Abstract
Bioassay-directed fractionation of an antiproliferative ethanol extract of the leaves and twigs of Piptocoma antillana (Asteraceae) afforded two new goyazensolide-type sesquiterpene lactones named 5-O-methyl-5-epiisogoyazensolide (1) and 15-O-methylgoyazensolide (2), together with the known compounds 1-oxo-3,10-epoxy-8-(2-methylacryloxy)-15-acetoxygermacra-2,4,11(13)-trien-6(12)-olide (3) and 5-epiisogoyazensolide (4). The structure elucidation of all compounds was carried out based on NMR and mass spectroscopic data analyses. The relative and absolute configurations of all the isolated compounds were determined from their CD and NOESY NMR spectra. Compounds 1–4 showed moderately potent antiproliferative activities against A2780 ovarian cancer cells, with IC50 values of 1.5±0.5, 0.6±0.3, 1.62±0.05, and 1.56±0.04 µM, respectively. They also displayed antimalarial activity against Plasmodium falciparum, with IC50 values of 6.2 ± 0.5, 2.2 ± 0.5, 8.0 ±0.4, and 9.0±0.6 µM, respectively.
Keywords: Antiproliferative activity, Antimalarial activity, Piptocoma antillana, Sesquiterpene lactone, Goyazensolide-type, Dereplication
The genus Piptocoma, a member of the Asteraceae family, was first discovered in the West Indies, and consisted initially of only three species. The genus was expanded in 2000, when Pruski described the Central and South American plant species from the genus Pollalesta as cogeneric with Piptocoma, and this raised the number of Piptocoma species to eighteen[2]. A literature search demonstrated that the only reports on the chemical constituents of the genus Piptocoma were in the last two years, describing the isolation of cytotoxic sesquiterpene lactones, phenylpropanol coumarates, and flavonoids from P. rufescens[3]. Goyazensolide (5), one of the isolated sesquiterpene lactones, was later shown to induce apoptosis in cancer cells in vitro and in vivo[4].
Our ongoing screening of extracts from the Natural Products Discovery Institute collection as part of a collaborative research program to explore the potential of the former Merck collection of extracts[5] indicated that a CH2Cl2 extract of the leaves and twigs of P. antillana exhibited significant antiproliferative activity against the A2780 human ovarian cancer cell line. We report herein the bioassay-guided isolation and structure identification of the components responsible for the antiproliferative activity, as well as the antiplasmodial activity of the same compounds.
An active CH2Cl2-soluble fraction obtained from liquid-liquid partition of the extract (100 mg) was subjected to dereplication studies using size-exclusion chromatography, reverse phase HPLC coupled with bioassay, high-resolution ESIMS (HRESIMS), 1HNMR, and a database search using the online Dictionary of Natural Product (DNP). The results indicated that the extract contained at least one new bioactive compound, and so a 2.0 g sample was investigated. Fractionation of this extract yielded an antiproliferative CH2Cl2 fraction which was further subjected to size-exclusion column chromatography on Sephadex LH-20 followed by normal phase silica gel column chromatography. The most active fractions from the silica gel column were subjected to C-18 HPLC to yield two new (1, 2) and two known (3, 4) bioactive sesquiterpene lactones.
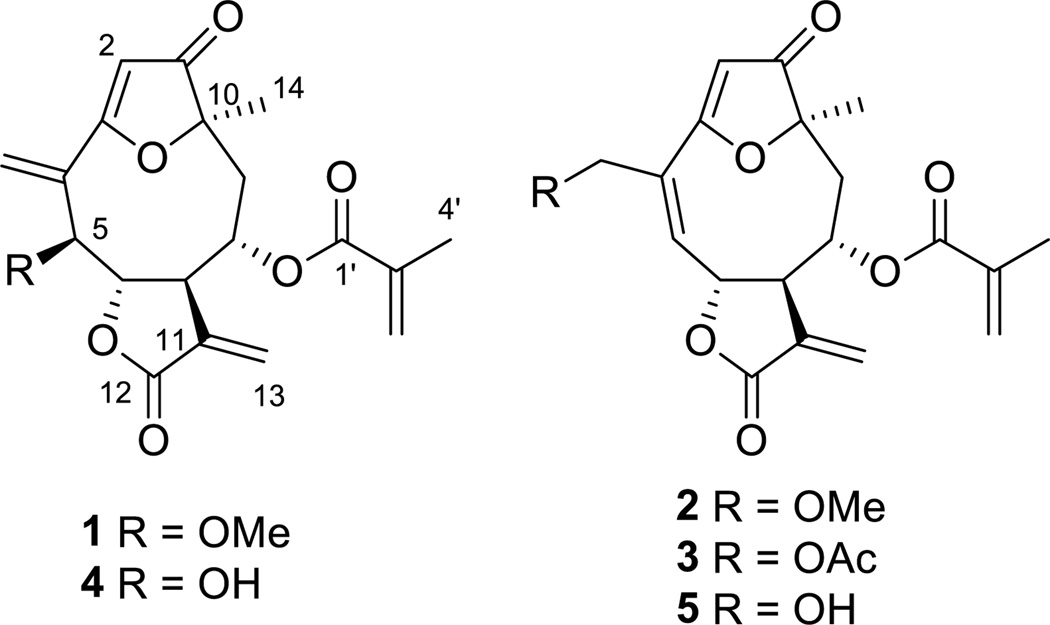
Compound 4 had the molecular formula C19H20O7 as determined by high resolution electrospray ionization mass spectroscopy (HRESIMS) analysis. It was determined to be 5-epiisogoyazensolide (4) by searching its molecular formula and key 1H NMR spectroscopic features in the DNP database and comparison of its full 1H NMR spectrum with reported literature data[6]. Compound 3 was identified as 1-oxo-3,10-epoxy-8-(2-methylacryloxy)-15-acetoxy-germacra-2,4,11(13)-trien-6(12)-olide (15-acetylgoyazensolide) by comparison of its spectroscopic data with values reported in the literature[7].
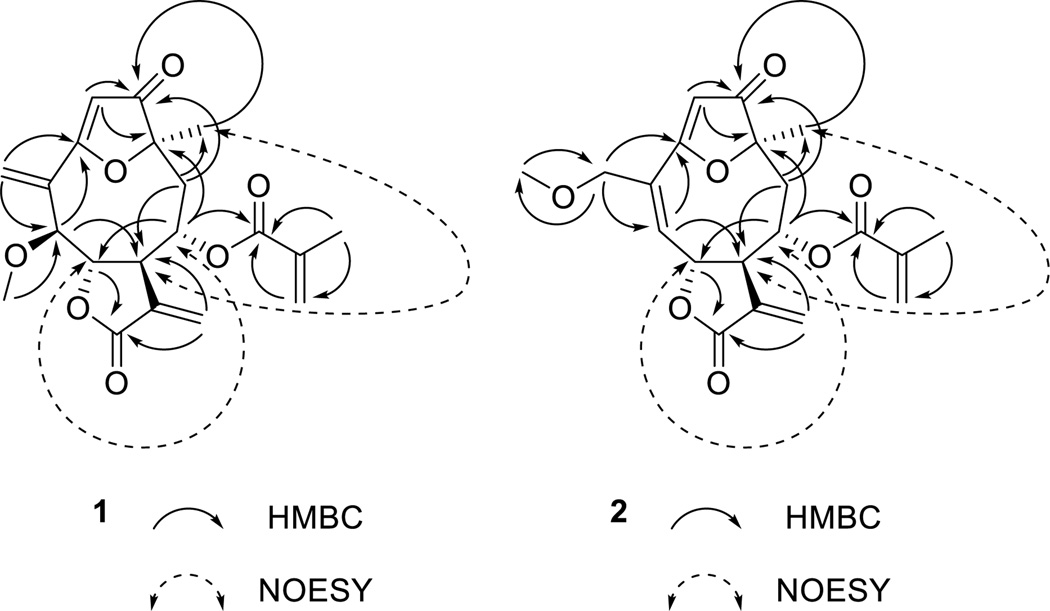
Compound 1 was obtained as an amorphous white powder and had the molecular formula C20H22O7 as indicated by HRESIMS analysis (m/z: 375.1461 [M+H]+, calcd. for C20H23O7+, 375.1438). Its IR spectrum showed absorptions characteristic of an α,β-unsaturated γ-lactone (1765 and 1640cm−1) and an α,β-unsaturated ester (1710 and 1645 cm−1) functions, as well as a dihydrofuran-3-one ring (1709 and 1582 cm−1)[3a, 6c, 7a, 8]. The similarity of the IR spectroscopic data of 1 with those of 4, in conjunction with the UV absorption characteristics at λmax 208 and 270 nm, suggested that 1 was also a furan ring-containing germacranolide similar to goyazensolide or furanoheliangolide[6c, 7a, 8]. Its 1H NMR spectrum (Table 1) displayed signals for a methyl group at δH1.53 (s, 3H, CH3-14), two exocyclic methylene groups as two doublets at δH 6.21 (J= 3.3 Hz, 1H) and 5.46 (J=2.9 Hz, 1H) and two singlets at δH6.00 and 5.78 (each 1H), representing (H2-13 and H2-15), and an olefinic proton at δH5.82 (s, 1H, H-2). These signals are all very similar to those in the goyazensolide skeleton of 4. In the 13C NMR spectrum, the presence of signals attributable to a conjugated ketone carbonyl at δC204.4 ppm (C-1), a lactone carbonyl at δC169.1(C-12), the carbons of an oxygen bearing alkene at δC 106.8 (C-2) and 185.9 (C-3) and of two exocyclic alkenes at δC 133.9 (C-11), 123.8 (C-13), 134.5 (C-4), and 127.1 (C-15), and three oxygen bearing methines at δC 84.5 (C-5), 85.1 (C-6), and 72.3 (C-8), supported the preliminary structural assignment[7c]. In the HMBC experiment, protons at δH6.00and 5.78 correlated with both C-3 (δC 185.9) and C-5 (δC 84.5), which allowed us to assign the two olefinic protons to be those of H2-15 (Fig. 2). In the same manner, the other pair of the exocylic methylene protons at δH 6.21 and 5.46 were determined to be those of H2-13 due to their correlations with δC169.1(C-12) and δC44.8(C-7). The 1H and 13C NMR spectra of 1 also revealed the expected signals from the goyazensolide methacrylate side chain, namely for a methyl group at δH1.82 (s, 3H, H-4'), two olefinic proton signals at δH 5.95 and 5.53 (m, 1H for each, H-3'), and four carbon resonances at δC 166.9 (C-1'), 135.4 (C-2'), 126.4 (C-3'), and 18.0 (C-4')[7c]. This assignment was confirmed by the HMBC correlations (Fig. 2) between the proton signals of H-4' with both C-1' and C-3'. The acyl group was located at C-8 by the long range HMBC cross peak observed between the signals at δH4.31 (ddd, J=11.8, 1.4, 1.3 Hz, 1H, H-8) and δC 166.9 (C-1'). The1H spectrum of 1 contained an additional proton signal at δH3.73 (s, 3H) as compared with the spectrum of 4. A carbon signal at δC57.2 and a deshielded proton signal at δH 4.23 (H-5) confirmed the presence of a methoxyl group at C-5 in 1 as compared with the hydroxyl group of 4. The HMBC correlation between δH3.73 (s, 3H) and δC 84.5 (C-5) substantiated the placement of the methoxyl group at the C-5 position in 1. The complete assignments of all protons and carbons of 1 (Table 1) were accomplished by further interpretation of the HMBC and NOESY spectra. The relative configurations at C-5, C-6, C-7, C-8, and C-10 of 1 were suggested by the analysis of a NOESY experiment (Fig. 2). The absolute configuration at C-7 was determined to be R by the negative Cotton effects at 225 and 268 nm observed in its CD spectrum. This is consistent with previous studies demonstrating that sesquiterpene lactones with a C-7, C-6 trans-fused α-methylene-γ-lactone moiety display negative Cotton effects in the range of 216–225 and 252–271 nm in their CD spectra, arising from the π→π * and n→π* transitions of the lactone ring, respectively[9]. Compound 1 was thus assigned as (5S,6S,7R,8S,10R)-1-oxo-3,10-epoxy-5-methoxy-8-(2-methylacryloxy)germacra-2,4(15),11(13)-trien-6,12-olide[10], or 5-O-methyl-5-epiisogoyazensolide.
Table 1.
1H and 13C NMR data for compounds 1 and 2.
| 1 | 2 | |||
|---|---|---|---|---|
| Posn | δHb | δCc | δHb | δCc |
| 1 | 204.4 C | 204.6 C | ||
| 2 | 5.82 s | 106.8 CH | 5.77 brs | 106.4 CH |
| 3 | 185.9 C | 184.4 C | ||
| 4 | 134.5 C | 131.8 C | ||
| 5 | 4.23 s | 84.5 CH | 6.27 dt (2.9, 1.4) | 137.3 CH |
| 6 | 4.74 d (5.2) | 85.1 CH | 5.33 ddt (5.5, 2.9, 1.4) | 81.3 CH |
| 7 | 4.21 m | 44.8 CH | 3.80 ddd (5.5, 3.2, 2.7) | 50.9 CH |
| 8 | 4.31 ddd (11.8, 1.4, 1.3) | 72.3 CH | 4.54 dt (11.8, 2.2) | 73.2 CH |
| 9 | 2.52 dd (13.7, 11.8) | 44.6 CH2 | 2.49 dd (13.9, 11.8) | 43.9 CH2 |
| 2.33 dd (13.7, 1.4) | 2.32 dd(13.9, 2.2) | |||
| 10 | 90.4 C | 89.8 C | ||
| 11 | 133.9 C | 133.1 C | ||
| 12 | 169.1 C | 168.6 C | ||
| 13 | 6.21 d (3.3) | 123.8 CH2 | 6.23 d (3.2) | 124.6 CH2 |
| 5.46 d (2.9) | 5.47 d (2.7) | |||
| 14 | 1.53 s | 21.2 CH3 | 1.54 s | 20.7 CH3 |
| 15 | 6.00 s | 127.1 CH2 | 4.16 ddd (12.8, 1.4, 1.3) | 72.7 CH2 |
| 5.78 s | 4.12 ddd (12.8,1.4, 1.3) | |||
| 1' | 166.9 C | 166.8 C | ||
| 2' | 135.4 C | 135.3 C | ||
| 3' | 5.95 m | 126.4 CH2 | 6.01 brs | 126.6 CH2 |
| 5.53 m | 5.55 m | |||
| 4' | 1.82 s | 18.0 CH3 | 1.83 s | 18.0 CH3 |
| 5-OMe | 3.73 s | 57.2 CH3 | ||
| 15-OMe | 3.40 s | 58.5 CH3 | ||
Assignments based on analysis of 2D NMR spectra.
Data (δ) measured at 500 MHz; s= singlet, br s=broad singlet, d=doublet, dd=doublet of doublets, ddd=doublet of doublets of doublets, dt=doublet of triplets, m=multiplet. J values are in Hz and are omitted if the signals overlapped as multiplets. The overlapped signals were assigned from HSQC and HMBC spectra without designating multiplicity.
Data (δ) measured at 125 MHz; CH3, CH2, CH, and C multiplicities were determined by HSQC experiment.
Figure 2.
Key HMBC and NOESY correlations of 1 and 2.
Compound 2 was isolated as an amorphous white powder with a molecular formula of C20H22O7 based on its HRESIMS spectrum. The close similarity of the UV, IR,1H and 13C NMR spectroscopic data of 2 with those of 1 and 3 demonstrated that all three compounds contain the same goyazensolide skeleton with a methacrylate side chain at the C-8 position. Comparison of the 1H NMR spectroscopic data of 2 with those of 3 indicated that the major differences between them were the lack of signals for the 15-acetyl group in 3 and the addition of signals at δH 3.40 (s, 3H) and δC 58.5 (15-OMe) for an O-methyl group. HMBC correlations from the H-15 with the methoxy carbon at δC 58.5 and from the methoxy protons δH 3.40 (s, 3H) with the deshielded oxygen bearing methylene carbon at δC 72.7 (C-15) confirmed that the methoxy group was at C-15. As in the case of 1, all proton and carbon signals of 2 were assigned by interpretation of the data obtained from HMBC and NOESY experiments (Table 1). In addition, comparison of the CD and NOESY spectrum of 2 with 1 indicated that both compounds have the same absolute configurations at their C6, C7, C8, and C10 stereogenic centers. Therefore, compound 2 was determined to be (6S,7R,8S,10R)-1-oxo-3,10-epoxy-15-methoxy-8-(2-methylacryloxy)germacra-2,4(5),11(13)-trien-6,12-olide[7a], or 15-O-methylgoyazensolide.
Compounds 1–4 were evaluated for their antiproliferative activity against the A2780 human ovarian cancer cell line. They showed micromolar activities with half-maximum inhibitory concentration (IC50) values of 1.5±0.5,0.6 ±0.3, 1.62 ±0.05, and1.56 ±0.04 µM, respectively. All the compounds were further evaluated for their antimalarial activity against P. falciparum Dd2 (a chloroquine/mefloquine-resistant strain), and showed IC50 values of 6.2 ± 0.5, 2.2 ± 0.5, 8.0±0.4, and 9.0 ± 0.6 µM, respectively.
Experimental section
General experimental procedures
IR and UV spectra were measured on MIDAC M-series FTIR and Shimadzu UV-1201 spectrophotometers, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 spectrometer in CDCl3, with CDCl3 as reference. Mass spectra were obtained on an Agilent 6220 mass spectrometer. Open column chromatography was performed using Sephadex LH-20 and silica gel (230-400 mesh, Silicycle Co. USA). Semi-preparative HPLC was performed using Shimadzu LC-10AT pumps coupled with a semipreparative Phenomenex C-18 column (5 μm, 250 × 10 mm), a Shimadzu SPD M10A diode array detector, and a SCL-10A system controller. All isolated compounds were purified to 95% purity or better, as judged by HPLC (both UV and ELSD detection) before determining bioactivity.
Plant material
Leaves and twigs of P. antillana Urb were collected by Hannah Stevens of the New York Botanical Garden near Manatí, Puerto Rico (18°4.385 N, 66°54.382 W). A voucher specimen is on deposit at the NYBG under the accession number HS 00246a.
Antiproliferative bioassay
The A2780 ovarian cancer cell line antiproliferative bioassay was performed at Virginia Tech as previously reported[11]. The A2780 cell line is a drug-sensitive ovarian cancer cell line[12].Paclitaxel was used as the positive control; it had an IC50 value of 0.024 µM.
Antimalarial bioassay
The effect of each compound on parasite growth of the Dd2 strain of P. falciparum was measured in a 72 h growth assay in the presence of compound as described previously with minor modifications[13]. Briefly, ring stage parasite cultures (1% hematocrit and 1% parasitemia) were grown for 72 h in the presence of increasing concentrations of the drug in a 5.05% CO2, 4.93% O2, and 90.2% N2 gas mixture at 37 °C. After 72 h in culture, parasite growth was determined by DNA quantitation using SYBR Green I.31 The half-maximal inhibitory concentration (IC50) calculation was performed with GraFit software using a nonlinear regression curve fitting. IC50 values are the average of three independent determinations with each determination in duplicate and are expressed ± SD. Artemisinin was used as the positive control; it had an IC50 value of 7 nM.
Extraction and Isolation
The dried and powdered leaves and twigs of P. antillana were exhaustively extracted with ethanol in two 24-hour percolation steps; successive partition of the concentrated extract with hexane and methylene chloride gave an active methylene chloride extract. For purposes of fractionation and purification, 2.0 g of the original ethanol extract designated 1000892-7G (IC50 12 µg/mL) was suspended in 90% aq. MeOH (300 mL), and extracted with hexanes (3 × 200 mL). Evaporation of the hexane-soluble fraction afforded 176 mg of residue. The 90% aq. MeOH layer was then diluted to 60% and extracted with CH2Cl2 (3 × 200 mL) to yield 479 mg of CH2Cl2-soluble fraction. The aqueous MeOH layer was concentrated to give 1.3 g of brown residue. The CH2Cl2 fraction was found to have antiproliferative activity with an IC50 value of 0.62 µg/mL, and was subjected to Sephadex LH-20 open column chromatography (CH2Cl2:MeOH, 1:1) to give 5 fractions. The most active fraction Fr 3 (IC500.5 µg/mL) was then divided into 6 sub-fractions by silica gel column chromatography (hexanes:EtOAc, 1:1). Further purification of the most active sub-fraction Fr 3-4 (IC500.3 µg/mL) was done by using HPLC on a C-18 column with a solvent gradient from H2O:CH3CN, 70:30 to 68:32 from 0 to 30 min, to 58:42 from 30 to 60 min, and ending with 100% CH3CN from 60 to 80 min. This process yielded compounds 4 (2.5 mg, tR31 min), 2 (1.5 mg, tR60 min), 1 (1.1 mg, tR64.5 min), and 3 (1.2 mg, tR66 min).
5-O-methyl-5-epiisogoyazensolide (1)
Amorphous powder, [α]D -95.6 (c 0.27, MeOH)
CD (c 0.031, MeOH, nm) λmax (Δε) 206 (+10), 225 (−4.0), 268 (−4.2), 320 (+2.5)
UV (MeOH) λmax nm (log ε): 270 (2.20), 208 (3.89)
IR (film) νmax cm−1: 1765, 1710, 1709, 1645, 1640, 1582, 1454 1H NMR (500 MHz, CDCl3): Table 1.
13C NMR (125 MHz, CDCl3): Table 1.
HRESIMS: m/z [M+H]+, calcd. for C20H23O7+: 375.1438; found: 375.1461.
5-O-methylgoyazensolide (2)
Amorphous powder, [α]D −121.8 (c 0.27, MeOH)
CD (c 0.031, MeOH, nm) λmax (Δε) 203 (+5.7), 216 (−1.8), 276 (−2.3), 318 (+2.9)
UV (MeOH) λmax nm (log ε) : 267 (2.67), 207 (4.01)
IR (film) νmax cm−1: 1769, 1710, 1709, 1653, 1587, 1456;
1H NMR (500 MHz, CDCl3): Table 1.
13C NMR (125 MHz, CDCl3): Table 1.
HRESIMS: m/z [M+H]+, calcd. for C20H23O7+: 375.1438; found: 374.1459.
Supplementary Material
Figure 1.
Structures of compounds 1–5.
Acknowledgments
This project was supported by the National Center for Complementary and Alternative Medicine under award 3U01TW000313-19S1 as a supplement to Cooperative Agreement U01 TW000313-19 from the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, with the International Cooperative Biodiversity Groups. J.D.W. was a recipient of a scholarship from the National Science Foundation S-STEM project (DUE-0850198). These supports are gratefully acknowledged. This work was also supported by the National Science Foundation under Grant No.CHE-0619382 for purchase of the Bruker Avance 500 NMR spectrometer and Grant No.CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Mr. B. Bebout for obtaining the mass spectra and Dr. H. Azurmendi for assistance with the NMR spectra. We gratefully acknowledge Dr. D. Stevenson, D. Atha, and H. Stevens of the New York Botanical Garden for the provision of plant material.
Footnotes
Supplementary data: 1H and 13C NMR spectra of compounds 1 and 2 and 1H NMR spectra of 3 and 4 are available in electronic form on the publisher’s website.
References
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 60. For Part 59, see Rasamison VE, Rakotondraibe H, Slebodnick C, Brodie PJ, Ratsimbason M, TenDyke K, Shen Y, Randrianjanaka LM, Kingston DGI. Nitrogen-Containing Dimeric nor-Multiflorane Triterpene from a Turraea sp. Organic Letters. 2014;16:2626–2629. doi: 10.1021/ol500775r.
- 2.Pruski JF. Compositae of the Guayana highland—X. Reduction of Pollalesta to Piptocoma (Vernonieae: Piptocarphinae) and consequent nomenclatural adjustments. Novon. 1996;6:96–102. [Google Scholar]
- 3.a) Ren Y, Acuna UM, Jimenez F, Garcia R, Mejia M, Chai H, Gallucci JC, Farnsworth NR, Soejarto DD, Carcache de Blanco EJ, Kinghorn AD. Cytotoxic and NF-κB inhibitory sesquiterpene lactones from Piptocoma rufescens. Tetrahedron. 2012;68:2671–2678. doi: 10.1016/j.tet.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ren Y, Jimenez F, Garcia R, Mejia M, Chai H, Farnsworth NR, Soejarto DD, Kinghorn AD. A cytotoxic dimeric furanoheliangolide from Piptocoma rufescens. Tetrahedron Letters. 2013;54:5457–5460. doi: 10.1016/j.tetlet.2013.07.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acuna UM, Shen Q, Ren Y, Lantvit DD, Wittwer JA, Kinghorn AD, Swanson SM, Carcache de Blanco EJ. Goyazensolide induces apoptosis in cancer cells in vitro and in vivo. International Journal of Cancer Research. 2013;9:36–53. doi: 10.3923/ijcr.2013.36.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis L. Marketing mother nature’s molecules. Chemical and Engineering News. 2012;90(8):30. [Google Scholar]
- 6.a) Lang G, Mayhudin NA, Mitova MI, Sun L, van der Sar S, Blunt JW, Cole ALJ, Ellis G, Laatsch H, Munro MHG. Evolving trends in the dereplication of natural product extracts: New methodology for rapid, small-scale investigation of natural product extracts. Journal of Natural Products. 2008;71:1595–1599. doi: 10.1021/np8002222. [DOI] [PubMed] [Google Scholar]; b) Wolfender J-L, Ndjoko K, Hostettmann K. The potential of LC-NMR in phytochemical analysis. Phytochemical Analysis. 2001;12:2–22. doi: 10.1002/1099-1565(200101/02)12:1<2::AID-PCA552>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]; c) Delgado G, Romo de Vivar A, Herz W. Sesquiterpene lactones from Viguiera species. Phytochemistry. 1982;21:1305–1308. [Google Scholar]
- 7.a) Batistada CF, Dias DA, Lopes JLC, Vichnewski W. Flavonoids and heliangolides from Lychnophora diamantinana. Phytochemistry. 1993;34:261–263. [Google Scholar]; b) Soares ACF, Silva AN, Matos PM, da Silva EH, Heleno VCG, Lopes NP, Lopes JLC, Sass DC. Complete 1H and 13C NMR structural assignments for a group of four goyazensolide-type furanoheliangolides. Quim. Nova. 2012;35:2205–2209. [Google Scholar]; c) Lunardello MA, Tomaz JC, Vichnewski W, Lopes JLC. Sesquiterpene lactones and flavonoids from Eremanthus mattogrossensis and Eremanthus eriopus. Journal of Brazilian Chemical Society. 1995;6:307–311. [Google Scholar]
- 8.Herz W, Kumar N. Sesquiterpene lactones of Calea zacatechichi and C. urticifolia. Phytochemistry. 1980;19:593–597. [Google Scholar]
- 9.a) Youssef D, Frahm AW. Circular dichroism of C-7, C-6 trans-fused guaianolides of Centaurea scoparia. Phytochemistry. 1996;41:1107–1111. [Google Scholar]; b) Stoecklin W, Waddell TG, Geissman TA. Circular dichroism and optical rotatory dispersion of sesquiterpene lactones. Tetrahedron. 1970;26:2397–2409. [Google Scholar]; c) Waddell TG, Stoecklin W, Geissman TA. Circular dichroism of sesquiterpene lactones. Tetrahedron Letters. 1969:1313–1316. [Google Scholar]
- 10.Valdes DA, Bardon A, Catalan CAN, Gedris TE, Herz W. Goyazensolides and isogoyazensolides from Argentine Centratherum punctatum ssp. punctatum. Biochemical Systematics and Ecology. 1998;26:805–808. [Google Scholar]
- 11.Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. Antiproliferative xanthones of Terminalia calcicola from the Madagascar rain forest. Journal of Natural Products. 2007;70:679–681. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Research. 1985;45:2110–2115. [PubMed] [Google Scholar]
- 13.a) Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrobial Agents Chemotherapy. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrobial Agents Chemotherapy. 2004;48:1803–18061. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.