Abstract
Sudden unexpected infant deaths (SUID) accounted for 1 in 3 postneonatal deaths in 2010. Sudden infant death syndrome and accidental sleep-related suffocation are among the most frequently reported types of SUID. The causes of these SUID usually are not obvious before a medico-legal investigation and may remain unexplained even after investigation. Lack of consistent investigation practices and an autopsy marker make it difficult to distinguish sudden infant death syndrome from other SUID. Standardized categories might assist in differentiating SUID subtypes and allow for more accurate monitoring of the magnitude of SUID, as well as an enhanced ability to characterize the highest risk groups. To capture information about the extent to which cases are thoroughly investigated and how factors like unsafe sleep may contribute to deaths, CDC created a multistate SUID Case Registry in 2009. As part of the registry, the Centers for Disease Control and Prevention developed a classification system that recognizes the uncertainty about how suffocation or asphyxiation may contribute to death and that accounts for unknown and incomplete information about the death scene and autopsy. This report describes the classification system, including its definitions and decision-making algorithm, and applies the system to 436 US SUID cases that occurred in 2011 and were reported to the registry. These categories, although not replacing official cause-of-death determinations, allow local and state programs to track SUID subtypes, creating a valuable tool to identify gaps in investigation and inform SUID reduction strategies.
Keywords: sudden infant death syndrome, sudden unexpected infant death, infant mortality, accidental suffocation, classification, child death review, surveillance
In the United States, ~1 in 7 infant deaths and 1 in 3 postneonatal deaths were attributed to sudden unexpected infant death (SUID) in 2010.1 The most frequently reported causes of SUID are sudden infant death syndrome (SIDS), ill-defined and unknown cause of mortality, and accidental sleep-related suffocation.2 Differentiating between these causes, especially SIDS and infant suffocation, can be challenging, because SUID case investigations are not always conducted in a standard manner, and universally accepted definitions or biological markers to distinguish SIDS from suffocation do not exist.3,4 To complicate matters, both SIDS and accidental sleep-related suffocations are frequently unwitnessed and occur in unsafe sleeping environments. SIDS is reserved for deaths that remain unexplained after a thorough case investigation.5 Accidental sleep-related suffocation relies on scene evidence of an infant being suffocated or strangulated by items or persons in a sleep environment. A standardized classification system with carefully delineated criteria that recognizes inconclusive evidence might assist in this differentiation. Moreover, such a system may allow for improved monitoring of SUID, enhance our ability to characterize the highest risk groups, and identify pathophysiologic and genetic mechanisms underlying these deaths.
Currently we rely on death certificates to monitor population estimates of SUID mortality. Unfortunately our ability to accurately monitor SIDS and other SUID is hindered by situations in which the cause of death reported on the death certificate may not be classified and coded as the certifier intended. For example, the Tenth Revision of the International Classification of Diseases (ICD-10) does not provide a unique coding category for the term SUID. Thus, reports of SUID are often coded as SIDS, even though certifiers may use them to mean different things.6 As a result of these challenges in investigating, reporting, and classifying deaths, we have an incomplete understanding of actual SUID trends and risk factors. Because vital records do not reveal the extent to which cases may have been thoroughly investigated, or how factors like unsafe sleep may contribute to death, the Centers for Disease Control and Prevention (CDC) created the SUID Case Registry in 2009.7–9
On January 1, 2010, the SUID Case Registry pilot program began actively collecting data in 5 states (Colorado, Georgia, Michigan, New Jersey, and New Mexico) using methods described elsewhere.9 On January 1, 2011, 2 additional states (Minnesota and New Hampshire) began collecting data. These states were awarded cooperative agreements with the CDC after a competitive proposal process. A major objective of this new SUID Case Registry was to create a classification system using standardized definitions to assign categories to cases reported in the case registry. Several SIDS and SUID definitions and classifications have recently been proposed,10–18 although not universally accepted, underscoring the need for consistent and standardized SUID reporting practices. This report describes the classification system used by the SUID Case Registry, including its standardized definitions and decision-making algorithm, and applies the categorization process to 436 SUID cases. These were cases that occurred in 2011 and were reported to the registry from participating states. The algorithm guides the assignment of cases into explained (“Suffocation with unsafe sleep factors”) and unexplained categories (“No autopsy or death scene investigation,” “Incomplete case information,” “No unsafe sleep factors,” “Unsafe sleep factors,” and “Possible suffocation with unsafe sleep factors”). These categories are not intended to, and do not, replace official cause-of-death determinations, but are meant to apply a common categorization process to cases to allow local and state programs to better track and understand SUID.
FLOW OF DATA FROM CASE IDENTIFICATION TO CATEGORIZATION
The SUID Case Registry program and how it builds on the infrastructure of child death review (CDR) teams has been previously reported.9 Briefly, the Registry is a population-based, multi-state surveillance program developed in partnership with the National Center for the Review and Prevention of Child Death (NCRPCD), formerly the National Center for Child Death Review.9 Local and state CDR teams operate under state legislation and program policies. However, with funding from CDC for increased staffing and resources along with technical assistance, grantees (ie, state health departments or their bona fide agents) comprehensively review all resident SUID cases.
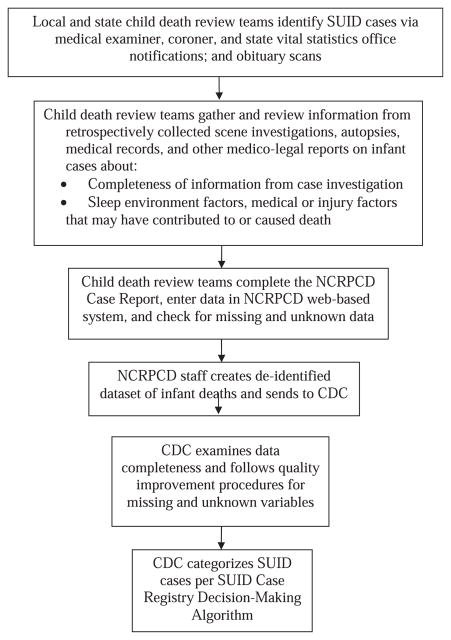
Figure 1 describes the flow of the SUID Case Registry data from the time of case identification to the time of SUID category assignment. SUID cases are identified by CDR teams using several methods, including notification from medical examiner or coroner offices and notification from state vital statistics offices. For each case, the multidisciplinary CDR team (including medical examiners, coroners, law enforcement, public health representatives, other health care providers, and social service representatives) reviews and discusses SUID case information from retrospectively collected death scene investigations, autopsies, medical records, and other medico-legal reports. The CDR team also identifies actionable strategies to help prevent future deaths and improve case investigation. The CDR team enters this information in the web-based NCRPCD Case Report,19 which includes a standard set of questions developed specifically to review SUID cases.20 The CDC receives de-identified data for all SUID cases reported from participating states and examines data for completeness. States are asked to reconcile any missing and unknown variables. Once the CDC receives the most complete information, CDC staff review cases and assign categories using the standardized definitions and decision-making algorithm. An explanation of these SUID categories and criteria used to define these groupings are described in Table 1. These categories can be used to identify risk factors and characteristics of infant deaths that could be potentially prevented, to improve scene investigation and autopsies, and to identify potential cases for clinical research. Personally identifying information is not available about cases, and resulting categorizations do not alter previously ascribed official cause-of-death determinations. In other words, these categories are strictly for surveillance purposes and do not replace official medical examiner or coroner cause-of-death determinations.
FIGURE 1.
Flow of SUID Case Registry data from case identification to categorization.
TABLE 1.
Definitions and Criteria for Assigning Cases to SUID Case Registry Categories
| Category | Criteria That Must Be Met |
|---|---|
| Unexplained: no autopsy or death scene investigation |
|
| Unexplained: incomplete case information |
|
| Unexplained: no unsafe sleep factors |
|
| Unexplained: unsafe sleep factors |
|
| Unexplained: possible suffocation with unsafe sleep factors |
|
| Explained: suffocation with unsafe sleep factors |
|
Complete case investigation is defined by the components of the death scene investigation and autopsy that were documented in the case report. For death scene investigation, detailed information about where and how the body was found was available. For autopsy, all 3 tests were performed and documented: (1) toxicology, (2) radiograph, and (3) pathology. Pathology can include histology, microbiology, or other pathology such as genetic testing, but not solely gross examination.
Safe sleep environment: supine position on a firm sleep surface including a crib, bassinet, portable crib, or pack-and-play. Sleep surface is free of soft objects, loose bedding, bumper pads, or any objects that could increase the risk for entrapment, suffocation, or strangulation out of the crib. Intentionally placing an infant to sleep in a car seat is considered unsafe. We derived these criteria from the 2011 AAP recommendations for a safe infant sleeping environment.23
An example of a concerning medical condition is an infant who has fever, vomiting, and lethargy in the 72 h before death.
DEVELOPING STANDARD DEFINITIONS FOR SUID CASE REGISTRY SURVEILLANCE
Although we considered other SUID classification systems,10–18 none completely fit the surveillance and programmatic purposes of the SUID Case Registry. In 2010, Sidebotham21 encouraged that any new categorical definitions be simple and improve on earlier classification systems. As such, our SUID Case Registry classification system incorporates the contributions of other colleagues.13,16,22 Similar to Randall et al16 and Pasquales-Styles et al,22 the SUID Case Registry system recognizes the importance of possible asphyxia or suffocation in sudden unexpected and unexplained infant death cases. Like Randall et al,16 our system also recognizes the degree of uncertainty that can accompany deaths in potentially asphyxiating environments. Also like others,13,16,17 we acknowledge the uncertainty resulting either from inadequate review of the death scene or incomplete forensic examination, and incorporated this uncertainty in our category definitions. To that end, like the Krous et al13 system and the Avon system,17 we have included a category, “no unsafe sleep factors,” to account for the proportion of unexplained deaths that occurred in an environment in which unsafe sleep factors were not identified. Our categories and definitions overlap with many of Randall et al,16 although our labels and criteria were created to meet the aims of the SUID Case Registry. Although we did not limit definitional categories to specific infant age groups (eg, postneonatal period or until 6 or 8 months of age)13 or require that a death occur during sleep like some earlier classifications,10,13,15 our categories allow further stratification by age and sleep status, depending on analytic needs.
In creating categories, we also considered the challenges reported with earlier categorization schemes.13,15–17 These challenges include reviewer diagnostic preferences, incomplete scene investigation and forensic investigation records, and lack of information about where and how the infant was found (including detailed factors about the sleeping environment), especially as related to airway obstruction. To reduce inconsistency among reviewers in assigning cases to categories, we created carefully defined criteria and questions to weigh evidence for each category. To address incomplete investigation data and limited contextual data about the sleep environment and potential airway obstruction, we developed categories to reflect this incomplete information. By quantifying the number of cases with incomplete investigation, programs can identify gaps and develop targeted interventions to improve scene and forensic investigation.
Assigning Categories Using the SUID Case Registry Decision-Making Algorithm
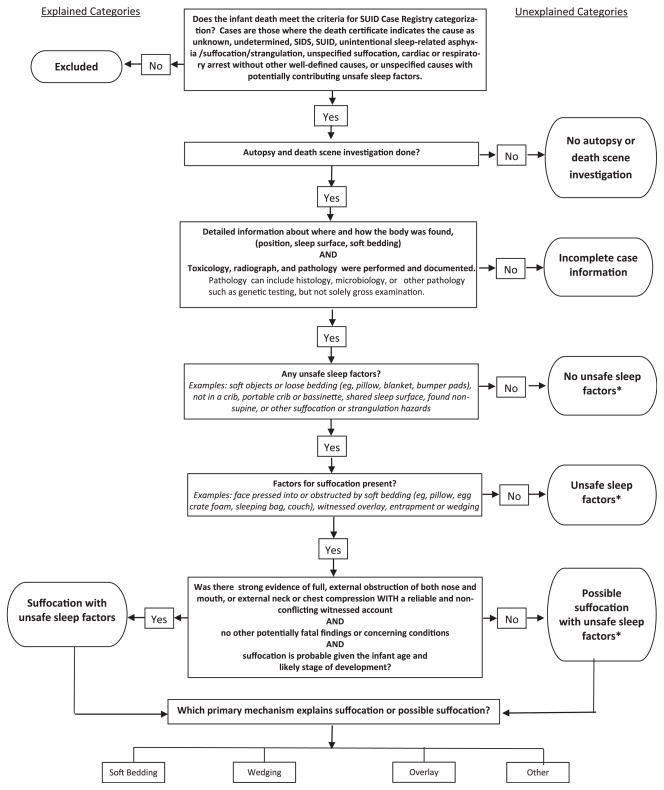
The SUID Case Registry Decision-Making Algorithm (Fig 2) serves as a systematic guide for assigning SUID cases to categories, based on information in the Case Registry reporting system. The algorithm begins with eligible SUID cases (ie, reviewed, entered, and verified complete). Case reviews are not final until a cause of death is assigned. Cases with a pending cause after the death year cohort closes are categorized as “incomplete case information.” Cases reported to the registry that are categorized as SUID include those in which the death certificate indicates that the cause was unknown, undetermined, SIDS, SUID, unintentional sleep-related asphyxia/suffocation/strangulation, unspecified suffocation, cardiac or respiratory arrest without other well-defined causes, or unspecified causes with potentially contributing unsafe sleep factors. Cases in which manner of death is reported as homicide, are excluded. Two trained members of the CDC’s SUID Case Registry program staff review and categorize each case by applying the algorithm that consists of a series of questions (Fig 2). Staff members work together to reach consensus about the assignment of a particular category. This process can be completed in ~7 minutes. In those cases in which the 2-member review team cannot concur, the case is conservatively grouped in the category reflecting the most uncertainty, eg, “Unexplained: Possible suffocation with unsafe sleep factors” versus “Explained: Suffocation with unsafe sleep factors.” After the initial review, all cases that are categorized as “Explained: Suffocation with unsafe sleep factors” and the few cases (<10 of those reported for our analysis) that cannot be reconciled are reviewed by 2 other program staff and a category is assigned by group consensus. The former cases are reviewed because of the high degree of difficulty of distinguishing these cases from unexplained, possible suffocation with unsafe sleep factors (see below). This secondary review takes ~5 minutes. Data are received quarterly and cases are categorized on an ongoing basis.
FIGURE 2.
Decision-making algorithm for assigning SUID case registry categories. *Category includes cases that may or may not have other potentially fatal findings concerning conditions, or competing cause of death, but how these factors contribute to death is uncertain.
To use the algorithm (Fig 2), one must apply the SUID Case Registry’s definition for a complete case investigation (includes both death scene investigation and autopsy) and a safe sleep environment (Table 1). At a minimum, the death scene investigation must provide sufficiently detailed information for the reviewers to envision where and how the body was found. For an autopsy to be considered complete, it must include evidence that the following tests were performed and documented: (1) toxicology, (2) radiograph, and (3) pathology. Pathology can include histology, microbiology, or other pathology such as genetic testing, but not solely gross examination. These minimal criteria were based on expert opinion and a National Association of Medical Examiners white paper.8 A safe sleep environment is 1 where: (1) the infant is found supine on a firm sleep surface including a crib or bassinet mattress, portable crib, or pack-and-play, and (2) the sleep surface is free of soft objects, loose bedding, bumper pads or any objects that could increase the risk for entrapment, suffocation, or strangulation. We derived these criteria from the 2011 AAP recommendations for a safe infant sleeping environment.23
Categories are based on the completeness of the case investigation, including death scene investigation, autopsy, and medical history. Categories are used to distinguish accidental sleep-related suffocations from other SUID groupings. Categories are further subdivided as “Explained” and “Unexplained” (Table 1 and Fig 2).
The 2 categories, “No autopsy or death scene investigation” and “Incomplete case information,” are the easiest to distinguish (Table 1 and Fig 2). The remaining categories, which aim to distinguish how or if an unsafe sleeping environment contributed to the death, are more difficult to differentiate, although each has complete case information by definition.
The category, “no unsafe sleep factors,” includes cases that may or may not have occurred during sleep or in a sleeping environment (Table 1 and Figure 2). For deaths occurring during sleep or in a sleeping environment, no potentially asphyxiating hazards were reported. In contrast, cases assigned “Unsafe sleep factors” are those found in an unsafe sleep environment, but the role of the potential suffocation or strangulation hazards relative to death is uncertain. This category includes cases in which the scene investigation does not provide any evidence of airway obstruction. For example, if an infant is sharing a sleep surface with an adult and there is no documentation that the adult overlaid the infant or that the infant’s airway was obstructed, the death is called “Unsafe sleep factors.” In such cases, the evidence about the airway is insufficient to categorize as explained or possible suffocation.
The most difficult task is distinguishing between “Unexplained: Possible suffocation with unsafe sleep factors” and “Explained: Suffocation with unsafe sleep factors.” To categorize a case as “Explained: Suffocation with unsafe sleep factors,” there must be strong evidence of suffocation (eg, report of full obstruction of nose and mouth or external compression of the neck or chest). Also, the event must be reliably witnessed with no conflicting reports or documented potentially fatal findings or other concerning medical conditions. Some examples are: (1) a 1-month-old infant found face down in a pillow with her nose and mouth fully obstructed; (2) a 2-month-old infant found with her head and face wedged between the cushions at the back of the sofa; and (3) a 4-month-old who is found lifeless in a twin bed with his head and body underneath his mother. On the other hand, deaths categorized as “Unexplained: Possible suffocation with unsafe sleep factors,” have some evidence that suffocation may have occurred, but information about a fully obstructed airway is weak or not based on a reliable witness account, or there is evidence of potentially fatal findings or other concerning conditions. An example of a case that would be assigned to “Unexplained: Possible suffocation with unsafe sleep factors” is an infant previously assumed to be healthy, but who has an atrial septal defect found at autopsy and was found face down on top of a pillow. Although the infant was found face down on a pillow with his nose and mouth obstructed, evidence of a competing potentially fatal finding (i.e, atrial septal defect) precludes assignment to the explained suffocation category. Importantly, in instances in which the reviewers cannot concur when differentiating between explained suffocation and unexplained possible suffocation, the case is assigned as an unexplained possible suffocation.
Mechanisms of Accidental Suffocation in a Sleep Environment
Cases categorized as possible and explained suffocation with unsafe sleep factors are further grouped by mechanism (Table 2). These mechanisms include: (1) overlay by a person, (2) soft bedding, (3) wedging/entrapment, and (4) others, such as suffocation by a plastic bag. When a single mechanism cannot be assigned for a particular case, more than 1 option may be selected and such cases are grouped together as “2 or more mechanisms identified.” Analysis of the mechanisms attributed to possible or explained suffocation might provide further insight into modifiable risk factors in the sleep environment and quantify the contribution of different mechanisms to suffocation deaths.
TABLE 2.
Definitions of Mechanisms for Suffocation or Possible Suffocation
| SUID Category | Definition |
|---|---|
| Mechanisms for suffocation | Factors in the sleep environment that caused or may have caused suffocation. |
| Overlay | Shared sleep surface with other person overlaying or rolling on top of or against infant while sleeping and obstructing airway or compressing the neck or chest area and preventing respiration. |
| Soft bedding | Soft or loose bedding, pillows, or stuffed toys on sleep surface obstructing airway. Infant found face down or other position with airway obstruction. |
| Wedging or entrapment | Wedging and entrapment of an infant between 2 objects such as a mattress and wall, bed frame, or furniture causing airway obstruction or compressing chest and preventing respiration. |
| Other | Other factors in the sleep environment causing an airway obstruction, for example, a situation in which an infant is sleeping in a car seat or stroller and the infant’s face or neck position results in an airway obstruction. |
Development of the Decision-Making Algorithm
The development of the algorithm was an iterative process and was conducted between 2009 and 2013. During its development, we applied the category definitions and algorithm to nearly 1000 cases reported in the SUID Case Registry system data files. In assigning cases to categories, the algorithm highlighted limitations in the ability of the case report to fully capture where and how a body was found and whether there was any obstruction of the airway, especially the nose and mouth. Because of this limitation, we modified the case report form with improved questions and wording and trained grantees how to incorporate these changes. Before finalizing, we applied the algorithm and category definitions to all cases reported to the registry in 2011 (the most recent year with completed data) that met the case definition. Minor revisions to wording were made to improve clarity.
Application of the Classification System
Table 3 shows the aggregated data from the initial 7 SUID Case Registry states (Colorado, Georgia, Michigan, Minnesota, New Hampshire, New Jersey, and New Mexico) and the categories to which the cases were assigned. Of the 436 SUID cases identified in 2011, most (n = 382; 88%) were classified as unexplained SUID and most occurred in an unsafe sleep environment (n = 320; 73%). “Suffocation with unsafe sleep factors” was assigned to 54 cases (12%), and 95 cases (22%) were assigned to “Possible suffocation with unsafe sleep factors.” In 5 states with 35 or more reported cases (Colorado, Georgia, Michigan, Minnesota, and New Jersey), the proportion of cases without a documented complete investigation varied, ranging widely from 5% to 55%. Also in these states, the highest proportion of deaths (with complete information) were assigned to the category “Unsafe sleep factors” (range, 45%–63%), followed by “Possible suffocation with unsafe sleep factors” (range, 20%–36%), “Suffocation with unsafe sleep factors” (range, 15%–25%), and “No unsafe sleep factors” (all states had <5 deaths in this category). Of the 66 possible and explained suffocation deaths in these states, the mechanism most frequently reported was soft bedding (n = 63), followed by overlay (n = 29), wedging/entrapment (n = 18), and other (10) (Table 4).
TABLE 3.
Number and Percentage of the 436 Cases Occurring in 2011 and Reported to the SUID Case Registry by SUID Category, by All Cases, and by Those With Complete Case Information Only
| SUID Category | Total Cases |
Percentage of Cases by
Statea
|
|||||
|---|---|---|---|---|---|---|---|
| Number | % | A | B | C | D | E | |
| All categories | |||||||
| Total n | 436 | 100% | b | b | b | b | b |
| Unexplained | |||||||
| No death scene investigation or autopsy | 11 | 3 | 0 | 3 | 10 | 0 | 3 |
| Incomplete case information | 101 | 23 | 5 | 33 | 27 | 55 | 8 |
| No unsafe sleep factors | 4c | <1 | 2 | 0 | 2 | 0 | 3 |
| Unsafe sleep environment | 171 | 39 | 51 | 30 | 40 | 21 | 44 |
| Possible suffocation with unsafe sleep factors | 95 | 22 | 23 | 23 | 13 | 14 | 28 |
| Explained | |||||||
| Suffocation with unsafe sleep factors | 54 | 12 | 18 | 11 | 10 | 11 | 15 |
| Incomplete informationd | 112 | 26 | 5 | 36 | 37 | 55 | 10 |
| Categories with complete information only | |||||||
| Unexplained | |||||||
| No unsafe sleep factors | 4c | 1 | 2 | 0 | 3 | 0 | 3 |
| Unsafe sleep environment | 171 | 53 | 54 | 46 | 63 | 45 | 49 |
| Possible suffocation with unsafe sleep factors | 95 | 29 | 25 | 36 | 20 | 30 | 31 |
| Explained | |||||||
| Suffocation with unsafe sleep factors | 54 | 17 | 19 | 17 | 15 | 25 | 17 |
States are represented by letters to maintain confidentiality. Percentages for states with fewer than 35 reported deaths are not calculated owing to small numbers and resulting unstable estimates.
The 436 cases reported by state in 2011 include: CO (44), GA (111), MI (142), MN (39), NH (6), NJ (63), and NM (31).
Three deaths occurred during a sleeping period and 1 did not.
Incomplete information: no death scene investigation or autopsy plus incomplete case information.
TABLE 4.
Number of Cases Categorized as “Possible Suffocation With Unsafe Sleep Factors” or “Suffocation With Unsafe Sleep Factors” by Mechanism, SUID Case Registry, 2011 Deaths
| Number of Casesa by Mechanismb
|
|||||
|---|---|---|---|---|---|
| Soft Bedding | Overlay | Wedging or Entrapment | Other | 2 or More | |
| All casesc | |||||
| Possible suffocation with unsafe sleep factors (n = 95) | 49 | 17 | 6 | 7 | 4 |
| Suffocation with unsafe sleep factors (n = 54) | 17 | 12 | 12 | 3 | 5 |
| Cases by state | |||||
| State A | |||||
| Possible suffocation with unsafe sleep factors (n = 26) | 1 | 0 | 4 | 2 | 0 |
| Suffocation with unsafe sleep factors (n = 20) | 3 | 3 | 1 | 1 | 3 |
| State B | |||||
| Possible suffocation with unsafe sleep factors (n = 33) | 27 | 1 | 4 | 2 | 2 |
| Suffocation with unsafe sleep factors (n = 16) | 7 | 4 | 6 | 0 | 1 |
| State C | |||||
| Possible suffocation with unsafe sleep factors (n = 8) | 5 | 3 | 1 | 1 | 2 |
| Suffocation with unsafe sleep factors (n = 6) | 2 | 3 | 2 | 0 | 1 |
| State D | |||||
| Possible suffocation with unsafe sleep factors (n = 6) | 6 | 0 | 0 | 0 | 0 |
| Suffocation with unsafe sleep factors (n = 5) | 2 | 1 | 0 | 2 | 0 |
| State E | |||||
| Possible suffocation with unsafe sleep factors (n = 11) | 6 | 1 | 2 | 2 | 0 |
| Suffocation with unsafe sleep factors (n = 6) | 3 | 0 | 3 | 0 | 0 |
Because fewer than 35 deaths were reported in NH and NM in 2011, data are not presented.
Multiple mechanisms can be assigned per case, but none of the 100 cases categorized had >2 mechanisms identified. Because each case can be assigned >1 mechanism, the sum of the mechanisms is greater than the total number of cases.
“All cases” includes reported cases from CO, GA, MI, MN, NH, NJ, and NM.
DISCUSSION
We have built on and strengthened the work of other investigators in defining and categorizing SIDS and other SUID for the purposes of the SUID Case Registry.10–17,19–21 Our classification system emphasizes the uncertainty about how suffocation or asphyxiation may have contributed to death and also accounts for unknown and incomplete information about the death scene and autopsy. Until comprehensive scene investigations are conducted on all SUID cases and standardized criteria are universally accepted by death certifiers, states and local public health programs can use information gathered from the SUID Case Registry and its classification system as a supplement to death certificate surveillance. We estimate that the current 9 states participating in the registry capture ~13% of all US SUID. The registry’s standardized categories allow programs to quantify the number of cases that: (1) have incomplete investigations; (2) occur in an unsafe sleep environment such as with soft bedding or with a shared sleep surface; and (3) are possible or explained suffocation cases by mechanism. By identifying and quantifying incomplete case information, local and state jurisdictions can identify strategies and target appropriate resources to improve scene and forensic investigations, as well as child death review programs. Additionally, these categories may help programs better understand the circumstances that potentially contribute to or actually cause suffocation or asphyxiation, which could lead to more strategic interventions.
Strengths
The SUID Case Registry’s process for assigning cases to categories of explained and unexplained SUID has several strengths. First, program staff and collaborators built on the work of other investigators10–17,19–21 and created categories that meet local and state surveillance and program needs. Second, categorization relies on standardized definitions of SUID subtypes and a simple decision-making algorithm with explicitly defined criteria with labels that are descriptive and easy to distinguish from 1 another. Finally, the system has been applied to hundreds of cases with assignment of categories based on group consensus by trained reviewers.
Another potential strength of these standardized categories may extend beyond the registry and its surveillance purposes. The categories could be used by researchers conducting epidemiologic and biomarker studies to define cases according to the degree of certainty about potentially contributing asphyxiation factors. Researchers could also use these categories to identify new mechanisms and factors associated with SUID. In addition, the SUID Case Registry categories can be easily grouped under existing ICD-10 codes for comparison with vital statistics data from death certificates. Specifically the categories “No autopsy or death scene investigation” and “Incomplete case information” could be grouped under the ICD-10 code R99, “Ill-defined and unknown cause of mortality.” The “explained” category, “Suffocation with unsafe sleep factors,” corresponds with ICD-10 code W75, “Accidental suffocation and strangulation in bed.” The remaining categories, all with complete case investigations but with varying degrees of uncertainty about contributing factors, could be grouped under the ICD-10 code R95, “SIDS.” Finally, if death certifiers eventually accepted these categories or a modified version, the categories could be used as a future rubric to make and report cause-of-death determinations on the death certificates.
Challenges in Categorizing Deaths
Although this classification system is largely automated, the qualitative part of the review is subjective and cannot be automated. To reduce potential bias from this qualitative analysis, our system applies standardized definitions, has trained reviewers who agree on categories through consensus, has a secondary review process, and relies on a guiding principle to err on the side of caution (ie, when in doubt, choose the category that reflects the least certainty). The greatest challenge in grouping SUID cases into defined categories is having sufficient evidence available about the sleep environment and airway. CDR teams must rely on retrospectively collected information about the death scene investigation. Of the 436 cases reported in 2011 to our surveillance system, 190 had a report of a scene re-enactment. Case reports with missing or unknown information for variables compromise data quality and completeness. In our data, one-quarter of the cases had incomplete information, although this varied widely between states. Missing and unknown information can lead to a different categorization outcome than the true category.
Because the states participating in the registry likely have greater access to critical death scene investigation data and are able to use more resources to eliminate missing information than non-funded states, generalization about the distribution of deaths in each category relative to other US states or populations may be limited. The CDC’s SUID Case Registry now operates in 9 states: Arizona, Colorado, Louisiana, Michigan, Minnesota, New Hampshire, New Jersey, New Mexico, and Wisconsin. If the registry were to become a national surveillance system, the quality of data about circumstances surrounding SUID could be enhanced in more states, and investigators and programs could have a deepened understanding of the magnitude of and characteristics associated with SUID.
CONCLUSIONS
The SUID Case Registry’s classification system has built on the work of other investigators in defining and categorizing SIDS and other SUID cases, while recognizing the uncertainty about the contribution of asphyxiation to the death and also accounting for variations in case investigations. This new system has been tested with hundreds of cases and has performed well when categorizing challenging cases. Finally, the system has key implications for both public health and clinical research. For public health, the classification system allows local and state programs to more accurately track the magnitude of specific types of SUID over time, creating a valuable tool to identify gaps in case investigation of SUID and an enhanced ability to identify the highest risk groups who might benefit from focused interventions or increased services. For clinical investigation, consistent reporting of SIDS and other SUID is crucial for identifying pathophysiologic and genetic mechanisms underlying these deaths.
Acknowledgments
We acknowledge the following contributors for their critical input and advice in conceptualizing this project: Kim Collins, MD, Timothy E. Corden, MD, John Fudenberg, D-ABMDI, Geoffrey Smith, MD, Bradley T. Thach, MD, and Gregory Wyatt. Also, special thanks to Shin Kim, MPH, for her contributions in conceptualizing the classification system and reviewing an early draft, and to Alexa Erck, MPH, for her assistance with analysis and thoughtful review of the final draft of the manuscript. Finally, we thank the NCRPCD and the CDR coordinators in the participating states for their support of this project by allowing access to the data contained in this report.
FUNDING: This work was supported in part by the Maternal and Infant Health Branch, Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, and in part by Federal funds from the Centers for Disease Control and Prevention under contract GS07F67053R.
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- CDR
child death review
- ICD-10
Tenth Revision of the International Classification of Diseases
- NCRPCD
National Center for the Review and Prevention of Child Death (formerly the National Center for Child Death Review)
- SIDS
sudden infant death syndrome
- SUID
sudden unexpected infant death
Footnotes
Dr Shapiro-Mendoza conceptualized and designed the classification system, coordinated and supervised the development and revision of categorization instruments, led the activities related to assignment of cases to categories, drafted, revised, and wrote the final version of the manuscript, and oversaw the project in general; Dr Camperlengo and Ms Ludvigsen participated in conceptualizing the categories, led activities to modify data collection instruments, provided technical assistance regarding review, data entry, and quality control for state grantees, carried out the initial analyses, and critically reviewed the manuscript; Ms Cottengim provided technical assistance regarding review, data entry, and quality control for state grantees, carried out the final analyses, and critically reviewed the manuscript; Ms Covington participated in conceptualizing the categories, designed the data collection instruments, provided technical assistance regarding review, data entry, and quality control for state grantees, carried out the initial analyses, and critically reviewed the manuscript; Drs Anderson, Andrew, Hauck, Kemp, and MacDorman participated in conceptualizing the categories and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
This work was presented in abstract form at the American Association of SIDS Prevention Physicians 22th Annual Conference, Naples, Florida, September 19 to 21, 2013.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page e240, online at www.pediatrics.org/cgi/doi/10.1542/peds.2014-0602.
FINANCIAL DISCLOSURE: Ms Covington’s agency, the Michigan Public Health Institute, received funds from EGS that originated at the Centers for Disease Control and Prevention in the amount of $233,076.48 for the period April 1, 2009 to May 31, 2011, to develop and support components of the SUID Case Registry described in the article (reference 09FED907750, contract GS07F67053R). The other authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) [Accessed May 2, 2014];Compressed Mortality File (CMF) on CDC WONDER Online Database. 2013 CMF 1999–2010, Series 20, No. 2P. Available at: http://wonder.cdc.gov/mortsql.html.
- 2.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163(8):762–769. doi: 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 3.Camperlengo LT, Shapiro-Mendoza CK, Kim SY. Sudden infant death syndrome: diagnostic practices and investigative policies, 2004. Am J Forensic Med Pathol. 2012;33(3):197–201. doi: 10.1097/PAF.0b013e3181fe33bd. [DOI] [PubMed] [Google Scholar]
- 4.Randall BB, Paterson DS, Haas EA, et al. Potential asphyxia and brainstem abnormalities in sudden and unexpected death in infants. Pediatrics. 2013;132(6) doi: 10.1542/peds.2013-0700. Available at: www.pediatrics.org/cgi/content/full/132/6/e1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol. 1991;11(5):677–684. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Shapiro-Mendoza CK, Chu SY, Camperlengo LT, Anderson RN. Differentiating cause-of-death terminology for deaths coded as sudden infant death syndrome, accidental suffocation, and unknown cause: an investigation using US death certificates, 2003–2004. J Forensic Sci. 2012;57(2):364–369. doi: 10.1111/j.1556-4029.2011.01937.x. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro-Mendoza CK, Kim SY, Chu SY, Kahn E, Anderson RN. Using death certificates to characterize sudden infant death syndrome (SIDS): opportunities and limitations. J Pediatr. 2010;156(1):38–43. doi: 10.1016/j.jpeds.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Corey TS, Hanzlick R, Howard J, Nelson C, Krous H NAME Ad Hoc Committee on Sudden Unexplained Infant Death. A functional approach to sudden unexplained infant deaths. Am J Forensic Med Pathol. 2007;28(3):271–277. doi: 10.1097/01.paf.0000257385.25803.cf. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro-Mendoza CK, Camperlengo LT, Kim SY, Covington T. The sudden unexpected infant death case registry: a method to improve surveillance. Pediatrics. 2012;129(2) doi: 10.1542/peds.2011-0854. Available at: www.pediatrics.org/cgi/content/full/129/2/e486. [DOI] [PubMed] [Google Scholar]
- 10.Beckwith JB. Defining the sudden infant death syndrome. Arch Pediatr Adolesc Med. 2003;157(3):286–290. doi: 10.1001/archpedi.157.3.286. [DOI] [PubMed] [Google Scholar]
- 11.Fleming PJ, Blair PS, Sidebotham PD, Hayler T. Investigating sudden unexpected deaths in infancy and childhood and caring for bereaved families: an integrated multiagency approach. BMJ. 2004;328(7435):331–334. doi: 10.1136/bmj.328.7435.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanzlick R, Hunsaker JC, III, Davis GJ. A Guide for Manner of Death Classification. Atlanta, GA: National Association of Medical Examiners; 2002. [Google Scholar]
- 13.Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome and un-classified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114(1):234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 14.Randall BB, Wadee SA, Sens MA, et al. A practical classification schema incorporating consideration of possible asphyxia in cases of sudden unexpected infant death. Forensic Sci Med Pathol. 2009;5(4):254–260. doi: 10.1007/s12024-009-9083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields LB, Hunsaker JC, III, Corey TS, Stewart D. Is SIDS on the rise? J Ky Med Assoc. 2007;105(8):343–353. [PubMed] [Google Scholar]
- 16.Randall B, Donelan K, Koponen M, Sens MA, Krous HF. Application of a classification system focusing on potential asphyxia for cases of sudden unexpected infant death. Forensic Sci Med Pathol. 2012;8(1):34–39. doi: 10.1007/s12024-011-9291-0. [DOI] [PubMed] [Google Scholar]
- 17.Blair PS, Byard RW, Fleming P. Proposal for an international classification of SUDI. Scand J Forens Sci. 2009;2009(1):6–9. [Google Scholar]
- 18.Blair PS, Byard RW, Fleming PJ. Sudden unexpected death in infancy (SUDI): suggested classification and applications to facilitate research activity. Forensic Sci Med Pathol. 2012;8(3):312–315. doi: 10.1007/s12024-011-9294-x. [DOI] [PubMed] [Google Scholar]
- 19.The National Center for Child Death Review. [Accessed March 27, 2014];Child Death Review Case Reporting System: Case Report 2.0. Available at: www.child-deathreview.org/reports/CDRCaseReport-Form02202008.pdf.
- 20.Covington TM. The US National Child Death review case reporting system. Inj Prev. 2011;17(suppl 1):i34–i37. doi: 10.1136/ip.2010.031203. [DOI] [PubMed] [Google Scholar]
- 21.Sidebotham P. Do we need a new definition for SIDS?: Commentary on “Sudden unexpected death in infancy and the dilemma of defining the sudden infant death syndrome” by Henry Krous. Curr Pediatr Rev. 2010;6(1):13–14. [Google Scholar]
- 22.Pasquale-Styles MA, Tackitt PL, Schmidt CJ. Infant death scene investigation and the assessment of potential risk factors for asphyxia: a review of 209 sudden unexpected infant deaths. J Forensic Sci. 2007;52(4):924–929. doi: 10.1111/j.1556-4029.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 23.Moon RY, Darnall RA, Goodstein MH, et al. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128(5) doi: 10.1542/peds.2011-2285. Available at: www.pediatrics.org/cgi/content/full/128/5/e1341. [DOI] [PubMed] [Google Scholar]