Abstract
Background
Low-income and racial/ethnic minority populations experience disproportionate colorectal cancer (CRC) burden and poorer survival. Novel behavioral strategies are needed to improve screening rates in these groups.
Purpose
To test a theoretically based “implementation intentions” intervention for improving CRC screening among unscreened adults in urban safety-net clinics.
Design
Randomized controlled trial.
Setting/participants
Adults (N=470) aged ≥50 years, due for CRC screening, from urban safety-net clinics were recruited.
Intervention
The intervention (conducted in 2009–2011) was delivered via touchscreen computers that tailored informational messages to decisional stage and screening barriers. The computer then randomized participants to generic health information on diet and exercise (Comparison group) or “implementation intentions” questions and planning (Experimental group) specific to the CRC screening test chosen (fecal immunochemical test or colonoscopy).
Main outcome measures
The primary study outcome was completion of CRC screening at 26 weeks based on test reports (analysis conducted in 2012–2013).
Results
The study population had a mean age of 57 years, and was 42% non-Hispanic African American, 28% non-Hispanic white, and 27% Hispanic. Those receiving the implementation intentions–based intervention had higher odds (AOR=1.83, 95% CI=1.23, 2.73) of completing CRC screening than the Comparison group. Those with higher self-efficacy for screening (AOR=1.57, 95% CI=1.03, 2.39), history of asthma (AOR=2.20, 95% CI=1.26, 3.84), no history of diabetes (AOR=1.86, 95% CI=1.21, 2.86), and reporting they had never heard that “cutting on cancer” makes it spread (AOR=1.78, 95% CI=1.16, 2.72) were more likely to complete CRC screening.
Conclusions
The results of this study suggest that programs incorporating an implementation intentions approach can contribute to successful completion of CRC screening even among very low-income and diverse primary care populations. Future initiatives to reduce CRC incidence and mortality disparities may be able to employ implementation intentions in large-scale efforts to encourage screening and prevention behaviors.
Introduction
Racial/ethnic minorities receive fewer colorectal cancer (CRC) screening tests and are less likely to be up to date with CRC screening than others.1–9 Age-eligible adults, particularly racial/ethnic minorities, report significant barriers to CRC screening, including the inconvenient or impractical nature of the tests,10–13 the embarrassing or unpleasant nature of the tests,12,13 fatalistic cancer beliefs,14,15 and fear of finding something wrong.12,16,17
A vast array of interventions and programs has been used to promote CRC screening.18–22 Although a growing body of studies has focused on low-income groups, such as urban minorities, challenges remain for assuring screening-related mortality reduction.23–25
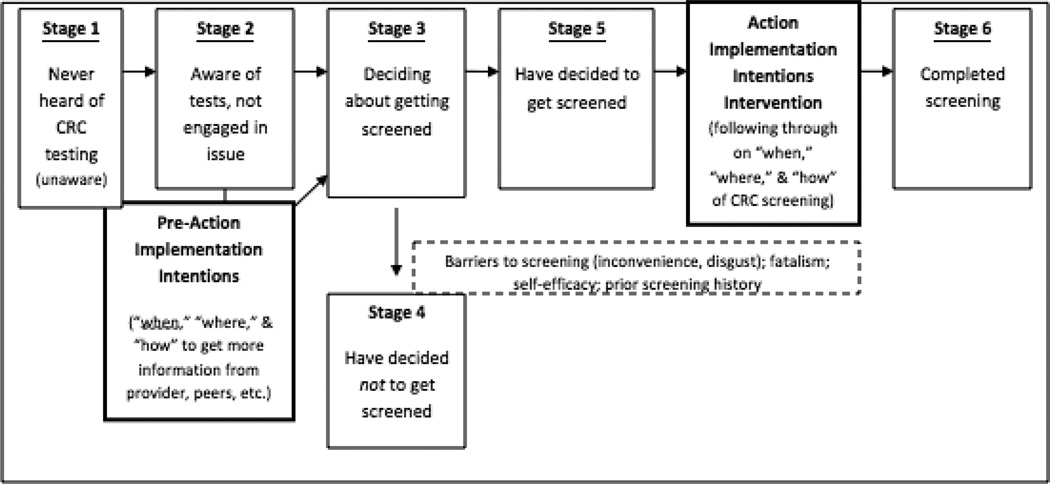
Health researchers have conceptualized the decision-making process for patients considering screening tests with many models.26,27 The Precaution Adoption Process Model (PAPM) is a stage theory of health behavior that includes stages for individuals who are “unaware” or have no knowledge of a health issue and also for those who have “decided against” engaging in a particular health behavior.28 In this study, PAPM and the concept of “implementation intentions”29(I-I) were used to model CRC screening behavior. This concept provides a theoretical grounding for behavioral interventions to construct tailored action plans that specify “when,” “where,” and “how” an intention (e.g., the intention to undergo CRC screening) will be implemented.30 A large number of I-I interventions have been conducted, showing positive behavioral results across populations. A limited number have focused on low-income/safety-net or minority populations.31–33 I-I can lead to initiation of action even when people are stressed, such as opiate addicts undergoing withdrawal, or when they experience high cognitive loads, such as multi-tasking.34 They have been found to reduce consultations for emergency contraception and unintended pregnancies among teenage girls attending a family planning clinic,35,36 increase cervical screening rates in women in rural England,37 and increase physical activity among low-income children and adults.31,32 Although recent studies suggest that interventions based on the I-I approach can enhance some types of cancer screening,37,38 no studies, to date, have tested I-I interventions for CRC screening. The primary aim of this RCT was to assess the efficacy of a touchscreen computer intervention using I-I for increasing completion of CRC screening.
Methods
Participants and Setting
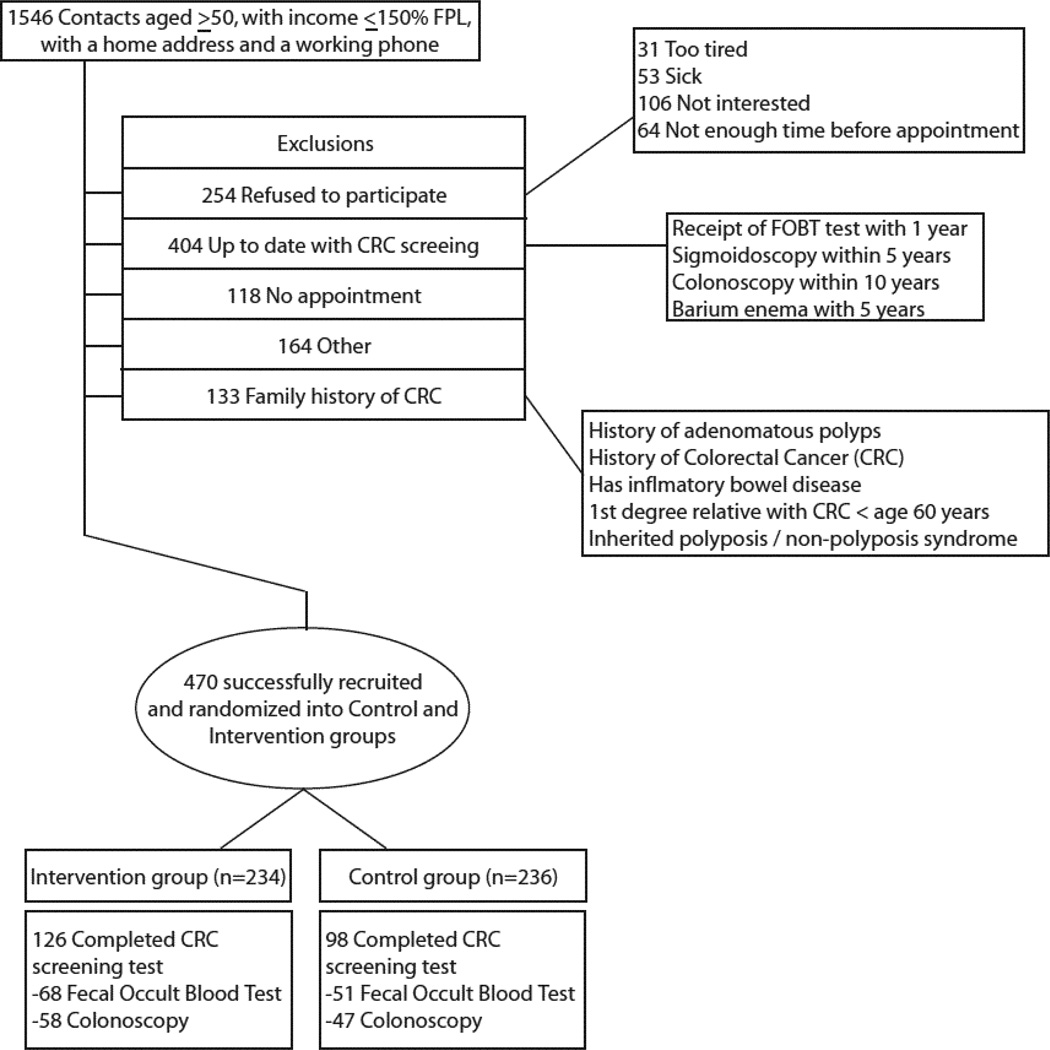
Four hundred seventy participants from nine safety-net clinics were recruited in a Midwestern metropolitan area. Health center staff from each clinic referred patients to kiosks in clinic waiting areas where research associates were available to facilitate eligibility screenings. These associates administered a verbal screening survey to determine patients’ study eligibility (Figure 1). All who were deemed eligible and agreed to participate provided informed consent and began study activities immediately. The study was restricted to patients aged ≥50 years with a provider visit the day of enrollment. Reasons for ineligibility are provided in Figure 1. Individuals were excluded if they had an income >150% of the Federal Poverty Level; lacked a home address and working telephone; had received a fecal occult blood test (FOBT) or fecal immunochemical test (FIT) within the last year, sigmoidoscopy or barium enema within the last 5 years, or colonoscopy within the last 10 years; had an acute medical illness; reported current gastrointestinal bleeding, history of colon polyps, history of CRC, a first-degree relative with CRC prior to age 60 years, an inherited polyposis/non-polyposis syndrome, inflammatory bowel disease; had another household member enrolled in the study; or had a cognitive impairment. The study was approved by the study team’s IRB.
Figure 1.
CONSORT flow diagram
Intervention
In 2009–2011, consented participants were randomized to one of two intervention groups: (1) an implementation intentions condition (Experimental group); or (2) a generic education condition (Comparison group). The Experimental group received information and education on CRC screening and responded to I-I planning questions specific to CRC screening. The Comparison group received information and education on CRC screening followed by questions on diet, exercise, and healthy living (as an attention control). All participants utilized an interactive, multimedia touchscreen computer that used either English or Spanish text with narration through headphones, as well as pictures and video targeted to the race/ethnicity of the participant. All clinic staff and healthcare providers were blinded to group assignment and not aware of the content differences between the two groups. Headphones and placement of kiosks in semi-private or private areas of clinics were used to prevent contamination between individuals assigned to different groups.
CRC screening education (provided via touchscreen computer to both groups) content was designed to be informational and intended to raise awareness among those with limited prior exposure to CRC information. Formative research activities (interviews, usability testing) informed the development of all components and were used to target the touchscreen materials to the appropriate audience.39 The touchscreen computer narrative and video components for both groups employed race/ethnicity-matched narration and video clips with detailed instruction on test completion. Scripted scenes were reproduced for the three major participant groups in our study: African Americans, Latinos, and whites. The computerized scenes for both groups included didactic instruction on use of FITs and completion of colonoscopy bowel preparation. Again, all educational components within the touchscreen system were identical for participants in both groups.
Only following presentation of educational content and assessment of decisional stage did the computer system differ for the two groups, with the Experimental group completing a series of questions and receiving information on I-I and the Comparison group completing items and receiving information on diet and physical activity behaviors. Computer system components, including assessment items, audio narrative, and brief multimedia instructional video clips were equivalent in length between the two groups.
For the Experimental group participants, PAPM staging items were used to tailor the I-I questions. For participants in the “unaware,” “unengaged,” or “undecided stages”, a tailored segment asked them to choose specific I-I for seeking out additional information on CRC screening from their healthcare provider, relatives, spouses, peers, or media. For those participants at more-advanced PAPM stages (deciding or decided to get screened), the segments asked participants to choose I-I for the “when,” “where,” and “how” they planned to complete a FIT or schedule, prep for, and complete a colonoscopy. All I-I questions were developed through a series of interviews testing the touchscreen system (Table 1).39
Table 1.
Implementation intentions question examples
| Question | Responses | |
|---|---|---|
| To remind myself to call and set up my colonoscopy appointment, I will…… |
|
|
| Some people need to take time off work or get childcare when they set up a colonoscopy appointment. It usually takes a full day to get the test, and recover from the pain medicine you receive during the test. I will request time off from work or childcare…․ |
|
|
| The day before my colonoscopy, to remind myself to start taking the laxative medicines, I will…… |
|
|
| I will get a ride to my colonoscopy from…․ |
|
|
| To remind myself to mail in my card with the stool samples, I will…. |
|
|
| I will mail the card with the two stool samples within…․ |
|
At completion of computer activities, all participants received a computer printout in their primary language (English or Spanish). Printouts for the Experimental group restated I-I entries from the computer, whereas Comparison group printouts restated diet and physical activity entries.
If the participant (from either group) reported “deciding to” pursue a screening test, research associates ensured that they received either a FIT kit or colonoscopy scheduling information and bowel preparation materials before leaving the clinic. Pre-packaged kits containing FIT cards, applicator brushes, and pre-addressed lab-return envelopes were provided to participants who preferred FIT. Kits contained brief text and pictorial instructions on the use of FIT. The study used the Enterix InSure™ FIT test because of test characteristics thought to maximize completion among the safety-net clinic populations targeted in the study.40 For participants preferring colonoscopy, research associates provided colonoscopy scheduling assistance after completion of the touchscreen module. If colonoscopy scheduling could not be completed immediately, participants were provided detailed scheduling information and were instructed to handle their own scheduling and call for a colonoscopy appointment later. No colonoscopy scheduling assistance was provided to participants in either group after this in-clinic assistance and research associates had no further contact with participants until a 13–26-week follow-up survey. To avoid contamination effects of the intervention and its I-I components, research associates and the computer did not encourage participants to discuss CRC screening with their healthcare provider. They told participants that they would talk to them after their provider visit to ask a few questions (exit interview). If participants asked questions about CRC screening, all research associates were trained to provide detailed answers, which helped avoid the need for deflection of questions from the research associate to the provider.
Participants in both groups were reimbursed with a $20 gift card and mailed an additional $20 gift card after completion of the follow-up survey. Costs for both FIT and colonoscopy were covered by the study in an effort to remove cost as a major barrier to obtaining screening. By paying for the tests, the study intended to explore the influence of I-I on screening behavior independent of cost barriers.
Data Collection and Measures
During eligibility screening, participants reported information about their health history and history of prior CRC screening. Next, during the touchscreen program they completed a baseline survey that assessed basic demographics, PAPM stage, perceived susceptibility to CRC, self-efficacy for CRC screening, cancer fatalism, CRC screening barriers, and screening test preference. These items were chosen based on prior work, conducted formative work, and an explicit theoretic model (Figure 2).39,41Table 2 provides examples and descriptions of these measures. After completing all computer activities and seeing their healthcare provider, all participants completed a 5-item exit survey to assess whether they had discussed CRC screening with their provider. Finally, all participants were re-contacted between 13 and 26 weeks post-enrollment to complete a final survey assessment. This survey and all computerized and verbally administered assessments were delivered in English or Spanish.
Figure 2.
The integrated Precaution Adoption Process Model and Implementation Intentions concept applied to colorectal cancer (CRC) screening in this study.
Table 2.
Fatalism, self-efficacy, susceptibility, barriers, and PAPM question examples
| Measure | Question/Responses |
|---|---|
| Fatalism (10 items) |
|
| Self-efficacy (1 item) | |
| CRC Susceptibility (3 items |
|
| Barriers (9 items for FIT, 11 items for Colonoscopy) |
|
FIT
|
|
Colonoscopy
|
|
| PAPM stage (5 items) |
|
PAPM stage was coded as 1–5 or “unaware,” “unengaged,” “deciding,” “decided not to screen,” or “decided to be screened” and the range of participants was from 1 to 5. Except PAPM staging, the score of each measure was the sum of items. Each fatalism item had 2 responses, coded as 0 = No and 1 = Yes, and the fatalism scores ranged from 13 to 30; self-efficacy ranged from 1 (very likely) to 4 (very unlikely); each CRC susceptibility item had responses of 1, 2, and 3, and CRC susceptibility scores ranged from 3 to 9; each barrier item had 4 responses: 1 = Doesn’t apply, 2 = applies a little, 3 = applies somewhat, and 4 = applies very much; FIT (n = 182) barrier scores ranged from 9 to 36, and colonoscopy barrier scores ranged from 11 to 44 (n = 327).
Outcome Measure
The primary study outcome measure was completion of either a FIT or screening colonoscopy. Colonoscopies were conducted at the university medical center’s endoscopy center and results were sent directly to study staff. Those choosing FIT tests mailed them to Quest Diagnostics, Inc. (Lenexa KS). Following processing, FIT test results were mailed and later electronically transmitted to study staff.
Independent variables were chosen based on theory (Figure 2) and prior studies that showed a relationship between these constructs and CRC screening behavior.41,42 They included cancer fatalism, perceived self-efficacy, PAPM stage, perceived risk of getting CRC, insurance coverage, education, employment, marital status, having a regular physician, heart disease, cancer, high blood pressure, asthma, and diabetes. All variables were measured by self-report from participants. Cancer fatalism items assessed attitudes toward getting cancer and the effectiveness of treatments. The Powe Cancer Fatalism Inventory14,41 was adapted with ten cancer fatalism items measured by Likert scale. These included: I think cancer will kill you no matter when it is found, and I think if I get checked for cancer, I am less likely to die from cancer. Individual items were not highly correlated and were included as separate variables in analysis. Separate CRC screening barriers items for FIT and colonoscopy tests were developed during formative studies leading up to this trial.43 Self-efficacy included one question for each screening test: Do you think you could complete a colonoscopy/stool blood test? adapted from prior multi-item self-efficacy measures.44,45 Perceived self-efficacy refers to participants’ self confidence in completing a task. For CRC susceptibility, a 3-item scale developed by Vernon et al.46 was used. Example items included: How likely do you think it is that you will develop colon cancer in the future? and How often do you worry about getting colon cancer? PAPM stages were determined to be “unaware,” “unengaged,” “undecided,” “deciding,” “decided not to screen,” or “decided to be screened” (Figure 2) based on questions asked for each test modality, such as: Have you thought about getting FIT/colonoscopy? and Do you want to get this test? Items were adapted from the work of Weinstein, Costanza, and Myers.28,47,48
Office Exit Interview Survey
Research staff administered a 5-item survey in English or Spanish to all study participants as they checked out of the primary care office after their healthcare provider visit. The survey asked if participants discussed CRC screening with their healthcare provider, whether their provider recommended a certain type of CRC screening test, and whether this was consistent with their expressed preference from the touchscreen assessment they had previously completed. Items were developed based on formative interview analysis.39
Thirteen- to 26-week Follow-Up Survey
All participants were contacted by phone to complete a brief survey. All participants were contacted at 13 weeks, and then systematically scheduled for additional follow up calls every 6 weeks (up to 26 weeks), if they described continued and ongoing efforts to schedule or complete CRC screening. The 13–26-week period was chosen based on local scheduling availability for colonoscopies and rescheduling timeframes typical for patients at the study endoscopy center. Participants who had yet to complete CRC screening at this point were re-asked PAPM staging questions. Those who had completed screening were asked to describe whether they felt the touchscreen computer intervention helped them complete screening and how. Those in the Experimental group were asked whether they felt that the I-I items helped them plan and carry out screening and how. Individuals received up to 15 phone calls, and, for those who could not be reached by phone, one home visit until they were contacted or were deemed unreachable. Unreachable individuals were sent a certified letter asking them to call project staff directly. Project staff worked with administrative staff in each of the safety-net clinics to re-check and update addresses and phone numbers for participants before they were deemed unreachable.
Data Analysis
Prior to initiating outcome analyses, frequency distributions for all variables, with particular attention to variable ranges and skewness were examined. Response values beyond defined ranges were checked for entry-error and coded as missing if they were invalid values. Variables considered in multivariable logistic regression included: age, cancer fatalism, self-efficacy, education, gender, marital status, ethnicity/race, insurance status, employment status, having asthma, having diabetes, experience of not having medical care owing to concern about cost, PAPM stage, and physician recommendation for screening. Variables were selected by backward elimination procedure using the Bayesian information criterion (BIC). Potential multicollinearity was examined by variance inflation factor (VIF). Interactions between these variables and group were examined but none was included according to BIC. Main effects for each of the clinics were included, to account for any clinic level variance that could have influenced our primary outcome. AORs for completion of screening were computed for each variable in the model. All analyses were performed in 2012–2013 using SAS, version 9.2 (SAS Institute Inc., Cary NC).
Results
Project staff screened 1,546 individuals to successfully recruit the 470 participants (Figure 1). The characteristics of the 470 participants who completed the program are shown in Table 4. The study population was predominately female (63.6%) and had a median age of 55 years. Approximately 27% reported Hispanic ethnicity. Race and ethnicity were combined into a single race/ethnicity variable. Non-Hispanic African Americans and whites accounted for 42% and 28% of the study, respectively (3% non-Hispanic other). Twenty-seven percent were married, 36% were divorced, and 16% were never married. The majority of the study population was uninsured (76.6%), and all participants were identified as low-income (≤150% Federal Poverty Level, per eligibility requirements). There were no statistically significant differences in demographics between the intervention conditions. One participant had an undefined response for the variable about having experience of not getting medical care owing to the concern of cost of care, and another had an undefined response for having heard of cutting on cancer. These two observations were coded as missing, thus the sample size was reduced to 468.
Table 4.
Baseline demographic characteristics by comparison condition
| Characteristic | Control (n=236) |
Experimental (n=234) |
p-value |
|---|---|---|---|
| Age, years, median (Inter-Quartile Range) | 55 (8) | 55 (9) | 0.24a |
| Ethnicity | 0.65b | ||
| Non-Hispanic, n=344 (73%) | 175 (74.2%) | 169 (72.2%) | |
| Hispanic, n=126 (27%) | 61 (25.8%) | 65 (27.8%) | |
| Ethnicity/Race | 0.07b | ||
| Hispanic, n=126 (27%) | 61 (25.8%) | 65 (27.8%) | |
| Non-Hispanic White, n=132 (28%) | 61 (25.8%) | 71 (30.3%) | |
| Non-Hispanic African American, n=198 (42%) | 108 (45.8%) | 90 (38.5%) | |
| Non-Hispanic Other, n=14 (3%) | 6 (2.5%) | 8 (3.4%) | |
| Marital Status | 0.025b | ||
| Married or living w/ partner, n=155 (33%) | 64 (27.1%) | 91 (38.9%) | |
| Divorced or separated, n=171 (36%) | 94 (39.8%) | 77 (32.9%) | |
| Widowed or never married, n=144 (31%) | 78 (33.1%) | 66 (28.2%) | |
| Education | 0.58b | ||
| High school or below, n=281 (60%) | 141 (59.7%) | 140 (59.8%) | |
| Some college or above, n=189 (40%) | 95 (40.3%) | 94 (40.2%) | |
| Employment | 0.15b | ||
| Full-time/part-time/seasonal | 65 (27.5%) | 74 (31.6%) | |
| Looking/homemaker/student/retired | 133 (56.4%) | 114 (48.7%) | |
| Disability | 38 (16.1%) | 46 (19.7%) | |
| Insured | 60 (25.4%) | 50 (21.4%) | 0.07b |
2-sample t-test;
Chi-squared test
Boldface indicates statistical significance (p<0.05).
During the touchscreen assessment, 89% of the total participants reported that they intended to complete CRC screening (89.7% of the Experimental group versus 88.1% of the Comparison group). The remaining 11% had either decided not to be screened or were still deciding.
Out of the total sample, 48% completed a CRC screening test (of those screened, 53% completed a FIT and 47% completed a colonoscopy). Participants who received I-I (Experimental group) were more likely to complete CRC screening than those in the Comparison group (54% to 42%, AOR=1.91, 95% CI=1.26, 2.89). The results of our multivariate analyses are shown in Table 5. Screening completion by race/ethnicity showed that 51.6% of Hispanics, 47.5% of non-Hispanic African Americans, and 47.7% non-Hispanic whites completed screening. Of those completing screening, 55.4% of Hispanics, 52.2% of non-Hispanic blacks, and 45.9% of non-Hispanic whites completed FIT, and 44.6% of Hispanics, 47.8% of non-Hispanic blacks, and 54.1% of non-Hispanic whites completed colonoscopy (differences across race/ethnicity were not significant).
Table 5.
Factors independently associated with colorectal cancer test completion based on logistic regression analysis
| Participant Characteristics | AOR (95% CI) | p-value |
|---|---|---|
| Group 1 – Implementation Intentions Condition | 1.91 (1.26–2.89) | <0.01 |
| Asthma | 2.71 (1.49–4.91) | <0.01 |
| No Diabetes | 1.76 (1.12–2.76) | 0.01 |
| Never Hearing of Cut on Cancer | 1.74 (1.10–2.75) | 0.02 |
| PAPM stage | 1.91 (0.89, 4.10) | 0.10 |
| FIT vs. no preference Colonoscopy vs. no preference | 4.07 (1.89, 8.80) | <0.01 |
Note: Boldface indicates statistical significance (p<0.05).
Other variables included in the final model were Age, preferred language, Cancer Fatalism, Education, Gender, Marital Status, clinic, ethnicity/race, insurance status, employment status, experience of not having medical care due to concern about cost of care, physician recommendation for screening, self-efficacy. VIF ranges from 1.31 to 2.08 for the main effects in Table 4.
Other factors associated with increased odds of completing CRC screening included no prior diagnosis of diabetes (AOR=1.76, 95% CI=1.12, 2.76), prior diagnosis of asthma (AOR=2.71, 95% CI=1.49, 4.91), never having heard that cutting on cancer causes it to spread (AOR=1.74, 95% CI=1.10, 2.75), and those who reported they were “very likely” to be able to screen if they chose to (self efficacy, AOR=1.56, 95% CI=1.01, 2.43). Participants who reported specific preference for either the FIT or colonoscopy screening test were more likely to complete screening than participants who reported no preference (AOR=1.91, 95% CI=0.89, 4.10 if preferred FIT; AOR=4.07, 95% CI=1.89, 8.80 if preferred colonoscopy). Race/ethnicity and language preference were not related to likelihood of completing CRC screening. Of the 126 participants in the Experimental Group that completed screening, 68 completed FIT and 58 completed colonoscopy. For the 98 screeners in the Comparison Group, 51 completed FIT and 47 completed colonoscopy. The FIT versus colonoscopy differences in completers across these groups were not significant.
Exit interviews (Table 3) showed that the majority of study participants talked to their doctor about CRC screening (309 of 468, 66%) during their office visit after completion of touchscreen computer activities. Almost all felt that they received all the information they needed (97%). For the 61% of all participants (249 of 408) that received a recommendation from their provider to get a CRC screening test, the majority received a recommendation for colonoscopy (54% colonoscopy, 13% FIT, 33% other). Almost all participants (99%) reported that they understood the messages they received on the touchscreen computer. The 61% of participants who received a provider recommendation for CRC screening were not more likely to complete CRC screening (50.6% completed screening among those talking to provider versus 44.5% among those who did not). There was no relationship between receipt of a provider recommendation and randomization condition.
Table 3.
Exit interview
| Question | Control (n=236) |
Experimental (n=234) |
p-value |
|---|---|---|---|
| Did you talk about colorectal cancer screening with your health care provider during your visit today? | 0.42a | ||
| No, n=159 (34%) | 84(18%) | 75(16%) | |
| Yes, n=309 (66%) | 151(32%) | 158(34%) | |
| If yes, did you feel you received all the information you needed? | 1.00b | ||
| No, n=9 (3%) | 4(1%) | 5(1%) | |
| Yes, n=300 (97%) | 147(48%) | 153(50%) | |
| Did your health care provider specifically recommend you get a colorectal cancer screening test? | 0.69a | ||
| No, n=159 (39%) | 83(20%) | 76(19%) | |
| Yes, n=249 (61%) | 125(31%) | 124(30%) | |
| If yes, which test? | 0.90a | ||
| Colonoscopy, n=125 (54%) | 60(26%) | 65(28%) | |
| FOBT, n=29 (13%) | 15(6%) | 14(6%) | |
| Other, n =77 (33%) | 36(16%) | 41((18%) | |
| Does this differ from what you chose on the computer? | 0.94a | ||
| No, n=150 (79%) | 72(38%) | 78(41%) | |
| Yes, n=39 (21%) | 19(10%) | 20(11%) | |
| Did you generally understand the touchscreen computer messages and information? | 0.25b | ||
| No, n=2 (0.4%) | 0(0%) | 2(1%) | |
| Yes, n=466 (99.6%) | 235(50%) | 231(49%) |
Chi-squared test;
Fisher’s Exact Test
The final retention rate was 85% for the follow-up survey. At follow-up, 64% of participants who completed a CRC test either scheduled their colonoscopy or began collecting FIT samples within 1 week of the intervention (data not shown). Most (72%) remembered the touchscreen computer somewhat to very well. Test completers reported the touchscreen computer as the most helpful to completing their CRC test (55% computer, 19% provider, 19% other, and 3% family/friends). Participants in the Experimental group were more likely to report I don’t want to have a colonoscopy than those in the Control group (20 participants versus five, respectively, p<0.05).
Discussion
The goal of this study was to test the efficacy of a novel, tailored, touchscreen computer program designed to increase uptake of CRC screening. The results of the multivariable analysis showed that participants in the Experimental group, who were guided through the formation of I-I for CRC screening, were more likely to follow through with screening than participants in the Comparison group who did not form CRC screening I-I. The process of having patients identify exactly how they “intend” to complete all actions involved in CRC screening may have helped them logistically complete screening. Because participants in both groups received the same CRC screening educational information and were at equal decisional stages for screening prior to delivery of I-I content, it is likely that I-I played a key role in driving screening completion differences. Efforts to construct the Comparison group intervention as a “behavioral control” resulted in both groups receiving similar levels of attention. Furthermore, the work “cost” of our I-I intervention, though not directly measured herein, was essentially equal to the work “cost” of formative/qualitative work necessary to customize the robust educational material required for both study arms. Likely barriers, such as colon preparation, which involves intake of large amounts of laxatives and many trips to the bathroom, may surprise or overwhelm patients when they are unprepared or do not have detailed expectations. Additionally, some patients may not know the amount of time involved, or the importance of finding transportation, child care, or requesting time off work prior to their appointment. In most medical facilities, colonoscopy will not be performed if patients arrive alone with no confirmed transportation assistance. Similarly, for FIT testing, detailed instructions and planning for the collection of stool samples may help to facilitate test completion. Future studies may be valuable in showing whether I-I are more or less impactful in low income groups. Results in this study are consistent with other research supporting the utility of I-I for promoting health behavior completion.36,49,50 I-I interventions can be quickly implemented in clinical settings, especially those such as community health centers, where staff and systems may be in place for such program initiation.
The cost of screening tests, often reported as important by patients,51 was not a factor in this study because participants were given access to CRC screening tests free of charge or co-pays. As expected, participants with a definite test preference (for FIT or colonoscopy) were more likely to complete screening. This may be because these participants already had engaged in decision-making processes or had some knowledge and awareness about the tests prior to enrollment.
Some work in underserved populations has revealed a belief that surgery to remove cancerous growths can cause cancer to spread.52 Participants who reported never having heard that cutting on cancer causes it to spread were more likely to complete screening. It is possible that those with no exposure to this common belief are uniquely health literate and thus more likely to complete screening. This fear was strong enough in the study population to remain significant despite built-in educational messages in both arms of the intervention debunking the myth. This could also reflect a lack of trust in the information presented about cancer surgery52,53 and suggests the need for further emphasis on improving factual knowledge.
Higher self-efficacy was associated with greater likelihood of test completion. This is consistent with a large body of research showing a link between self-efficacy and various health behaviors.54
The findings that those with asthma diagnoses and those without diabetes were more likely to complete screening are difficult to interpret. Reporting a diagnosis of cancer, heart disease, or high blood pressure was unrelated to screening. It is unclear how factors like familiarity with the healthcare system or the burden of symptomatic disease may have affected these groups. Unlike many prior studies,55–57 results showed that prior provider recommendations were unrelated to the impact of the experimental or control intervention. This may relate to the study’s coverage of test costs or the limited impact providers may have on behaviors in the safety-net setting, where patients may have many needs and complex comorbidities to address.
The study has several important limitations. Owing to recruitment and intervention delivery in safety-net clinics, the study was unable to completely blind all study personnel to the group assignment of patients. Unintended bias could have influenced main findings. Bias could also have been introduced from the relatively high participation refusal rate among patients approached in the nine clinics. The study may have missed an opportunity to influence even greater screening by encouraging study participants to talk to their providers about CRC screening after receiving the intervention. Another primary study limitation was failure of the hospital endoscopy scheduling department to provide consistent Spanish-speaking scheduling support to participants. Process monitoring revealed that research assistants often received calls from Hispanic participants regarding trouble scheduling their colonoscopies. Study staff were instructed to advise the participants to ask for a Spanish speaking scheduler but staff were not allowed to assist in scheduling appointments for the patients as the study sought to reflect “normal” processes associated with the healthcare system. Lastly, the coverage of screening costs limits the generalizability of the findings, but demonstrates the potential of I-I free of this barrier to test completion. These cost-neutral results may be valuable, as new Affordable Care Act insurance provisions under health reform require all new plans, whether employer-provided or otherwise, to cover CRC screening tests without co-pays or co-insurance.
Acknowledgments
This study would not have been possible without the direct support of the Wyandotte County Safety-Net Clinic Coalition, as well as providers and staff from Swope Wyandotte, Swope West, Swope Central, KC CARE Clinic, Southwest Blvd. Family Health Care, Duchesne Clinic, Mercy and Truth Clinics, and Silver City Health Clinic. Quest Diagnostics, Inc. supported this study through provision and processing of InSure FIT kits. Finally, this study is a tribute to the late Marci Kramish Campbell, PhD, a groundbreaking scientist in the field of nutrition, communications, technology, and health behavior and an inspiration to many of us who were fortunate to call her a mentor, colleague, and collaborator. Our work on the concept of implementation intentions was the direct result of the insight and input from of Dr. Campbell and we hope to honor her always through top-quality science and interventions to reduce health disparities.
The study was supported by grant No. CA123245 from the National Cancer Institute. All opinions expressed herein are the sole responsibility of the authors and do not reflect the views of the NIH. Fecal immunochemical tests were provided for the study by Enterix Inc., 236 Fernwood Avenue, Edison NJ 08337. Laboratory analysis of InSure© test specimens was provided by Quest Diagnostics, Inc., Lenexa, KS 66219. Trial Registry Name: ClinicalTrials.gov: ClinicalTrials.gov identifier: NCT00594113; registered January 3, 2008. University of Kansas Medical Center IRB approval August 24, 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
References
- 1.Ko CW, Kreuter W, Baldwin LM. Effect of Medicare coverage on use of invasive colorectal cancer screening tests. Arch Intern Med. 2002 Dec 9–23;162(22):2581–2586. doi: 10.1001/archinte.162.22.2581. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin SS, Thompson TD, Seeff L, Richards T, Stallings F. Breast, cervical, and colorectal carcinoma screening in a demographically defined region of the southern U.S. Cancer. 2002 Nov 15;95(10):2211–2222. doi: 10.1002/cncr.10933. [DOI] [PubMed] [Google Scholar]
- 3.Holmes-Rovner M, Williams GA, Hoppough S, Quillan L, Butler R, Given CW. Colorectal cancer screening barriers in persons with low income. Cancer Pract. 2002 Sep-Oct;10(5):240–247. doi: 10.1046/j.1523-5394.2002.105003.x. [DOI] [PubMed] [Google Scholar]
- 4.Nadel MR, Blackman DK, Shapiro JA, Seeff LC. Are people being screened for colorectal cancer as recommended? Results from the National Health Interview Survey. Prev Med. 2002 Sep;35(3):199–206. doi: 10.1006/pmed.2002.1070. [DOI] [PubMed] [Google Scholar]
- 5.Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001 Nov 21;93(22):1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 6.Behavioral Risk Factor Surveillance System, 1999. Survey data. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Washington, DC: United States Department of Health and Human Services; 1999. [Google Scholar]
- 7.Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health. 2000 Jun;25(3):263–278. doi: 10.1023/a:1005104406934. [DOI] [PubMed] [Google Scholar]
- 8.Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Prev Med. 2000 Oct;31(4):410–416. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- 9.Paskett ED, Rushing J, D'Agostino R, Jr, Tatum C, Velez R. Cancer screening behaviors of low-income women: the impact of race. Womens Health. 1997 Fall-Winter;3(3–4):203–226. [PubMed] [Google Scholar]
- 10.Bejes C, Marvel MK. Attempting the improbable: offering colorectal cancer screening to all appropriate patients. Fam Pract Res J. 1992 Mar;12(1):83–90. [PubMed] [Google Scholar]
- 11.Myers RE, Ross EA, Wolf TA, Balshem A, Jepson C, Millner L. Behavioral interventions to increase adherence in colorectal cancer screening. Med Care. 1991 Oct;29(10):1039–1050. doi: 10.1097/00005650-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Arveux P, Durand G, Milan C, et al. Views of a general population on mass screening for colorectal cancer: the Burgundy Study. Prev Med. 1992 Sep;21(5):574–581. doi: 10.1016/0091-7435(92)90065-p. [DOI] [PubMed] [Google Scholar]
- 13.Hunter W, Farmer A, Mant D, Verne J, Northover J, Fitzpatrick R. The effect of self-administered faecal occult blood tests on compliance with screening for colorectal cancer: results of a survey of those invited. Fam Pract. 1991 Dec;8(4):367–372. doi: 10.1093/fampra/8.4.367. [DOI] [PubMed] [Google Scholar]
- 14.Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995 Oct;18(5):385–392. [PubMed] [Google Scholar]
- 15.Hoogewerf PE, Hislop TG, Morrison BJ, Burns SD, Sizto R. Health belief and compliance with screening for fecal occult blood. Soc Sci Med. 1990;30(6):721–726. doi: 10.1016/0277-9536(88)90257-2. [DOI] [PubMed] [Google Scholar]
- 16.Vernon SW, Acquavella JF, Yarborough CM, Hughes JI, Thar WE. Reasons for participation and nonparticipation in a colorectal cancer screening program for a cohort of high risk polypropylene workers. J Occup Med. 1990 Jan;32(1):46–51. doi: 10.1097/00043764-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Vernon SW, Gilstrap EL, Jackson GL, Hughes JI. An intervention to increase participation in a work site cancer screening program. Health Values. 1992;16:3–9. [Google Scholar]
- 18.Cole SR, Young GP. Effect of dietary restriction on participation in faecal occult blood test screening for colorectal cancer. Med J Aust. 2001 Aug 20;175(4):195–198. doi: 10.5694/j.1326-5377.2001.tb143094.x. [DOI] [PubMed] [Google Scholar]
- 19.Joseph A. Compliance with fecal occult blood testing: the role of restrictive diets. Am J Public Health. 1988 Jul;78(7):839–841. doi: 10.2105/ajph.78.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson MH, Pye G, Thomas WM, Hardcastle JD, Mangham CM. Haemoccult screening for colorectal cancer: the effect of dietary restriction on compliance. Eur J Surg Oncol. 1994 Oct;20(5):545–548. [PubMed] [Google Scholar]
- 21.Robinson MH, Marks CG, Farrands PA, Bostock K, Hardcastle JD. Screening for colorectal cancer with an immunological faecal occult blood test: 2-year follow-up. Br J Surg. 1996 Apr;83(4):500–501. doi: 10.1002/bjs.1800830420. [DOI] [PubMed] [Google Scholar]
- 22.Thomas WM, Pye G, Hardcastle JD, Mangham CM. Faecal occult blood screening for colorectal neoplasia: a randomized trial of three days or six days of tests. Br J Surg. 1990 Mar;77(3):277–279. doi: 10.1002/bjs.1800770313. [DOI] [PubMed] [Google Scholar]
- 23.Khankari K, Eder M, Osborn CY, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410–1414. doi: 10.1007/s11606-007-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz ML, Fisher JL, Fleming K, Paskett ED. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45–52. doi: 10.1158/1055-9965.EPI-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glanz K, Rimer BK. Theory at a glance. A guide for health promotion practice. Washington, D.C.: National Cancer Institute. National Institutes of Health. U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 27.Trauth JM, Ling BS, Weissfeld JL, Schoen RE, Hayran M. Using the transtheoretical model to stage screening behavior for colorectal cancer. Health education & behavior : the official publication of the Society for Public Health Education. 2003 Jun;30(3):322–336. doi: 10.1177/1090198103030003007. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein ND, Lyon JE, Sandman PM, Cuite CL. Experimental evidence for stages of health behavior change: the precaution adoption process model applied to home radon testing. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1998 Sep;17(5):445–453. doi: 10.1037//0278-6133.17.5.445. [DOI] [PubMed] [Google Scholar]
- 29.Gollwitzer PM. Goal achievement: The role of intentions. European Review of Social Psychology. 1993;4:141–185. [Google Scholar]
- 30.Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54(7):493–503. [Google Scholar]
- 31.Armitage CJ, Arden MA. A volitional help sheet to increase physical activity in people with low socioeconomic status: A randomised exploratory trial. Psychol Health. 2010 Dec;25(10):1129–1145. doi: 10.1080/08870440903121638. [DOI] [PubMed] [Google Scholar]
- 32.Armitage CJ, Sprigg CA. The roles of behavioral and implementation intentions in changing physical activity in young children with low socioeconomic status. J Sport Exerc Psychol. 2010 Jun;32(3):359–376. doi: 10.1123/jsep.32.3.359. [DOI] [PubMed] [Google Scholar]
- 33.Engelman KK, Cupertino AP, Daley CM, et al. Engaging diverse underserved communities to bridge the mammography divide. BMC public health. 2011;11:47. doi: 10.1186/1471-2458-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandstatter V, Lengfelder A, Gollwitzer PM. Implementation intentions and efficient action initiation. Journal of personality and social psychology. 2001 Nov;81(5):946–960. doi: 10.1037//0022-3514.81.5.946. [DOI] [PubMed] [Google Scholar]
- 35.Martin J, Sheeran P, Slade P, Wright A, Dibble T. Implementation intention formation reduces consultations for emergency contraception and pregnancy testing among teenage women. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009 Nov;28(6):762–769. doi: 10.1037/a0016200. [DOI] [PubMed] [Google Scholar]
- 36.Martin J, Sheeran P, Slade P, Wright A, Dibble T. Durable effects of implementation intentions: reduced rates of confirmed pregnancy at 2 years. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2011 May;30(3):368–373. doi: 10.1037/a0022739. [DOI] [PubMed] [Google Scholar]
- 37.Sheeran P, Orbell S. Using implementation intentions to increase attendance for cervical cancer screening. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2000 May;19(3):283–289. doi: 10.1037//0278-6133.19.3.283. [DOI] [PubMed] [Google Scholar]
- 38.Rutter DR, Steadman L, Quine L. An implementation intentions intervention to increase uptake of mammography. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2006 Oct;32(2):127–134. doi: 10.1207/s15324796abm3202_10. [DOI] [PubMed] [Google Scholar]
- 39.Greiner KA, Geana MV, Epp A, et al. A computerized intervention to promote colorectal cancer screening for underserved populations: theoretical background and algorithm development. Technology and health care : official journal of the European Society for Engineering and Medicine. 2012;20(1):25–35. doi: 10.3233/THC-2011-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006 Nov 1;107(9):2152–2159. doi: 10.1002/cncr.22230. [DOI] [PubMed] [Google Scholar]
- 41.Greiner KA, James AS, Born W, et al. Predictors of fecal occult blood test (FOBT) completion among low-income adults. Preventive medicine. 2005 Aug;41(2):676–684. doi: 10.1016/j.ypmed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997 Oct 1;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 43.Greiner KA, Born W, Nollen N, Ahluwalia JS. Knowledge and perceptions of colorectal cancer screening among urban African Americans. Journal of general internal medicine. 2005 Nov;20(11):977–983. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers RE, Vernon SW, Tilley BC, Lu M, Watts BG. Intention to screen for colorectal cancer among white male employees. Prev Med. 1998 Mar-Apr;27(2):279–287. doi: 10.1006/pmed.1998.0264. [DOI] [PubMed] [Google Scholar]
- 45.McQueen A, Tiro JA, Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008 Sep;17(9):2231–2237. doi: 10.1158/1055-9965.EPI-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997 Oct;6(10):825–832. [PubMed] [Google Scholar]
- 47.Costanza ME, Luckmann R, Stoddard AM, et al. Applying a stage model of behavior change to colon cancer screening. Prev Med. 2005 Sep-Oct;41(3–4):707–719. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007 Nov 1;110(9):2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 49.Webb TL, Sheeran P, Totterdell P, Miles E, Mansell W, Baker S. Using implementation intentions to overcome the effect of mood on risky behaviour. The British journal of social psychology / the British Psychological Society. 2010 Nov 3; doi: 10.1348/014466610X533623. [DOI] [PubMed] [Google Scholar]
- 50.van Osch L, Reubsaet A, Lechner L, de Vries H. The formation of specific action plans can enhance sun protection behavior in motivated parents. Prev Med. 2008 Jul;47(1):127–132. doi: 10.1016/j.ypmed.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 51.Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005 Nov;20(11):989–995. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James A, Daley CM, Greiner KA. "Cutting" on cancer: attitudes about cancer spread and surgery among primary care patients in the U.S.A. Soc Sci Med. 2011 Dec;73(11):1669–1673. doi: 10.1016/j.socscimed.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Born W, Engelman K, Greiner KA, et al. Colorectal cancer screening, perceived discrimination, and low-income and trust in doctors: a survey of minority patients. BMC public health. 2009;9:363. doi: 10.1186/1471-2458-9-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins RM. Self-efficacy: a predictor but not a cause of behavior. Journal of behavior therapy and experimental psychiatry. 1992 Dec;23(4):251–256. doi: 10.1016/0005-7916(92)90047-m. [DOI] [PubMed] [Google Scholar]
- 55.Ye J, Xu Z, Aladesanmi O. Provider recommendation for colorectal cancer screening: examining the role of patients' socioeconomic status and health insurance. Cancer epidemiology. 2009 Oct;33(3–4):207–211. doi: 10.1016/j.canep.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Palmer RC, Midgette LA, Dankwa I. Colorectal cancer screening and African Americans: findings from a qualitative study. Cancer control : journal of the Moffitt Cancer Center. 2008 Jan;15(1):72–79. doi: 10.1177/107327480801500109. [DOI] [PubMed] [Google Scholar]
- 57.Jibara G, Jandorf L, Fodera MB, DuHamel KN. Adherence to physician recommendation to colorectal cancer screening colonoscopy among Hispanics. J Gen Intern Med. 2011 Oct;26(10):1124–1130. doi: 10.1007/s11606-011-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012 May 15;118(10):2726–2734. doi: 10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]