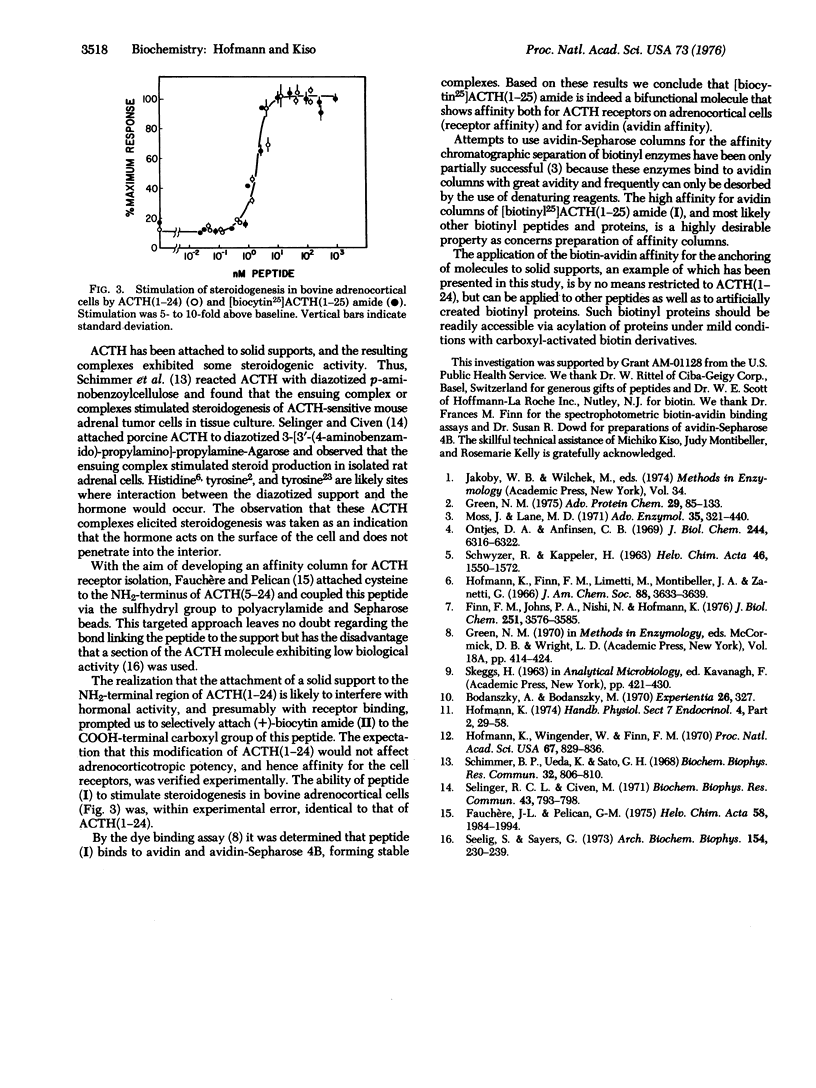
A novel approach to affinity columns is described that is based on the high avidity of biotinylated molecules for avidin attached to solid supports. Biocytin amide [Nepsilon-(+)-biotinyllysine amide] was coupled to the COOH-terminal carboxyl group of corticotropin(1-24) [ACTH(1-24)] to form [biocytin25]ACTH(1-25) amide. The ability of this peptide to stimulate steroidogenesis of bovine adrenocortical cells was within experimental error identical to that of ACTH(1-24). The peptide also binds to avidin and avidin-Sepharose, forming stable complexes. Thus, with biotin as the anchor, the adrenocorticotropically active segment of the ACTH molecule was attached to a solid support in a targeted manner. The general applicability of this principle for the attachment of peptides and proteins to solid supports is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodanszky A., Bodanszky M. Sepharose-avidin column for the binding of biotin or biotin-containing peptides. Experientia. 1970 Mar 15;26(3):327–327. doi: 10.1007/BF01900128. [DOI] [PubMed] [Google Scholar]
- Civen M., Selinger R. C. ACTH diazotized to agarose: effects on isolated adrenal cells. Biochem Biophys Res Commun. 1971 May 21;43(4):793–799. doi: 10.1016/0006-291x(71)90686-3. [DOI] [PubMed] [Google Scholar]
- Fauchère J. L., Pelican G. M. Chemisch-spezifische Kopplung von Adrenocorticotropin- und Angiotensin-II-Derivaten an Polymerträger für die Affinitätschromatographie. Helv Chim Acta. 1975 Nov 5;58(7):1984–1994. doi: 10.1002/hlca.19750580713. [DOI] [PubMed] [Google Scholar]
- Finn F. M., Johns P. A., Nishi N., Hofmann K. Differential response to adrenocorticotropic hormone analogs of bovine adrenal plasma membranes and cells. J Biol Chem. 1976 Jun 25;251(12):3576–3585. [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Finn F. M., Limetti M., Montibeller J., Zanetti G. Studies on polypeptides. XXXIV. Enzymic properties of partially synthetic De(16-20)- and De(15-20)-ribonucleases S'1-3. J Am Chem Soc. 1966 Aug 5;88(15):3633–3639. doi: 10.1021/ja00967a030. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Wingender W., Finn F. M. Correlation of adrenocorticotropic activity of ACTH analogs with degree of binding to an adrenal cortical particulate preparation. Proc Natl Acad Sci U S A. 1970 Oct;67(2):829–836. doi: 10.1073/pnas.67.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Lane M. D. The biotin-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Ontjes D. A., Anfinsen C. B. Synthetic studies of structure-function relationships in staphylococcal nuclease. Synthetic analogues of fragment P2. J Biol Chem. 1969 Dec 10;244(23):6316–6322. [PubMed] [Google Scholar]
- Schimmer B. P., Ueda K., Sato G. H. Site of action of adrenocorticotropic hormone (ACTH) in adrenal cell cultures. Biochem Biophys Res Commun. 1968 Sep 6;32(5):806–810. doi: 10.1016/0006-291x(68)90312-4. [DOI] [PubMed] [Google Scholar]
- Seelig S., Sayers G. Isolated adrenal cortex cells: ACTH agonists, partial agonists, antagonists; cyclic AMP and corticosterone production. Arch Biochem Biophys. 1973 Jan;154(1):230–239. doi: 10.1016/0003-9861(73)90053-2. [DOI] [PubMed] [Google Scholar]


