Abstract
Caveolin-1 (Cav-1), the signature protein of caveolae is expressed in several cell types in the adult retina and is linked to ocular pathologies including uveitis, diabetic retinopathy, and primary open angle glaucoma. Genetic ablation of Cav-1 causes retinal functional deficits due to disruptions in environmental homeostasis. To better understand Cav-1 function in the retina, we examined its expression/localization during postnatal retinal development. From P0–P5, Cav-1 was detected only in the developing superficial retinal vessels, in hyaloid and choroidal vasculature, and in the retinal pigment epithelium (RPE). At P7, staining began to be observed centrally in radial cells in the neuroretina, and this staining increased dramatically by P9/10 in identifiable Müller glia. Prominent vascular staining continued throughout development. These results support the idea that Cav-1 is an indicator of Müller glial differentiation and suggests that it plays an important role in Müller cell function.
Keywords: Caveolin-1, Retina, Müller glia, Vasculature, Development, Differentiation
3.1 Introduction
Caveolin-1 (Cav-1) is the primary structural protein of specialized, 50–100 nm flask-shaped caveolae membrane domains [1]. Cav-1 is intrinsically involved in multiple caveolar functions including lipid trafficking, endocytosis, mechanotransduction, and cell signaling [1, 2]. Our understanding of Cav-1 and caveolae function in the eye is limited. Changes in Cav-1 expression are associated with blood-retinal barrier breakdown in experimental diabetic retinopathy [3] and with chronic inflammation in posterior uveitis [4]. Polymorphisms in the CAV1 gene are also linked to primary open angle glaucoma [5]. We recently reported that loss of Cav-1 compromises retinal environmental homeostasis leading to retinal functional deficits [6]. In adult retinas, Cav-1 protein is expressed in several cell types including RPE, Müller glia, photoreceptors, and vascular cells [6–8]. At the transcript level, Cav-1 is dramatically enriched in Müller glia compared to retinal neurons [9] and our immunohistochemical staining confirms this prominent expression in Müller glia in adult retinas [6]. Intriguingly, Cav-1 mRNA expression in FACS-purified Müller cells increases in a temporal pattern matching that of markers of Müller glial differentiation [10], but whether other cell types express Cav-1 during retinal development is not known.
The purpose of the present study was to determine the localization of Cav-1 protein during postnatal retinal development. The temporal and spatial expression indicated that differentiating and adult Müller glia and retinal vasculature are the major cell types expressing Cav-1. These results support the idea that Cav-1 is an indicator of Müller glial maturation and suggest that it plays an important role in the function of differentiated Müller glia.
3.2 Methods
Mice
C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) mice were used for these studies. All procedures were carried out according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by Institutional Animal Care and Use Committees of the University of Oklahoma Health Sciences Center and Dean McGee Eye Institute.
Immunohistochemistry and Confocal Microscopy
Mice were euthanized at the indicated postnatal ages, eyes were fixed in Prefer fixative (Anatech, Ltd., Battlefield, MI), embedded in paraffin, and 5-μm sections were cut. Immunohistochemistry was performed as previously described [6] with the following antibodies: rabbit anti-Cav-1 (1:100, BD Biosciences, San Jose, CA); rat anti-CD31 (1:300, Dianova GmbH, Hamburg, Germany); and mouse antibodies against glutamine synthetase (GS; 1:500, clone GS-6) and rhodopsin (1:500, clone 4D2) from Millipore (Billerica, MA), and synaptic vesicle glycoprotein 2 (SV2, 1:500, clone 10H3, gift from Erik Floor, University of Kansas). Immunoreactivity was detected with Alexa Fluor-labeled secondaries (Life Technologies, Grand Island, NY) and nuclei were stained with DAPI or propidium iodide. Pseudocolors were assigned to images as follows: Cav-1 (green), other proteins (red), nuclei (blue).
3.3 Results
3.3.1 Cav-1 is Expressed by the Vasculature During Retinal Development
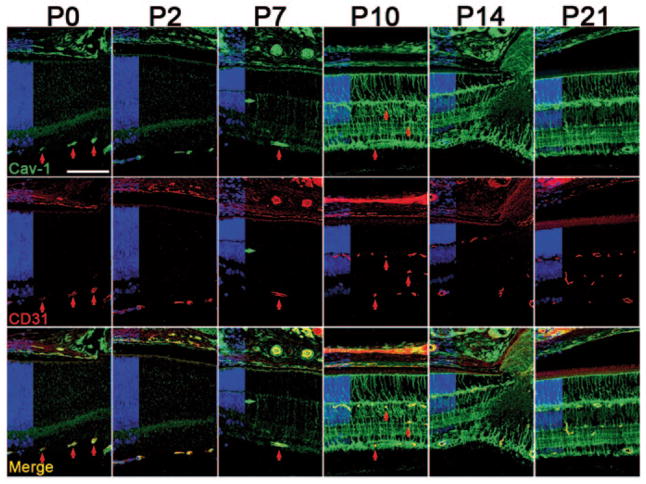
Mouse retinal vasculature develops postnatally with the superficial vascular plexus forming from the optic nerve head (ONH) and progressing to the retinal periphery by P8. From P7, superficial capillaries sprout perpendicularly toward the outer retina to form deep and intermediate capillary plexuses in the outer and inner plexiform layers which are interconnected by P21. At early postnatal days, Cav-1 is predominantly colocalized with the endothelial marker, CD31, in superficial retinal vessels (vertical red arrows in Fig. 3.1 highlight representative vessels) and choroidal vasculature. It is also detected in vesicular structures at the apical RPE. At P7, weak, nonvascular radial staining in the neuroretina begins to be observed (horizontal green arrow in P7 panels). Cav-1 immunoreactivity remains prominent in retinal vessels throughout development but is less apparent as Cav-1 expression in presumptive Müller glia increases between P7 and P21.
Figure 3.1.
Caveolin-1 (Cav-1) is expressed in developing vessels and increases in the neuroretina from P7–P10. Cav-1 (green) colocalizes with the endothelial marker, cluster of differentiation 31 (CD31, red) in vessels throughout postnatal retinal development. Red vertical arrows highlight several vessels at various developmental stages. The green horizontal arrow at P7 indicates a CD31-negative radial cell. Nuclear layers are indicated in blue on the left of each panel. (Scale bar = 100 μm)
3.3.2 Cav-1 Expression Increases Dramatically in Neuroretina as Müller glia Mature
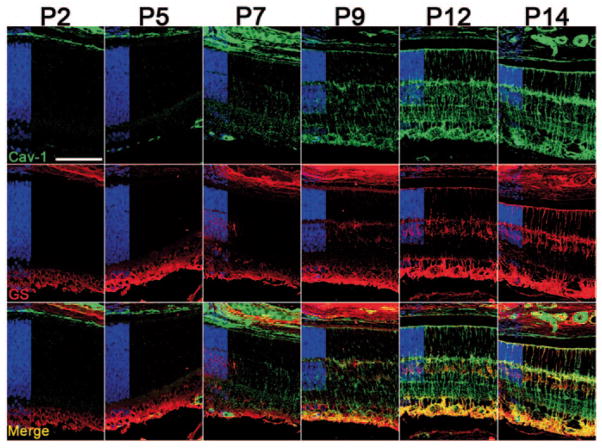
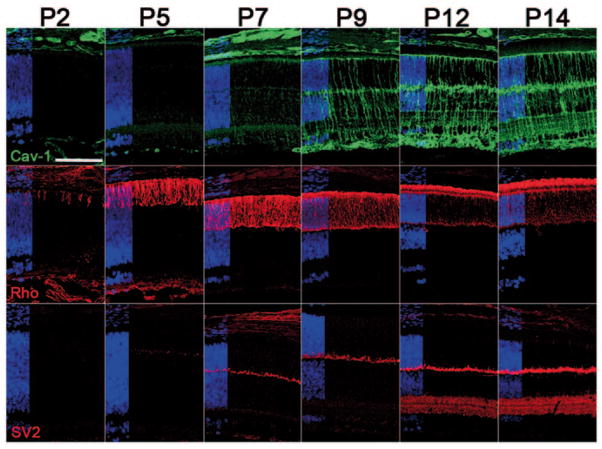
As shown in Fig. 3.1, nonvascular Cav-1 staining in the neuroretina was first detected in radial cells at P7. This staining was initially most pronounced near the ONH and decreased toward the retinal periphery (not shown), but eventually a radial expression pattern with Müller glial morphology was apparent panretinally. The morphology of Cav-1-localized cells and the temporal expression, coinciding with the timing of Müller glial differentiation [11], suggested that these nonvascular Cav-1-positive cells were Müller cells. To confirm this, we co-labeled with the Müller glial marker, GS (Fig. 3.2). Prior to P9, no specific GS immunoreactivity was detected in retinal sections. The staining observed in the inner retina at early time points was indistinguishable from sections incubated without primary antibodies (not shown) suggesting that this represents secondary antibody reaction with endogenous murine immunoglobulins. Specific GS-positive immunoreactivity could be localized to Müller cell bodies in the inner nuclear layer by P9 when Cav-1 staining was clearly detected in Müller glia. At P12, characteristic GS immunoreactivity could be observed in the same cells that express Cav-1. Perfect colocalization is not achieved as GS is a cytosolic enzyme and Cav-1 is an integral protein but it is clear from Fig. 3.2 that Cav-1 and GS are both present in the same cells. In addition to GS, we also labeled sections with rod photoreceptor and synaptic markers, rhodopsin and SV2, respectively. As shown in Fig. 3.3, these markers did not display the same localization or temporal expression as Cav-1.
Figure 3.2.
Caveolin-1 (Cav-1)-positive radial cells (green) detected after P7 are also positive for glutamine synthetase (GS, red), a marker of mature Müller glia. Nuclear layers are indicated in blue. (Scale bar = 100 μm)
Figure 3.3.
Caveolin-1 (Cav-1) immunoreactivity (green) does not spatially or temporally associate with rod photoreceptors or synaptic development. Middle panels are labeled for rhodopsin (red) and lower panels for the synaptic vesicle protein (SV2, red). Nuclear layers are indicated in blue. (Scale bar = 100 μm)
3.4 Discussion
Based on the timing and localization of immunoreactivity and the morphology of immunopositive cells, we conclude that Müller glia and vascular endothelium are the principal cell types that express Cav-1 in the retina. The pronounced immunoreactivity in the developing retinal vasculature is consistent with the well-established abundant expression of Cav-1 and numerous caveolae detected in vascular endothelium in other tissues [1, 2]. The expression in the developing retinal vasculature is not surprising given that caveolae in retinal vascular endothelium have been suggested as sites of vascular endothelial growth factor (VEGF) signaling [8].
Less is understood about Cav-1 function in cells derived from neuroretinal progenitors. Our results agree with those of Roesch et al. [9], who identified Cav-1 as a Müller-cell-enriched transcript in individual adult Müller cells and with our previous immunolocalization of Cav-1 in adult retinas [6]. The temporal increase in Cav-1 in maturing Müller glia agrees precisely with the timing of expression of Cav-1 mRNA in fluorescence-activated cell sorting (FACS)-isolated Müller cells [10]. Importantly, Cav-1 expression increases dramatically between P7 and P10 at the time when Müller cells increase expression of markers of functional maturation, e.g., Kir4.1, aquaporin-4 [10], and GS (this study). Collectively, these results suggest that Cav-1 plays an important role in Müller cell function in the fully developed retina.
Müller glia span the entire retina, contact all retinal neurons, and form the outer and inner limiting membranes. They perform many functions including: regulation of ion homeostasis, neurotransmitter recycling, neuroprotection, retinal structure scaffolding, and possibly neuronal regeneration [12]. Given the myriad functions of Müller glia [12], we can presently only speculate on which of these might be Cav-1 associated. We have recently reported reduced electroretinogram (ERG) responses and changes in ion homeostasis in retinas from Cav-1 null mice [6]. We speculate that these deficits result from loss of Cav-1-dependent functions in Müller cells.
Acknowledgments
This work was supported by NIH Grants EY019494, RR017703, and P30EY021725, and by Research to Prevent Blindness, Inc. (unrestricted grant and Sybil B. Harrington Special Scholar Award for Macular Degeneration).
Abbreviations
- Cav-1
Caveolin-1
- RPE
Retinal pigment epithelium
- GS
Glutamine synthetase
- SV2
Synaptic vesicle glycoprotein 2
- ONH
Optic nerve head
- CD31
Cluster of differentiation 31
Contributor Information
Xiaowu Gu, Department of Ophthalmology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Oklahoma Center for Neuroscience, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Dean McGee Eye Institute, 608 Stanton L. Young Blvd., DMEI B423, Oklahoma City, OK 73104, USA.
Alaina Reagan, Department of Ophthalmology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Oklahoma Center for Neuroscience, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Dean McGee Eye Institute, 608 Stanton L. Young Blvd., DMEI B423, Oklahoma City, OK 73104, USA.
Allen Yen, Department of Ophthalmology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Dean McGee Eye Institute, 608 Stanton L. Young Blvd., DMEI B423, Oklahoma City, OK 73104, USA.
Faizah Bhatti, Department of Ophthalmology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Department of Pediatrics, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Alex W. Cohen, Department of Ophthalmology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Dean McGee Eye Institute, 608 Stanton L. Young Blvd., DMEI B423, Oklahoma City, OK 73104, USA
Michael H. Elliott, Email: Michael-Elliott@ouhsc.edu, Department of Ophthalmology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Oklahoma Center for Neuroscience, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA. Dean McGee Eye Institute, 608 Stanton L. Young Blvd., DMEI B423, Oklahoma City, OK 73104, USA. Department of Physiology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
References
- 1.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 3.Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009;89:4–15. doi: 10.1016/j.exer.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Hauck SM, Dietter J, Kramer RL, Hofmaier F, Zipplies JK, Amann B, et al. Deciphering membrane-associated molecular processes in target tissue of autoimmune uveitis by label-free quantitative mass spectrometry. Mol Cell Proteomics. 2010;9:2292–2305. doi: 10.1074/mcp.M110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, McClellan ME, Tanito M, Garteiser P, Towner R, Bissig D, et al. Loss of caveolin-1 impairs retinal function due to disturbance of subretinal microenvironment. J Biol Chem. 2012;287:16424–16434. doi: 10.1074/jbc.M112.353763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora RC, Bonilha VL, Shin BC, Hu J, Cohen-Gould L, Bok D, Rodriguez-Boulan E. Bipolar assembly of caveolae in retinal pigment epithelium. Am J Physiol Cell Physiol. 2006;290:C832–C843. doi: 10.1152/ajpcell.00405.2005. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB. VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci. 1999;40:157–167. [PubMed] [Google Scholar]
- 9.Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6:e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]