Abstract
Introduction
We report the outcomes of a long-term surveillance programme for individuals with a family history of colorectal cancer.
Methods
The details of patients undergoing a colonoscopy having been referred on the basis of family history of colorectal cancer were entered prospectively into a database. Further colonoscopy was arranged on the basis of the findings. The outcomes assessed included incidence of cancer and adenoma identification at initial and subsequent colonoscopy.
Results
The records of 2,293 patients (917 men; median patient age: 51 years) were entered over 22 years, giving data on 3,982 colonoscopies. Eight adverse events (0.2%) were recorded. Twenty-seven cancers were found at first colonoscopy and thirteen developed during the follow-up period. There were significantly more cancers identified in those with more than one first-degree relative with cancer than in other groups (p=0.01). The number of adenomas identified at subsequent surveillance colonoscopies remained constant with between 9.3% and 12.0% of patients having adenomas that were removed. Two-thirds (68%) of patients with cancer and three-quarters (77%) with adenomas fell outside the British Society of Gastroenterology (BSG) 2006 guidelines.
Conclusions
Repeated colonoscopy continues to yield significant pathology including new cancers. These continue to occur despite removal of adenomas at prior colonoscopies. The majority of patients with cancers and adenomas fell outside the BSG 2006 guidelines; more would have fallen outside the 2010 guidelines.
Keywords: Colonoscopy, Family history, Adenoma, Colorectal cancer
Colorectal cancer (CRC) is the second most common cause of cancer related death in the UK and people with a family history are at greater risk of developing the disease. 1–3 Colonoscopy can identify cancers as well as identifying and removing premalignant lesions, adenomatous polyps. Colorectal adenomas are extremely common, affecting up to 40% of adults by the age of 60 years, 4 but since the lifetime cumulative incidence of CRC is 5%, only a small proportion of these adenomas become malignant. 5 At present, it is not possible to predict which adenomas will transform into carcinomas although increasing size, degree of dysplasia and multiplicity are risk factors for malignancy. 6
The British Society of Gastroenterology (BSG) has published guidelines for the screening of individuals at risk because of their family history 3 and follow-up of adenomas, which is risk stratified according to the findings at colonoscopy. 5 These guidelines were revised and became more specific in 2006 and 2010. 7
The concept that colonoscopy and adenoma excision may prevent CRC is supported by observational data. 8 Our audit examined the validity of this concept in a study with extended observation and repetitive colonoscopy. We present 22 years of experience from our colonoscopic screening programme for relatives of patients with CRC. An earlier analysis has already been published. 9
Methods
This study was performed on the records of patients routinely attending a UK district general hospital between 1990 and 2011. Patients were included if they were referred on the basis of family history, regardless of whether they were symptomatic. Exclusions included a personal history of CRC, ulcerative colitis, Crohn’s disease and familial adenomatous polyposis.
The study started in 1990 before any specific guidelines for screening patients with a family history of CRC were introduced. The unit policy was to offer a colonoscopy to anyone referred with a family history who was over 40 years old or at 10 years before the age of the youngest relative when he or she developed cancer. If nothing was found, they underwent a colonoscopy every five years. If an adenoma was found, a colonoscopy was performed at one year. If the results were negative, another colonoscopy was performed at 3 years and, if still clear, at 5-yearly intervals until age 80. Colonoscopy was performed after bowel preparation with sedation and non-invasive cardiorespiratory monitoring. At colonoscopy, polyps were removed by diathermy and examined histologically.
The BSG guidelines introduced in 2002 (and updated in 2006 and 2010) did not appreciably alter the unit policy for patients already enrolled in the study but they were applied gradually to new entrants. Our study therefore presents a group of patients of whom many would lie outside the current guidelines for investigation.
Colonoscopy reports were entered into a prospective database alongside patient demographics, the findings of subsequent colonoscopies, histology and follow-up details. The UK cancer registers were searched for cancers occurring in patients lost to follow-up, not on the waiting list or not seen at the hospital in the previous 18 months. The BSG 2006 guidelines for family history were applied during analysis for comparative purposes.
The chi-squared test was used to compare independent groups where the outcome was categorical (in this case proportions). A p-value of <0.05 was taken as statistically significant.
Results
A total of 2,293 patients (917 men [40%], median age: 51 years, range: 17–86 years; 1,376 women [60%], median age: 51 years, range: 16–91 years) were enrolled in the screening programme between 1990 and 2011 (Table 1). Another 217 patients referred to the screening programme were excluded from the study at referral: 65 did not have a family history of CRC, 12 had a diagnosis of familial adenomatous polyposis, 11 had ulcerative colitis, 11 had CRC and 3 were considered too old. One hundred and sixteen patients were offered a colonoscopy but declined. Patients had up to 9 colonoscopies, giving a total of 3,982 colonoscopies performed. The caecum was reached in 87% of all colonoscopies.
Table 1.
Age and sex at colonoscopy
| Finding at colonoscopy | n | Male | Female | Median age (range) |
|---|---|---|---|---|
| Cancer | 38 | 18 (1.1%) | 20 (0.8%) | 61 (38–82) |
| Adenoma | 397 | 200 (12.4%) | 197 (8.3%) | 57 (18–86) |
| Neither | 3,547 | 1,401 (86.5%) | 2,146 (90.8%) | 44 (16–90) |
| Total | 3,982 | 1,619 | 2,363 | 51 (16–90) |
Note: Two further cancers were only found after enquiry at the UK cancer registers.
Occurrence of adenomas
Overall, 397 adenomas were found in 3,982 colonoscopies. Those with adenomas at the first colonoscopy (median age: 55 years) were on average 17 years older than those without adenomas (median age: 38 years).
The percentage of patients with adenomas found at each subsequent colonoscopy remained fairly constant, between 10% and 14% (Fig 1). Of the 230 patients with an adenoma at the first colonoscopy (‘scope 1’), 34 (15%) had an adenoma at scope 2. Of the 2,036 patients with no adenoma at scope 1, adenomas were found sporadically in these initially ‘clear’ patients at subsequent colonoscopies with no particular pattern.
Figure 1.
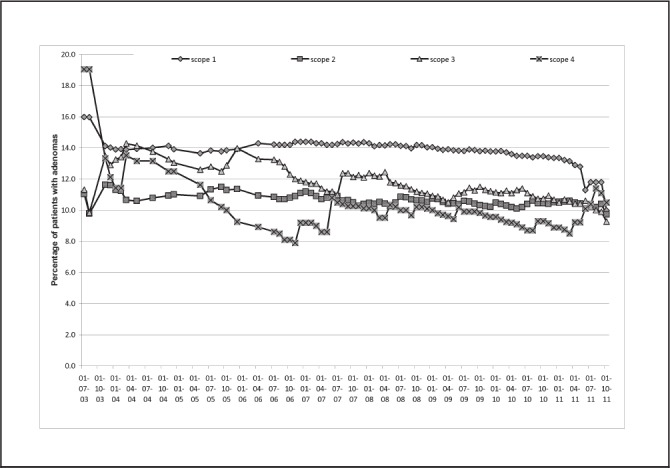
Percentage of patients with a family history of colorectal cancer found to have one or more adenomas on successive colonoscopies (scopes 1–4), illustrated over time since 2003
The site of adenoma is shown in Table 2. In 119 patients (30%), adenomas were found proximal to the splenic flexure in isolation.
Table 2.
Site of cancers and adenomas in relation to the splenic flexure on colonoscopy for patients with a family history of colorectal cancer
| Finding at colonoscopy | Above splenic flexure | Below splenic flexure | Both above and below splenic flexure | Site not clearly stated | Total |
|---|---|---|---|---|---|
| Cancer | 8 | 27 | 3 | 0 | 38 |
| % of all cancers | 21% | 71% | 8% | 0% | |
| Adenomas | 119 | 236 | 35 | 7 | 397 |
| % of all adenomas | 30% | 60% | 8% | 2% |
Note: Two further cancers were only found after enquiry at the UK cancer registers.
Colorectal cancer
A total of 40 patients (1.7%) were found to have CRC during this study: 27 (1.2%) at scope 1, six (0.6%) at scope 2, three (0.7%) at scope 3 and two (5.6%) at scope 5 (Table 1). Two patients developed cancer who were lost to follow-up but were found by searching the UK cancer registers. One of these developed CRC at fifteen months and the other at five years after the last colonoscopy. Both died subsequently of the disease.
Of those patients who had cancers found during follow-up colonoscopies, this was a metachronous cancer in one patient at scope 2 and in another patient at scope 5. Two patients with adenomas at scope 1 had cancer at scope 2. Of six patients clear at scope 1, three had cancer at scope 2 and another three at scope 3.
The cancer site is shown in Table 2. Eight cancers (20%) were proximal to the splenic flexure.
Family history
The number of relatives and finding of adenomas and cancer at colonoscopy for patients with affected first-degree relatives (FDRs) and second-degree relatives (SDRs) are shown in Table 3. Over half (n=1,167, 51%,) only had one FDR, 288 (13%) had one FDR and one SDR, 185 (8%) had one FDR and more than one SDR, and 180 (8%) had two FDRs.
Table 3.
Number of relatives and finding of adenomas and cancer at colonoscopy for patients with a family history of colorectal cancer
| Relatives | n | Cancer found at colonoscopy | Adenoma found at colonoscopy |
|---|---|---|---|
| 1 SDR | 102 | 2 (2.0%) | 20 (19.6%) |
| 2 SDRs | 62 | 1 (1.6%) | 18 (29.0%) |
| >2 SDRs | 46 | 2 (4.3%) | 3 (6.5%) |
| 1 FDR | 1,167 | 17 (1.5%) | 204 (17.5%) |
| 1 FDR + 1 SDR | 288 | 4 (1.4%) | 43 (14.9%) |
| 1 FDR + >1 SDR | 185 | 2 (1.1%) | 39 (21.1%) |
| 2 FDRs | 180 | 6 (3.3%) | 41 (22.8%) |
| 2 FDRs + 1 SDR | 33 | 2 (6.1%) | 4 (12.1%) |
| 2 FDRs + >1 SDR | 18 | 0 (0%) | 1 (5.6%) |
| >2 FDRs | 30 | 2 (6.7%) | 8 (26.7%) |
| >2 FDRs + 1 or >1 SDR | 5 | 0 (0%) | 1 (20.0%) |
| Not stated | 177 | 2 (1.1%) | 15 (8.5%) |
| Total | 2,293 | 40 (1.7%) | 397 (17.3%) |
Statistical comparisons
- 5 cancers in those with SDRs (2.3%) vs 33 in those with FDRs (1.7%) (p=0.9)
- 41 adenomas in those with SDRs (19.5%) vs 340 in those with FDRs (17.9%) (p=0.7)
- 23 cancers in those with 1 FDR (1.4%) vs 10 in those with >1 FDR (3.8%) (p=0.01)
- 286 adenomas in those with 1 FDR (21%) vs 54 in those with >1 FDR (21%) (p=0.6)
FDR = first-degree relative; SDR = second-degree relative
With respect to the identification of adenomas at colonoscopy, our data did not support a direct relationship between number or closeness of affected relatives and frequency of adenomas (Table 3). In fact, the percentage of identifiable adenomas remained quite constant, between 17% and 21%, when compared across categories of involved relatives.
There was no significant difference in the number of cancers detected between those with only SDRs (2.3%) and those with FDRs (1.7%). However, when the number of cancers found was compared between those with only one FDR (1.4%) and those with more than one FDR (3.8%), the difference was significant (p=0.01).
British Society of Gastroenterology guidelines
Nineteen patients (70%) with CRC and 161 (70%) with an adenoma at scope 1 were not eligible for colonoscopy according to the 2006 BSG guidelines. Overall, 1,645 colonoscopies (72%) occurred in patients outside the guidelines.
Follow-up, adverse reactions and deaths
The mean follow-up duration was 3.9 years (range: 0–18 years). Sixty-seven patients (age range: 44–75 years) died during the follow-up period. During the 3,982 procedures, 8 adverse events (0.2%) were recorded with 4 patients suffering haemorrhage and 3 possible perforations. All recovered.
Discussion
A number of genetic disorders including Lynch syndrome and familial adenomatous polyposis are associated with a high risk of gastrointestinal malignancy, and should undergo endoscopic surveillance. The Bowel Cancer Screening Programme provides population level screening in the UK using the faecal occult blood test (http://www.cancerscreening.nhs.uk/bowel/). Nevertheless, controversy still exists over screening regimens for those with an increased risk due to family history. The prevalence of a family history of one or more affected FDRs in the UK is 4–10%. 3 However, if BSG guidelines are followed, less than 1% of the population fulfil family history criteria for intervention. 7 This study reports surveillance with wider inclusion criteria. In 2,293 patients referred for colonoscopy with a family history of CRC, 40 cancers were found and 68% of these patients with cancer fell outside the 2006 BSG guidelines.
Colonoscopy allows identification of the group of patients who require regular surveillance, and aims to remove precancerous lesions and identify cancers as well as reassuring a much larger group that their risk of developing cancer is low. 7 In this study, 30% of adenomas and 20% of cancers were found proximal to the splenic flexure with no lesion distal to the flexure. This suggests flexible sigmoidoscopy would miss a significant number of individuals with pathology if used as a screening tool in those with a family history. It has been suggested that there is an increased incidence of right-sided colonic neoplastic change in patients with a familial predisposition. 10
The proportion of people aged 55 in the general population with at least 1 adenoma has been reported as 4–21% but adenomas with a significant risk of developing malignancy are only found in 2–6%. 7 Adenomas have been suggested to occur at a younger age in groups at higher risk due to family history although they remain rare under the age of 50. 11 Findings published in 2012 suggest that polypectomies not only reduce the incidence of CRC but also the risk of death from the disease. 12
This study reports two patients who developed cancer on follow-up colonoscopy despite previous removal of adenomas and six patients who developed cancer after an initially clear colonoscopy. These patients represent a clinical reality that lends support to continuing surveillance colonoscopies in those with a family history. Such interval cancers may represent missed lesions or an accelerated adenoma–carcinoma sequence. Overall, 25–50% of small adenomas and even 4% of cancers may be missed at the initial colonoscopy. 13
In this study, the percentage of patients with adenomas found at each subsequent colonoscopy remained fairly constant, between 10% and 15%. Repeated endoscopy has been found to increase the detection rate of cancers and advanced adenomas in studies 14 and population series 15 with the addition of a second test increasing the detection by 26% in women and 34% in men.
If repeated colonoscopy is the best form of detecting CRC in those with a family history, the next question is who to target for screening. Colonoscopy among FDRs of patients with CRC has been shown to yield higher rates of neoplasia than in control groups of the general population. 16,17 In this series, a 17.5% adenoma rate was found in those who had one FDR. There was a significantly higher rate of cancers identified in those with more than one FDR than in those with only one FDR.
A subject would only fit into the surveillance guidelines produced by the BSG if he or she had more than one FDR or one who was less than 45 years old. In this study, 68% of patients with cancer and 77% of patients with adenomas fell outside the BSG 2006 guidelines. A policy of colonoscopies for all patients in a defined age range with any family history of CRC would allow a significant number of precancerous and cancerous lesions to be identified. Repeated colonoscopies continue to yield pathology with a persistent number of adenomas found and new cancerous lesions.
The value of this study lies in the number of patients involved and the length of the follow-up period, allowing it to potentially challenge some of the assumptions on which UK guidelines are based. Although the results have enabled risk stratification for different levels of family history, there is no true control group. While population studies can give an assessment of the baseline incidence of CRC on colonoscopy (1.14–1.186% in one trial), 18 it is not known how many patients were at risk in these populations. A direct comparison between those at risk and those without family history would allow better risk stratification and justification for a greater level of surveillance.
In this study, 3,982 colonoscopies enabled detection of 38 cancers. If a 50% cure rate is assumed, 19 lives could have been saved over the study period. A cost of £550 per colonoscopy results in a cost of £115,000 per life saved. This positive achievement has to be considered in relation to the fact that 3,547 of the 3,982 colonoscopies (89%) were negative. Nevertheless, it is difficult to quantify the reassurance that may be given to those who may have personal experience of a relative with CRC. This study did rely on patients volunteering for referral and self-reporting of family history. However, it does report the outcomes of real life referrals that reach the endoscopy suite. Future developments, including identification of certain genetic polymorphisms, may allow a personalised estimation of risk and a more specific surveillance strategy.
Conclusions
Repeated colonoscopy continues to yield significant pathology including new cancers. These continue to occur despite removal of adenomas at prior colonoscopies. The majority of patients with cancers and adenomas fell outside the BSG 2006 guidelines; more would have fallen outside the 2010 guidelines.
Acknowledgements
CSG has been supported for the past ten years by the Lady Sobell Gastrointestinal Unit Trust, Wexham Park Hospital.
The results that form the basis of this paper were presented by JMG in his presidential address to the Royal Society of Medicine Coloproctology Section held in London, October 2012.
References
- 1. Fuchs CS, Giovannucci EL, Colditz GA et al A prospective study of family history and the risk of colorectal cancer. N Engl J Med 1994; 331: 1,669–1,674. [DOI] [PubMed] [Google Scholar]
- 2. Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: the Utah Population Database. J Natl Cancer Inst 1994; 86: 1,618–1,626. [DOI] [PubMed] [Google Scholar]
- 3. Dunlop MG. Guidance on large bowel surveillance for people with two first degree relatives with colorectal cancer or one first degree relative diagnosed with colorectal cancer under 45 years. Gut 2002; 51: v17–v20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lieberman DA, Weiss DG, Bond JH et al Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med 2000; 343: 162–168. [DOI] [PubMed] [Google Scholar]
- 5. Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002; 51: v6–v9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stryker SJ, Wolff BG, Culp CE et al Natural history of untreated colonic polyps. Gastroenterology 1987; 93: 1,009–1,013. [DOI] [PubMed] [Google Scholar]
- 7. Cairns SR, Scholefield JH, Steele RJ et al Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups. Gut 2010; 59: 666–689. [DOI] [PubMed] [Google Scholar]
- 8. Winawer SJ, Zauber AG, O’Brien MJ et al Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med 1993; 328: 901–906. [DOI] [PubMed] [Google Scholar]
- 9. Gilbert JM, Vaizey CJ, Cassell PG, Holden J. Feasibility study of colonoscopy as the primary screening investigation in relatives of patients with colorectal cancer. Ann R Coll Surg Engl 2001; 83: 415–419. [PMC free article] [PubMed] [Google Scholar]
- 10. Banerjea A, Clark S, Dorudi S. The changing face of familial colorectal cancer. BMJ 2005; 330: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradshaw N, Holloway S, Penman I et al Colonoscopy surveillance of individuals at risk of familial colorectal cancer. Gut 2003; 52: 1,748–1,751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zauber AG, Winawer SJ, O’Brien MJ et al Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bressler B, Paszat LF, Vinden C et al Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology 2004; 127: 452–456. [DOI] [PubMed] [Google Scholar]
- 14. Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ 2005; 331: 1,047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weissfeld JL Schoen RE, Pinsky PF et al Flexible sigmoidoscopy in the randomized prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial: added yield from a second screening examination. J Natl Cancer Inst 2012; 104: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephenson BM, Murday VA, Finan PJ et al Feasibility of family based screening for colorectal neoplasia: experience in one general surgical practice. Gut 1993; 34: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puente Gutiérrez JJ, Marín Moreno MA, Domínguez Jiménez JL et al Effectiveness of a colonoscopic screening programme in first-degree relatives of patients with colorectal cancer. Colorectal Dis 2011; 13: e145–e153. [DOI] [PubMed] [Google Scholar]
- 18. Hardcastle JD, Chamberlain JO, Robinson MH et al Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348: 1,472–1,477. [DOI] [PubMed] [Google Scholar]


