Abstract
Background
Staphylococcus aureus is associated with chronic mastitis in cattle, and disease manifestation is usually refractory to antibiotic therapy. Biofilm production is a key element of S. aureus pathogenesis and may contribute to the treatment failure that is consistently reported by veterinarians. Minas Gerais State is the largest milk-producing state in Brazil, and the characterization of bacterial isolates is an important aspect of disease control for dairy farmers. Here, we investigated the potential of S. aureus isolated from bovine mastitis to produce slime and biofilm in a skim-milk medium and classified the isolates according to their agr type.
Results
Slime was detected using the Congo Red agar (CRA) test in 35.18% (19/54) of the strains; however, 87.04% (47/54) of the strains were considered biofilm-positive based on crystal violet staining. Compared to TSB supplemented with 0.25% glucose, skim milk significantly increased the production of biofilm, but this effect was only observed in slime-producing strains. The bacteria belonged to agr groups I (12/54), II (34/54), III (6/54), and IV (2/54), and bacteria in agr group III were found to be stronger biofilm producers than those in groups I and II. Again, milk had a significant influence only on slime-positive agr I and II isolates, revealing an association between milk and slime.
Conclusions
The present study demonstrated that skim-milk medium and slime production are two factors that together influence biofilm formation by bovine strains of S. aureus. A predominance of bacteria belonging to agr group II was observed, and bacteria from agr group III showed the highest proportion of biofilm producers. The majority of bacteria characterized in this study formed biofilm in milk, which suggests that biofilm formation has an important role in the virulence of S. aureus isolated from bovine intramammary infections.
Keywords: Staphylococcus aureus, Biofilm, Slime, Skim-milk medium; agr type
Background
Staphylococcus aureus is a pathogen that frequently causes mastitis in bovine herds worldwide. In Brazil, which is considered by the Food and Agriculture Organization (FAO) as the fourth largest milk producer in the world [1], S. aureus infections are also a major concern with respect to the welfare of dairy cattle. The presence of this pathogen in a dairy herd was first reported in Brazil in 1978 by Muller et al. [2]; since then, the pathogen has been found in herds distributed throughout the country [3,4]. In Minas Gerais State, over 220,000 farms are engaged in milk production, indicating the supremacy of this region in the milk production sector [5]. However, subclinical mastitis accounts for a high percentage of the disease manifestations in Minas Gerais [6], and epidemiological studies have shown that the prevalence of S. aureus can reach nearly 50%.
Veterinarians describe intramammary infections caused by S. aureus as a subclinical manifestation that usually evolves into a chronic state [7]. One possible reason for the persistence of the pathogen in the udder is the formation of biofilms, which are bacterial communities attached to surfaces that are embedded in matrices mainly composed of polysaccharides [8]. The correlation between biofilms and the persistence of S. aureus of bovine origin has been previously described [9]. However, Simojoki et al. [10] evaluated nearly 200 coagulase-negative staphylococci associated with bovine mastitis and found that neither biofilm nor slime production are correlated with persistent infection. Another complaint by veterinarians is the low efficacy of antimicrobial treatments for infections caused by S. aureus [7], although it has been reported that inhibiting bacterial biofilm production reduces bacterial resistance in in vivo assays [11]. Recently, Beenken et al. [12] demonstrated that reduced biofilm production by S. aureus in a murine model was correlated with increased susceptibility to daptomycin.
The production of biofilm depends on the ability of bacteria to attach to abiotic/biotic surfaces, proliferate, and produce an extracellular matrix, which is mainly formed by polysaccharide intercellular adhesion (PIA) in S. aureus [13]. PIA is the major component of the extra-polysaccharide matrix, also known as slime, and is encoded by the ica operon (icaABCD). Deletion of this operon results in impairment of biofilm formation and the production of PIA in vitro [14]. An indirect relationship between the ica locus and the accessory gene regulator (agr) locus, a quorum-sensing system that regulates the expression of several virulence traits in S. aureus, has been found. Some reports have shown that low agr activity is needed to support biofilm development but that the dispersion of bacterial cells relies on the secretion of proteases, which is stimulated by agr activation [15,16].
The agr system can be used to divide S. aureus into four groups. Buzzola et al. [17] found a high prevalence (88%) of agr group I in bovine mastitis isolates from Argentina, and this group was also prevalent (69%) in the studies of Gilot and van Leeuwen [18], which describe the analysis of isolates from different countries. In contrast, 81% of the field strains collected by Melchior et al. [19] in the Netherlands belonged to agr type II, while 9% belonged to agr I. In this last report, the authors showed that agr II strains produce more biofilm in milk serum than agr I strains and suggested clinical implications for these observations, such as the adaptation of agr II to the extracellular niche.
In this study, we evaluated the ability of Brazilian isolates to produce slime and biofilm and classified them according to their agr type. Our results show that milk and slime production are two factors that have a positive effect on biofilm production by bovine isolates of S. aureus, regardless of their agr type.
Results
Fifty-four bovine isolates of S. aureus were screened for their ability to produce slime and biofilm in two different media. Streptococcus agalactiae was used as a negative control for slime and biofilm production due to the phenotype of its colonies on Congo Red agar (CRA; red-smooth colonies) and because it showed the same optical density (OD630) value as the medium without bacteria in microplate assays. Nineteen (35.18%) and thirty-five (64.82%) isolates were classified as slime producers and non-slime producers, respectively, according to the phenotype of the colonies on CRA. The majority of the isolates (47/54) produced biofilm in TSBg (Trypticase soy broth containing 0.25% glucose), a medium that is known to stimulate biofilm production [20]. Skim milk also promoted the production of biofilm by 77.7% (42/54) of the isolates. However, the average production of biofilm by the 54 isolates in TSBg was lower when compared with their average biofilm production in skim-milk medium (P < 0.001) (Figure 1A). The difference was also significant (P < 0.001) when skim milk was used as the growth medium for the slime-producing strains (Figure 1B). Conversely, milk did not have a positive effect on the non-slime producers.
Figure 1.
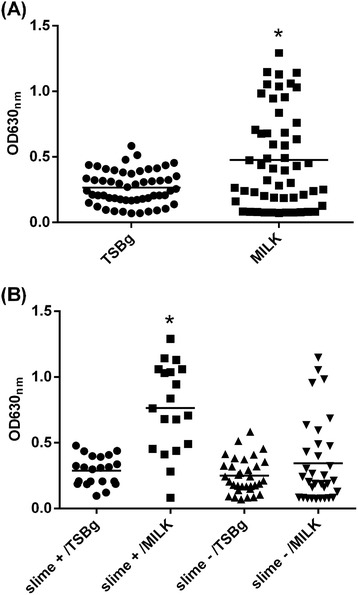
Skim milk and slime increase the production of biofilm by bovine Staphylococcus aureus strains. Bacteria were grown in polystyrene microtiter plates in TSB supplemented with 0.25% glucose or skim-milk medium, and biofilm production was visualized by crystal violet staining. Isolates cultivated in skim-milk medium (A) and isolates with a slime-positive phenotype grown in skim-milk medium (B) showed increases in biofilm production. The horizontal bar represents the average biofilm production. The experiments were performed in triplicate. * P < 0.001.
According to the agr type, the strains were classified as follows: agr I (12/54), agr II (34/54), agr III (7/54), and agr IV (2/54). Biofilm production by agr group I did not differ significantly regardless of the medium used (TSBg or milk) (P = 0.557) (Figure 2A). Nevertheless, skim milk promoted biofilm production by agr group II.
Figure 2.
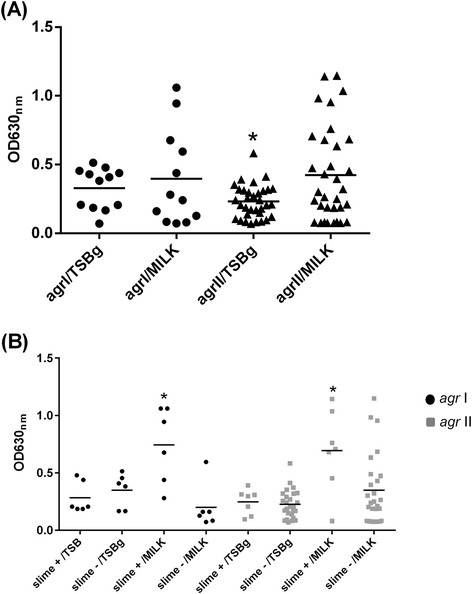
Biofilm formation by agr type I and II Staphylococcus aureus strains. Bacteria grown in skim-milk medium (A) and slime-positive strains cultivated in skim-milk medium (B) showed increases in biofilm production. The crystal violet staining method was used to detect biofilm production in polystyrene microplates. The experiments were performed in triplicate. * P < 0.05.
An effect of the culture medium on the amount of biofilm formed by the slime-positive agr I strains was observed (P = 0.028), suggesting that slime also exerted an influence on the production of biofilm (Figure 2B). The same result was not observed in strains that were classified as non-slime producers. The results for agr group II were similar; again, only the slime-positive group showed an increase in biofilm production in milk (p = 0.015). No significant difference between the slime-positive or slime-negative strains was detected in TSBg (p = 0.699). There was a significant difference in biofilm production between slime-positives from agr I and agr II groups when grown in skim-milk medium (agr I = 0.6, agr II = 0.69) compared to growth in TSBg (agr I = 0.26, agr II = 0.24).
Despite the small number of agr III isolates, milk had a positive effect (P < 0.05) on biofilm production (Figure 3) regardless of slime production. It should be noted that the average biofilm production of agr III (0.89) was higher than that of the other groups (0.6 and 0.69) in milk. All isolates (54/54) tested positive for the presence of the icaAD genes (data not shown).
Figure 3.
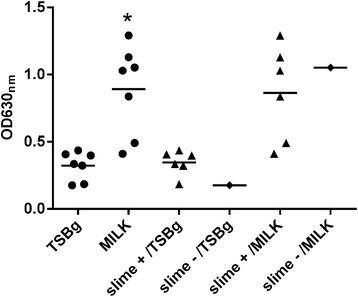
Biofilm production by agr group III Staphylococcus aureus strains is significantly increased in skim-milk medium. Bacteria were grown in polystyrene microtiter plates in TSB supplemented with 0.25% glucose (TSBg) or skim-milk medium (Milk). The crystal violet staining method was used to detect biofilm production in the polystyrene microplates. The experiments were performed in triplicate. * P < 0.05.
Discussion
In this study, the production of biofilm and slime by S. aureus isolated from mastitic cows was investigated. Although other authors have used biofilm and slime synonymously [21,22], slime is in fact a component of biofilm. “Slime formation” was once the term used for biofilm formation [23]; however, if we revisit the review published by Hall-Stoodley et al. [24], slime is defined as the extracellular polymeric substance, also known as EPS, that is mainly formed by PIA in S. epidermidis and S. aureus, although DNA and proteins can also be found in this material. Biofilm was defined by Costerton et al. [25] as a population of cells that is attached to a surface and enclosed by a matrix. In contrast, slime is the protective matrix that surrounds this population and has a fundamental role in the biofilm structure, as it maintains adhesion between the cells and acts as a protective barrier against the host immune system and biocides [24].
Among the S. aureus isolates tested in the present study, a minority (19/54) were found to be slime-positive, even though the majority (47/54) were considered to be biofilm producer. Only two slime-positive isolates (3885 and 688) did not produce biofilm in TSBg, yet biofilm was observed when they were grown in skim milk. This low correlation between biofilm and slime may be due to limitations of the CRA method, a qualitative test used to categorize bacterial strains as slime producers or non-slime producers based on the appearance of the colonies. A positive result is indicated by black colonies. The black color supposedly manifests due to a greater association between the thick layer of exopolysaccharides and the Congo Red stain; this association is decreased in slime-negative strains, resulting in a lighter color (red- or pink-Bordeaux). It is known that the thickness of the polysaccharide layer that surrounds the bacterial cell wall differs among S. aureus strains [26,27]. Thus, it is plausible that the CRA test lacks the appropriate sensitivity needed to discriminate strains that form thinner extracellular layers from those that do not produce the layer, instead placing both phenotypes in the same category (slime-negative). If we consider that a thinner layer still allows cell adhesion and promotes biofilm formation, the results from the CRA test would be similar those obtained in the crystal violet staining assay (87.04%).
The slime-producing abilities of bovine isolates of S. aureus have been reported to range from 11.42% to 91.42% [22,28], and differences in the criteria used for the interpretation of the CRA test (color, morphology or both) could explain this discrepancy [28]. A low correlation between the CRA test and the crystal violet assay was found by some authors, who attributed these findings to differences in the culture conditions used [29,30].
Biofilm production was increased in a skim-milk medium. Lactose and milk whey also contribute to capsule polysaccharide and biofilm formation in S. aureus [19,31]. Recently, Varhimo et al. [32] reported that milk components stimulated biofilm formation in Streptococcus uberis. When added at a low concentration into TSB medium, milk or lactose was also found to upregulate ica operon genes in two strains of S. aureus associated with bovine mastitis [33]; in one strain, milk also promoted an increase in the transcription of surface proteins such as Bap, the biofilm-associated protein. These studies suggest that bovine isolates of S. aureus adapt to the milieu found in the udder, with milk influencing biofilm production and hence promoting bacterial survival.
Milk had a positive effect on the agr group II and III strains but not on the agr I strains. It was previously shown that strains belonging to agr group I have an increased ability to invade and persist in MAC-T cells [17,34]; unlike other agr groups that might be more adapted for survival outside mammary gland cells, biofilm production might not be an important factor for the persistence of agr I bacteria in the udder. Strong biofilm producers were observed among other strains growing in extracellular niches, such as those isolated from catheter-associated infections, and found to belong to agr genotype II [15]. In our study, the few isolates from agr group III were considered to be strong biofilm producers in milk. However, this analysis should be extended to more isolates to confirm this pattern. Indeed, agr group III is always less prevalent in S. aureus isolated from bovine mastitis compared to agr groups I and II [17,19,18], and this may explain why agr group III is less studied. Similar to agr II, agr III isolates are less likely to be internalized by MAC-T cells [17]. This feature, combined with the high biofilm production observed in the present study, can suggest a better adaptation of these isolates to the extracellular milieu.
Biofilm formation is a protective mechanism used by bacteria to avoid antimicrobials, and this could contribute to mastitis treatment failure. Field isolates of S. aureus found to be susceptible to several antibiotics based on CLSI testing methods were considered highly resistant when grown in biofilms [35]. According to Raza et al. [36], the EPS secreted by the bacteria acts as a barrier that may play a role in this resistance, preventing the adsorption and penetration of antimicrobials. Alternatively, the EPS matrix could neutralize or bind these compounds, promoting their dilution to subinhibitory concentrations before they reach the cells [24]. Biofilms are composed of dormant and active cell subpopulations, and this difference in bacterial physiology can also influence the efficacy of antibiotics [37] and hence the outcome of mastitis therapy.
An interesting observation was the significant increase in biofilm formation by slime-producing bacteria in skim-milk medium, indicating a potential association between slime and milk. If we consider the invasive potential of agr I isolates [17], the presence of slime and milk could promote a change in the bacterial lifestyle that increases the chances of survival in the extracellular medium. Slime-producing strains of agr II also showed improved biofilm formation following growth in skim-milk medium. As another role of the EPS matrix is to increase adherence to the cell surface [38], this could stimulate the formation of biofilm by bovine isolates and, consequently, bacterial resistance to antimicrobials.
In this study, we showed that 87.04% (47/54) of the bovine isolates that were analyzed produced biofilm, which was well above the values that were found in herds from the USA (68.57%) [29], India (29.41%) [22], Portugal (37.5%) [30], and Poland (57.6%) [39]. However, unlike the study in Poland [39], which analyzed a population representing different genotypes with diverse abilities to form biofilms, the genetic background of the strains was not assessed in our study, which may have influenced the outcome. The ability of some genotypes to produce more biofilm than others has been demonstrated elsewhere [40]. Our results showed that all of the isolates carried the icaA and icaD genes, which was also reported for the S. aureus isolates from American herds [29]. Thus, the presence of these two genes in our isolates and in the American isolates could explain why our values were similar to those in the American study. In contrast, only 35.29% (36/102) of the strains harbored both genes in the study carried out in India [22]. Because the ica operon is responsible for the production of PIA, it is expected that ica-positive isolates are more likely to produce biofilm. The expression of these genes should be assessed in future studies to evaluate their actual contribution to biofilm production. Coexpression of the icaA and icaD genes is related to high activity of N-acetylglucosaminyltransferase, which is involved in PIA biosynthesis in Staphylococcus epidermidis [41]. In coagulase-negative staphylococci, there was concordance between the expression of icaAD and biofilm production [42].
Conclusions
The present study demonstrated that slime-producing strains can produce more biofilm when grown in a skim-milk medium, suggesting an association between milk and slime. A predominance of bacteria belonging to agr group II was observed, and bacteria from agr group III were the best biofilm producers. All isolates were icaAD-positive, and most of them formed biofilm in milk. Considering the large number of biofilm-producing bacteria found in this work, it is suggested that biofilm production represents a challenge in the control of bovine mastitis in Minas Gerais.
Methods
Bacterial isolates
The 54 S. aureus isolates used in this study were kindly provided by Embrapa Dairy Cattle, Juiz de Fora, Minas Gerais. They were collected between 1996 and 2011 from cows with subclinical mastitis belonging to dairy herds located in Minas Gerais State. S. aureus was identified by microbiological methods and biochemical tests [43]. The reference strains Staphylococcus epidermidis NRS101, S. aureus NRS133 and S. aureus NRS155 were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) and were used as positive controls in the biofilm assays (NRS101) or agr typing (NRS133 and NRS155). Streptococcus agalactiae, which was isolated from a mastitic cow, served as the negative control. The bacteria were grown in brain heart infusion broth (BHI, HiMedia, Mumbai, India) or Trypticase soy broth (TSB; HiMedia) at 37°C with agitation. Skim milk powder (HiMedia) was also used as a growth medium in the biofilm assay. All isolates used in this study were stored at -70°C in BHI containing 40% glycerol.
Slime production on Congo Red agar
Polysaccharide-producing S. aureus strains were determined by cultivation on agar plates containing Congo Red (Sigma-Aldrich, Oakville, Ontario, Canada) [22]. Initially, the bacteria were inoculated in BHI broth and incubated at 37°C for 16 h with agitation. The cultures were then streaked onto CRA plates and incubated at 37°C for 24 h and subsequently kept at room temperature for 48 h and 72 h. Only isolates growing as black colonies with a dry and crystalline consistency were considered to be slime producers. S. epidermidis NRS101 and Streptococcus agalactiae were used as positive and negative controls, respectively. The assay was repeated three times for each isolate.
Biofilm production assay
Biofilm formation was assessed in 96-well polystyrene tissue culture microplates as previously described [29] with modifications. The bacterial isolates were inoculated into TSB and incubated at 37°C for 16 h, and the optical density (OD600nm) was adjusted to 0.1. Subsequently, the inoculum was diluted 1:40 in TSB containing 0.25% glucose (TSBg) or skim-milk medium in a final volume of 200 μL per well, and the microplate was incubated at 37°C for 24 h without agitation. The medium was then discarded, and the wells of each plate were gently washed three times with 200 μL of sterile PBS (pH 7.4), dried at 45°C for 20 min and then stained with 50 μL of 1% crystal violet for 15 minutes. Each well was washed three times with 200 μL of sterile distilled water, followed by drying at 45°C for 20 min; 200 μL of 100% ethanol was then added. A 150-μL aliquot was removed from each well and transferred to a new microplate, and the absorbance at 630 nm was measured using a microplate reader (VersaMax Molecular Devices, Sunnyvale, California, USA). Wells filled with TSBg or skim-milk medium were used as blanks to correct for background staining by subtracting the value of the blank from each experimental value. The bacterial isolates were classified as biofilm-positive if their average OD values were higher than the average OD value of the negative control (Streptococcus agalactiae) (OD630nm values < 0.1). S. epidermidis NRS101 was used as the positive control. Each isolate was tested in triplicate, and the assay was repeated three times.
Polymerase chain reaction
Genomic DNA from the bacterial isolates was extracted according to Pospiech & Neumann [44]. The DNA quality and quantity were analyzed by gel electrophoresis. A total of 150 ng μL-1 of DNA was used in amplification reactions with specific primers (Table 1). The reactions contained 1X reaction buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 1U GoTaq DNA polymerase (Promega, Madison, Wisconsin, USA), 1 μM each primer and Milli-Q water to increase the reaction volume to 20 μL. The cycling conditions were either the same as those suggested by Ciftci et al. [45] to amplify the icaA and icaD genes or the same as those suggested by Campbell et al. [46] to amplify different agr groups. S. aureus NRS133 and NRS155 were used as positive controls for agr-types I and II, respectively. The amplicons were resolved by 1.5% agarose gel electrophoresis on gels containing 0.5 μg mL-1 ethidium bromide. Images were registered using the L-PIX Imaging system (Loccus Biotecnologia, São Paulo, São Paulo, Brazil).
Table 1.
Primers used in this study
| Gene | Primer sequence | Concentration (μM) | Product size (bp) | Tm (°C) | Reference |
|---|---|---|---|---|---|
| icaA | F- CCTAACTAACGAAAGGTAG | 1.0 | 1315 | 49 | [45] |
| R- AAGATATAGCGATAAGTGC | |||||
| icaD | F- AAACGTAAGAGAGGTGG | 1.0 | 381 | 49 | [45] |
| R- GGCAATATGATCAAGATA | |||||
| agr-type I | F-ATCGCAGCTTATAGTACTTGT | 1.0 | 578 | 53 | [46] |
| R-CTTGATTACGTTTATATTTCATC | |||||
| agr-type II | F-AACGCTTGCAGCAGTTTATTT | 1.0 | 814 | 57 | [46] |
| R-CGACATTATAAGTATTACAACA | |||||
| agr-type III | F-TATATAAATTGTGATTTTTTATTG | 1.0 | 893 | 53 | [46] |
| R-TTCTTTAAGAGTAAATTGAGAA | |||||
| agr-type IV | F-GTTGCTTCTTATAGTACATGTT | 1.0 | 757 | 53 | [46] |
| R-CTTAAAAATATAGTGATTCCAATA |
Statistical analysis
Data analyses were performed using the statistical programs SAS/STAT® [47] and SPSS [48]. Descriptive statistics (the arithmetic mean, standard deviation and standard error) were used to assess biofilm production in different media (TSB or skim milk) and by different agr groups (I, II, or III). To compare the mean biofilm production values according to the medium and agr group, a two-way factorial ANOVA was used; the least significant difference (LSD) test was employed for comparison of the means. The two-way factorial model used for the slime-positive isolates and biofilm producers in TSBg and skim milk was as follows: Yij = m + MEDi + AGRJ + MEDxAGRk + eijk, where Yij = observed values (slime), m = constant associated with each observation, MEDi = the fixed effect of the medium i (milk = 0 and TSB = 1), AGRJ = the fixed effect of the agr group (agr II = 0 and agr I and III = 1), MED x AGRk = the interaction of the medium and the agr group, and eijk = random error. The OD values for the production of biofilm in TSB or skim milk were categorized as negative (OD ≤ 0.1) or positive (OD > 0.1). We used Fisher's exact test and the Kappa index to evaluate the association and level of agreement between the positive and negative results for biofilm production and the positive and negative results for slime production (SPSS, 1998). The variation in slime production was assessed by generalized linear models. The values for slime production were zero (0) for a negative result and one (1) for a positive result. Slime production by each isolate was measured. The biofilm production in the respective growth medium was categorized as positive or negative, and isolates were categorized as “agr I and III” or “agr II”.
Acknowledgements
Staphylococcus aureus NRS133, S. aureus NRS155, and S. epidermidis NRS101 were obtained through the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under NIAID/NIH Contract # HHSN272200700055C. The authors are grateful to Embrapa Dairy Cattle, which provided all other S. aureus and Streptococcus agalactiae isolates used in the study. The authors are also grateful for the reviewers’ valuable comments, which improved the manuscript.
Funding
This project was supported by Grant APQ01054-10 provided by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Abbreviations
- CRA
Congo Red agar
- FAO
Food and agriculture organization
- PIA
Polysaccharide intracellular adhesion
- EPS
Extracellular polymeric substance
- OD
Optical density
- BHI
Brain heart infusion
- TSB
Trypticase soy broth
- TSBg
Trypticase soy broth containing 0.25% glucose
- NARSA
Network on antimicrobial resistance in Staphylococcus aureus
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: RCK, MHF-K, and AOBR. Performed the experiments: RCK and MJCS. Analyzed the data: MHF-K, MJCS, GNS, and AOBR. Contributed reagents/materials/analytic tools: GNS and AOBR. Wrote the paper: MHF-K, GNS, and AOBR. All authors read and approved the final manuscript.
Contributor Information
Mary Hellen Fabres-Klein, Email: mhfabres@gmail.com.
Mário Junior Caizer Santos, Email: mariocaizer@yahoo.com.br.
Raphael Contelli Klein, Email: rcontelli@gmail.com.
Guilherme Nunes de Souza, Email: guilherme.souza@embrapa.br.
Andrea de Oliveira Barros Ribon, Email: abribon@ufv.br.
References
- 1.Food and Agriculture Organization (FAO) The State of Food and Agriculture 2010-2011. Rome: Food and Agriculture Organization of United Nations; 2011. [Google Scholar]
- 2.Müller EE. Estudo da prevalência da mastite bovina. Semina. 1978;1:47–48. [Google Scholar]
- 3.Freitas JA, Pedroso SCS, Barroso R, Aguiar RV, Monteiro FJC. Ocorrência de mastite em rebanhos leiteiros bovinos e bubalinos no estado do Pará. Rev Bras Cienc Agr. 2009;52:189–194. [Google Scholar]
- 4.Martins RP, da Silva JAG, Nakazato L, Dutra V, Almeida Filho ES. Prevalência e etiologia infecciosa da mastite bovina na microrregião de Cuiabá, MT. Cienc Anim Bras. 2010;11:81–187. [Google Scholar]
- 5.Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa da Pecuária Municipal. Rio de Janeiro. 2011;39:1-63.
- 6.Chagas LGS, Melo PC, Barbosa NG, Guimarães EC, Brito DD. Occurrence of bovine mastitis caused by Staphylococcus sp. Streptococcus sp. and Candida sp. in a rural area of Indianópolis – Minas Gerais, Brazil. Biosci J. 2012;28:1007–14.
- 7.Barkema HW, Schukken YH, Zadoks RN. Invited review: the role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus Mastitis. J Dairy Sci. 2006;89:1877–1895. doi: 10.3168/jds.S0022-0302(06)72256-1. [DOI] [PubMed] [Google Scholar]
- 8.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 9.Cucarella C, Tormo MA, Ubeda C, Trotonda MP, Monzón M, Peris C, et al. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect Immun. 2004;4:2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simojoki H, Hyvönen P, Plumed Ferrer C, Taponen S, Pyörälä S. Is the biofilm formation and slime producing ability of coagulase-negative staphylococci associated with the persistence and severity of intramammary infection? Vet Microbiol. 2012;158:344–352. doi: 10.1016/j.vetmic.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Giacometti A, Cirioni O, Gov Y, Ghiselli R, Del Prete MS, Mocchegiani F, et al. RNA III inhibiting peptide inhibits in vivo biofilm formation by drug-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1979–1983. doi: 10.1128/AAC.47.6.1979-1983.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beenken KE, Spencer H, Griffin LM, Smeltzer MS. Impact of extracellular nuclease production on the biofilm phenotype of Staphylococcus aureus under in vitro and in vivo conditions. Infect Immun. 2012;80:1634–1638. doi: 10.1128/IAI.06134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cafiso V, Bertuccio T, Santagati M, Demelio V, Spina D, Nicoletti G, et al. agr-Genotyping and transcriptional analysis of biofilm-producing Staphylococcus aureus. FEMS Immunol Med Microbiol. 2007;51:220–227. doi: 10.1111/j.1574-695X.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 16.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzzola FR, Barbagelata MS, Caccuri RL, Sordelli DO. Attenuation and persistence of and ability to induce protective immunity to a Staphylococcus aureus aroA mutant in mice. Infect Immun. 2007;74:3498–3506. doi: 10.1128/IAI.01507-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilot P, van Leeuwen W. Comparative analysis of agr locus diversification and overall genetic variability among bovine and human Staphylococcus aureus isolates. J Clin Microbiol. 2004;42:1265–1269. doi: 10.1128/JCM.42.3.1265-1269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melchior MB, van Osch MHJ, Graat RM, van Duijkeren E, Mevius DJ, Nielen M, et al. Biofilm formation and genotyping of Staphylococcus aureus bovine mastitis isolates: evidence for lack of penicillin-resistance in Agr-type II strains. Vet Microbiol. 2009;137:83–89. doi: 10.1016/j.vetmic.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Croes S, Deurenberg RH, Boumans ML, Beisser PS, Neef C, Stobberingh EE. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 2009;9:229–238. doi: 10.1186/1471-2180-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo YS, Lee DY, Rayamahji N, Kang ML, Yoo HS. Biofilm-forming associated genotypic and phenotypic characteristics of Staphylococcus spp. isolated from animals and air. Res Vet Sci. 2008;85:433–438. doi: 10.1016/j.rvsc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Dhanawade NB, Kalorey DR, Srinivasan R, Barbuddhe SB, Kurkure NV. Detection of intercellular adhesion genes and biofilm production in Staphylococcus aureus isolated from bovine subclinical mastitis. Vet Res Commun. 2010;34:81–89. doi: 10.1007/s11259-009-9326-0. [DOI] [PubMed] [Google Scholar]
- 23.Rohde H, Frankenberger S, Zähringer U, Mack D. Structure, function and contribution of polysaccharide intercellular adhesin (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur J Cell Biol. 2010;89:103–111. doi: 10.1016/j.ejcb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 25.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 26.Baselga R, Albizu I, De la Cruz M, Del Cacho E, Barberan M, Amorena B. Phase variation of slime production in Staphylococcus aureus: implications in colonization and virulence. Infect Immun. 1993;61:4857–4862. doi: 10.1128/iai.61.11.4857-4862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida K, Takahashi M, Ohtomo T, Minegishi Y, Ichiman Y, Haga K, et al. Application of fluorescent antibody for detecting capsular substances in Staphylococcus aureus. J Appl Bacteriol. 1979;46:147–152. doi: 10.1111/j.1365-2672.1979.tb02592.x. [DOI] [PubMed] [Google Scholar]
- 28.Dubravka M, Lazic S, Branka V, Jelena P, Bugarski D, Zorica S. Slime production and biofilm forming ability by Staphylococcus aureus bovine mastitis isolates. Acta Vet. 2010;60:217–226. doi: 10.2298/AVB1003217M. [DOI] [Google Scholar]
- 29.Vasudevan P, Nair MKM, Annamalai T, Venkitanarayanan KS. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet Microbiol. 2003;92:179–185. doi: 10.1016/S0378-1135(02)00360-7. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, et al. Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol. 2006;118:133–140. doi: 10.1016/j.vetmic.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Poutrel B, Gilbert FB, Lebrun M. Effects of culture conditions on production of type 5 capsular polysaccharide by human and bovine Staphylococcus aureus strains. Clin Diagn Lab Immun. 1995;2:166–171. doi: 10.1128/cdli.2.2.166-171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varhimo E, Varmanen P, Fallarero A, Skogman M, Pyorala S, Iivanainen A, et al. Alpha- and β-casein components of host milk induce biofilm formation in the mastitis bacterium Streptococcus uberis. Vet Microbiol. 2011;149:381–389. doi: 10.1016/j.vetmic.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Xue T, Chen X, Shang F. Effects of lactose and milk on the expression of biofilm-associated genes in Staphylococcus aureus strains isolated from a dairy cow with mastitis. J Dairy Sci. 2014;97:6129–6134. doi: 10.3168/jds.2014-8344. [DOI] [PubMed] [Google Scholar]
- 34.Bardiau M, Detilleux J, Farnir F, Mainil JG, Ote I. Associations between properties linked with persistence in a collection of Staphylococcus aureus isolates from bovine mastitis. Vet Microbiol. 2014;169:74–79. doi: 10.1016/j.vetmic.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Melchior MB, Fink-Gremmels J, Gaastra W. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J Vet Med B Infect Dis Vet Public Health. 2006;53:326–332. doi: 10.1111/j.1439-0450.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 36.Raza A, Muhammad G, Sharif S, Atta A. Biofilm producing Staphylococcus aureus and bovine mastitis: a review. Mol Microbiol Res. 2013;3:1–8. [Google Scholar]
- 37.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Falcieri E, Vaudaux P, Huggler E, Lew D, Waldvogel F. Role of bacterial exopolymers and host factors on adherence and phagocytosis of Staphylococcus aureus in foreign body infection. J Infec Dis. 1987;155:524–531. doi: 10.1093/infdis/155.3.524. [DOI] [PubMed] [Google Scholar]
- 39.Szweda P, Schielmann M, Milewski S, Frankowska A, Jakubczak A. Biofilm production and presence of ica and bap genes in Staphylococcus aureus strains isolated from cows with mastitis in the eastern Poland. Pol J Microbiol. 2012;61:65–69. [PubMed] [Google Scholar]
- 40.Fox LK, Zadoks RN, Gaskins CT. Biofilm production by Staphylococcus aureus associated with intramammary infection. Vet Microbiol. 2005;107:295–299. doi: 10.1016/j.vetmic.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Gerke C, Kraft A, Süssmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 42.Liberto MC, Matera G, Quirino A, Lamberti AG, Capicotto R, Puccio R, et al. Phenotypic and genotypic evaluation of slime production by conventional and molecular microbiological techniques. Microbiol Res. 2009;164:522–528. doi: 10.1016/j.micres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Brito MAVP, Carvalho MGM, Brito JRF. Esquema simplificado para identificação de estafilococos coagulase. Cienc Rural. 2002;32:79–82. doi: 10.1590/S0103-84782002000100014. [DOI] [Google Scholar]
- 44.Pospiech A, Neumann BA. A versatile quick-prep of genomic DNA from Gram positive bacteria. Trends Genet. 1995;11:217–218. doi: 10.1016/S0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- 45.Ciftci A, Findik A, Onuk EE, Savasan S. Detection of methicillin resistance and slime factor production of Staphylococcus aureus in bovine mastitis. Braz J Microbiol. 2009;40:254–261. doi: 10.1590/S1517-83822009000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46:678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SAS Institute Inc. SAS/STAT user’s guide version 6. 4th ed. Cary.NC: SAS Institute Inc; 1990. p. 1022p.
- 48.Statistical Package for the Social Science (SPSS) Statistical Package for the Social Science, versão 8.0. Chicago: SPSS Inc; 1998. [Google Scholar]


