Abstract
Introduction
Boerhaave’s syndrome represents the most lethal of all gastrointestinal perforations. In 2009 a treatment algorithm was published based on current level 4 evidence indicating that all septic patients should be treated surgically, early presentations without sepsis endoscopically and delayed presentations without sepsis conservatively. No provision was made for septic patients unfit for surgical intervention. Using a case series, we demonstrate how minimally invasive endoscopic therapies can be used successfully to manage such a cohort.
Methods
Between September 2008 and January 2010, five patients presented to Wishaw General Hospital with Boerhaave’s syndrome, all with an associated septic profile and none fit for surgery. They were managed using minimally invasive endoscopic therapies including endoscopic placement of oesophageal stents, elimination of mediastinal/pleural contamination using video assisted thorascopic lavage, management of subsequent collections using sinus tract endoscopy and minilaparotomy with transhiatal endoscopic drainage, and closure of oesophagocutaneous fistulas using the Surgisis® (Cook Surgical, Bloomington, IN, US) anal fistula plug sited endoscopically with a rendezvous technique.
Results
Oesophageal re-epithelialisation and resolution of sepsis was achieved in all five cases on days 50, 50, 51, 59 and 103. Four patients are alive today. The fifth died on day 109 in hospital as a consequence of co-morbidity. Two patients required oesophageal dilatation for benign oesophageal strictures.
Conclusions
Minimally invasive endoscopic therapy can be used successfully to achieve oesophageal re-epithelialisation and resolution of sepsis in patients unfit for surgical intervention. It offers a feasible treatment for patients not accounted for in today’s literature and expands on currently described endoscopic therapies.
Keywords: Boerhaave’s syndrome, Oesophageal stent, Surgisis® anal fistula plug
A spontaneous oesophageal rupture, also known as Boerhaave’s syndrome, is regarded as the most lethal of all gastrointestinal perforations. It carries a mortality rate of close to 100% without treatment. 1 There is no treatment consensus in the literature, reflecting the rarity of the condition and also the spectrum of disease that it represents. In 2009 de Schipper et al published the only literature review and treatment algorithm for Boerhaave’s syndrome, based on level 4 evidence, highlighting the need for further multicentre trials to consolidate management. 2
At present, early diagnosis in patients with a septic profile favours surgical management and in those without, endoscopic therapy. 2 Conservative management is reserved for delayed presentations with contained, wide necked perforations without sepsis and for those who demonstrate tolerance to pleural contamination amenable to radiological drainage. 3 Those failing to progress on conservative management primarily owing to the evolution of sepsis should be reconsidered for surgical intervention. With regard to endoscopic therapy, the literature description is limited to oesophageal stents, for which there is a variable prognosis, and also to patients who present early without a septic profile. 2 However, we describe the successful management of Boerhaave’s syndrome using minimally invasive endoscopic therapies to treat septic patients (presenting both early and late) who were deemed not fit for surgical intervention. Using a case series, we illustrate the successful technique of minimally invasive endoscopic therapy.
Case series
Five patients presented to Wishaw General Hospital with a spontaneous oesophageal rupture between September 2008 and January 2010 (Table 1). Case 1 was a 30-year-old male alcoholic with brittle end stage diabetes and known oesophagitis. Case 2 was a 31-year-old female alcoholic with no known oesophageal disease. Both these patients were overtly septic with a delay in presentation and diagnosis.
Table 1.
Case series details
| Case | Age / sex | Co-morbidity | Presentation | Diagnosis | Sepsis | Organ failure | APACHE II points | APACHE II (predicted mortality) |
|---|---|---|---|---|---|---|---|---|
| 1 | 30 M | Diabetes mellitus type 1 Asthma Chronic renal failure Alcohol excess Smoker Oesophagitis |
>48 hours | >72 hours | Septic shock | Multiple organ failure | 32 | 76.0% |
| 2 | 31 F | Alcohol excess Smoker | >48 hours | >72 hours | Septic shock | Multiple organ failure | 31 | 73.3% |
| 3 | 74 F | Hypertension | <24 hours | <48 hours | SIRS | Respiratory failure | 35 | 85.1% |
| 4 | 75 M | Hypertension | <24 hours | <48 hours | SIRS | Respiratory failure | 37 | 86.8% |
| 5 | 75 F | Colorectal cancer | <24 hours | <48hours | SIRS | Respiratory failure | 38 | 88.4% |
SIRS = systemic inflammatory response syndrome
Case 3 was a 74-year-old woman, case 4 a 75-year-old man and case 5 a 75-year-old woman. All three of these patients had no significant medical co-morbidity or known oesophageal disease but, despite an early presentation and diagnosis, were found to be septic with single organ failure.
For cases 3, 4 and 5, a clinical diagnosis was confirmed radiologically on computed tomography (CT). For cases 1 and 2, CT provided a radiological diagnosis where the clinical picture had been unclear. All five patients had their diagnosis confirmed endoscopically as part of their minimally invasive endoscopic therapy.
For cases 1 and 2, a delayed presentation with sepsis should have favoured surgical intervention, either in the form of an oesophagectomy for case 1, or closure around a T-tube or a diversion procedure for case 2. For cases 3 and 4, an early diagnosis without any significant medical co-morbidity should have favoured surgical intervention in the form of primary closure of the defect. In all four cases, however, the surgical and anaesthetic teams deemed them not fit for surgical intervention because of the degree of associated sepsis and organ failure, and additionally for cases 1 and 2, their associated medical co-morbidity (Table 1). For case 5, an initial attempt at primary closure of the oesophageal defect was made thoracoscopically but the procedure was terminated owing to an inability to tolerate single lung ventilation. As a consequence, all five patients were treated using minimally invasive endoscopic therapies.
The Acute Physiology and Chronic Health Evaluation (APACHE) II is a severity score and mortality estimation tool developed from a large sample of intensive care unit (ICU) patients in the US. It comprises 12 physiological and 2 disease related variables. The APACHE II calculates its score based on the worst recorded value for each variable within a patient’s first 24 hours of admission to the ICU. ClinCalc.com provides an online electronic APACHE II calculator, which was used in our cohort to calculate APACHE II scores. By taking into consideration the indication for ICU admission, ClinCalc.com converts APACHE II scores to a percentage risk of mortality.
Minimally invasive endoscopic therapy: the technique
In all cases, minimally invasive endoscopic therapy requires a multidisciplinary approach to patient care and, in particular, a close working relationship between surgeon and intensivist. All patients require aggressive resuscitation, following which varying degrees of physiological support will be required (ie invasive ventilation and inotropic therapy). Intravenous antimicrobials and antisecretories are provided to all patients, as is enteral nutrition.
Following these initial interventions, all patients undergo an oesophagogastroduodenoscopy (OGD) under general anaesthesia. This allows confirmation of the diagnosis as well as an assessment regarding the site and size of the perforation. The primary goal of minimally invasive endoscopic therapy is to seal the oesophageal perforation. Van Heel et al described the successful use of plastic covered self-expandable metallic stents to cover benign oesophageal perforations. 4 However, in four of our patients, the Proximal Release Ultraflex™ oesophageal stent (Boston Scientific, Natick, MA, US) was inadequate for restoring oesophageal continuity as all four demonstrated an ongoing oesophageal leak on initial post-stenting contrast study. Furthermore, in three of these patients, this leak was associated with the development of either a further mediastinal or pleural collection.
In our initial case series, all four Proximal Release Ultraflex™ oesophageal stents were subsequently changed to Polyflex® oesophageal stents (Boston Scientific), which appeared to provide increased radial force, reinstating oesophageal continuity as confirmed fluoroscopically prior to the introduction of oral alimentation. Following successful stenting of the oesophageal defect, a nasojejunal feeding tube was sited, as was a nasogastric drainage tube. The drainage tube was kept on low continuous suction to ensure the site of the healing perforation was kept dry and the jejunal feeding commenced with the addition of methylene blue. The presence of this in the drainage tube or the chest drains that were to be sited later was an indicator that the rate of feeding was too high or that there was an ongoing leak from the site of the oesophageal perforation.
Case 2 suffered latterly with an inability to initiate a safe swallow. This may have in part reflected the length of time he endured both nasogastric and nasojejunal tubes, and a formal drainage gastrostomy was therefore fashioned, as was a formal feeding jejunostomy.
The secondary goal of minimally invasive endoscopic therapy is to eliminate any associated contamination from either the mediastinal or pleural cavities. This could be achieved endoscopically by irrigating the contaminated fields through the perforation site at the time of the initial OGD. This was performed in case 5 owing to the size of the oesophageal perforation and relatively localised extent of contamination. In the remaining four cases, either bilateral or unilateral video assisted thoracoscopic lavage (VATS) of the pleural cavities was performed under the same initial anaesthetic, again depending on the extent and distribution of any associated contamination. All cavities irrigated were drained percutaneously via a standard 32Fr Portex® chest drain (Smiths Medical, Ashford, UK) inserted under direct vision using either the endoscope (case 5) or the thoracoscope. This provided ongoing drainage and minimised the risk of further mediastinal or pleural collections. Supportive therapy was subsequently withdrawn.
In four of the cases, a formal tracheostomy was required to aid weaning. Others weaned from both inotropes and antiarrythmics. Case 1 required regular physician review to manage his furosemide dependent renal failure and brittle end stage diabetes.
The tertiary goal of minimally invasive endoscopic therapy is the aggressive investigation and management of late secondary complications, primarily the presence of both mediastinal and pleural collections. Such collections can be targeted through a combination of percutaneous drainage under radiological guidance, repeat VATS, sinus tract endoscopy and mini-laparotomy with endoscopic transhiatal drainage of mediastinal collections.
Two of our patients went on to develop an empyema, one of which was thought to have arisen as a consequence of an ongoing oesophageal leak from the Proximal Release Ultraflex™ oesophageal stent. The first failed to resolve with percutaneous drainage and both were ultimately treated with repeat unilateral VATS, which was again followed by the insertion of a chest drain under direct vision. The Portex® 12Fr Seldinger chest drainage kit (Smiths Medical, Ashford, UK) with irrigation adaptor was used to allow ongoing irrigation of the abscess cavity.
Two mediastinal collections developed as a late complication. One of these was thought to have arisen because of an ongoing oesophageal leak from the Proximal Release Ultraflex™ oesophageal stent. The first was treated using sinus tract endoscopy. The left-sided 32Fr Portex® chest drain was removed and a GIF-H260 endoscope (Olympus, Southend-on-Sea, UK) was introduced into the mediastinal cavity via the tract of the chest drain, entering and irrigating the mediastinal collection under vision. On this occasion, the oesophageal stent could be visualised through the healing perforation. Again, the Portex® 12Fr Seldinger chest drainage kit with irrigation adaptor was sited to allow ongoing irrigation of the abscess cavity.
The second mediastinal collection was not accessible using sinus tract endoscopy and the abscess cavity was accessed through a mini-laparotomy sited in the epigastrium. A GIF-H260 endoscope was used to access the mediastinal collection transhiatally. The cavity was irrigated and a transhiatal Portex® 12Fr Seldinger chest drainage kit with irrigation adaptor was sited under vision to allow ongoing irrigation of the abscess cavity. In all cases, the cavities were irrigated until resolution of sepsis had been obtained.
The final stage of minimally invasive endoscopic therapy is to ensure that oesophageal continuity has been restored. A repeat OGD was performed following resolution of sepsis at a minimum of six weeks. The Polyflex® oesophageal stents were removed and the oesophageal mucosa was inspected. In three of the five patients, a small subcentimetre residual defect was identified as an internal opening of an oesophagocutaneous fistula, the fistula traversing both the mediastinum and pleural cavity to exit the skin at the lateral chest wall, the distal portion of the fistula being occupied by a chest drain. These fistulas were closed using the Surgisis® (Cook Surgical, Bloomington, IN, US) anal fistula plug (Fig 1), positioned endoscopically across the oesophageal defect using a rendezvous technique (Fig 2).
Figure 1.
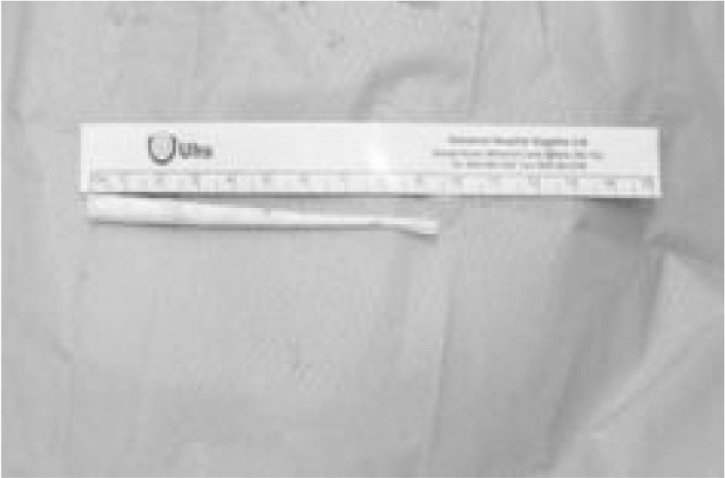
Surgisis® anal fistula plug
Figure 2.
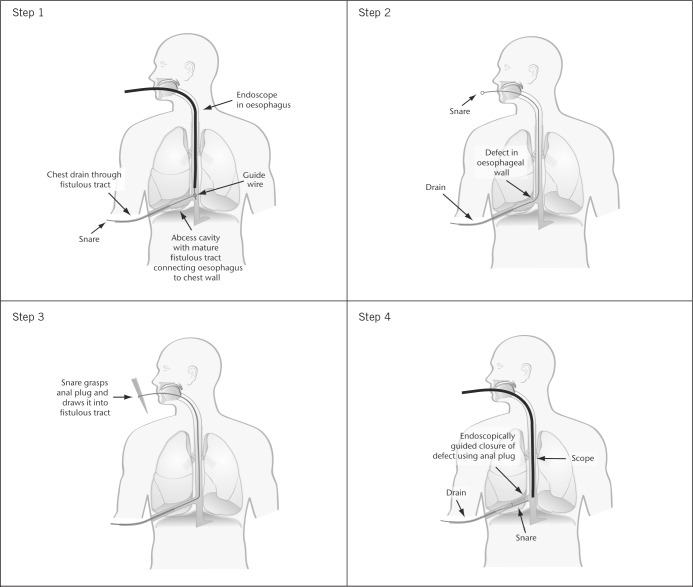
Rendezvous procedure steps 1–4
Step 1: An upper gastrointestinal endoscopy is performed, positioning the endoscope proximal to the oesophageal defect. A guidewire is passed through the endoscope and allowed to enter the oesophageal lumen. An endoscopic snare is introduced into the fistula tract via the left-sided chest drain until its position is such that the guidewire can be snared. The snare traverses the tubing of the chest drain laterally and the mediastinal abscess cavity medially to enter the oesophageal lumen through the residual defect in the oesophagus. With the passage of time, the mediastinal abscess cavity has significantly reduced in size, resulting in a relatively narrowed tract leading up to the site of the oesophageal perforation.
Step 2: The oesophagus is extubated, pulling the snare up through the oesophagus to emerge from the mouth.
Step 3: The snare can then be opened and closed around the fistula plug.
Step 4: The snare is pulled back into the oesophagus, carrying the fistula plug down with it into position. The endoscope is reintroduced to ensure the fistula plug traverses the oesophageal defect under vision. Once positioned correctly, the snare is released and withdrawn from the chest drain. The chest drain is left in situ for 24–48 hours to ensure that the fistula output has ceased and oral alimentation is reintroduced on day 5.
Results
In all five cases, endoscopic evidence of oesophageal re-epithelialisation was achieved on days 50, 50, 51, 59 and 103. Four of our patients are alive today, two of whom have required oesophageal dilatation of a resultant benign oesophageal stricture. Our 30-year-old male patient with significant medical co-morbidity, known oesophageal disease, a delayed presentation in extremis with a further delay in diagnosis failed to survive. He died on day 109 as a consequence of end stage diabetes, furosemide dependent renal failure, aspiration pneumonia and an intra-abdominal perforation from a change of percutaneous endoscopic gastrostomy tube, for which he was deemed not fit for laparotomy (Table 2).
Table 2.
Case series outcomes
| Case | Age / sex | Oesophageal re-epithelialisation | Length of hospital stay | Oesophgeal stricture | Present day status |
|---|---|---|---|---|---|
| 1 | 30 M | Day 103 | 109 days | No | Died on day 109 |
| 2 | 31 F | Day 59 | 65 days | Yes | Alive |
| 3 | 74 F | Day 50 | 57 days | Yes | Alive |
| 4 | 75 M | Day 51 | 55 days | No | Alive |
| 5 | 75 F | Day 50 | 61 days | No | Alive |
Conclusions
We have shown how new and innovative minimally invasive endoscopic therapies can be used to achieve oesophageal re-epithelialisation in a cohort of patients with Boerhaave’s syndrome. This is of particular importance as it challenges current beliefs that endoscopic therapy should be reserved for patients who present early and in whom there is no associated sepsis. We have demonstrated success in patients presenting both early and late, all having an associated septic profile.
It is also important that our patients represent a cohort not currently accounted for in the treatment algorithm of de Schippers et al 2 (ie those with a septic profile, precluding them from conservative management, although their condition renders them unfit for surgical intervention). We have shown that minimally invasive endoscopic therapy offers a real treatment alternative for such patients, without which they would be facing a mortality rate approaching 100%. 1 We have also expanded on previously described endoscopic therapies for the management of Boerhaave’s syndrome. Minimally invasive endoscopic therapy requires a dedicated multidisciplinary team approach to managing Boerhaave’s syndrome but the results are rewarding.
References
- 1. Boervaave Syndrome. Medscape reference. http://emedicine.medscape.com/article/171683 (cited August 2013).
- 2. de Schipper JP, Pull ter Gunne AF, Oostvogel HJ, van Laarhoven CJ. Spontaneous rupture of the oesophagus: Boerhaave’s syndrome in 2008. Dig Surg 2009; 26: 1–6. [DOI] [PubMed] [Google Scholar]
- 3. Griffin SM, Lamb PJ, Shenfine J et al Spontaneous rupture of the oesophagus. Br J Surg 2008; 95: 1,115–1,120. [DOI] [PubMed] [Google Scholar]
- 4. van Heel NC, Haringsma J, Spaander MC et al Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol 2010; 105: 1,515–1,520. [DOI] [PubMed] [Google Scholar]